Abstract
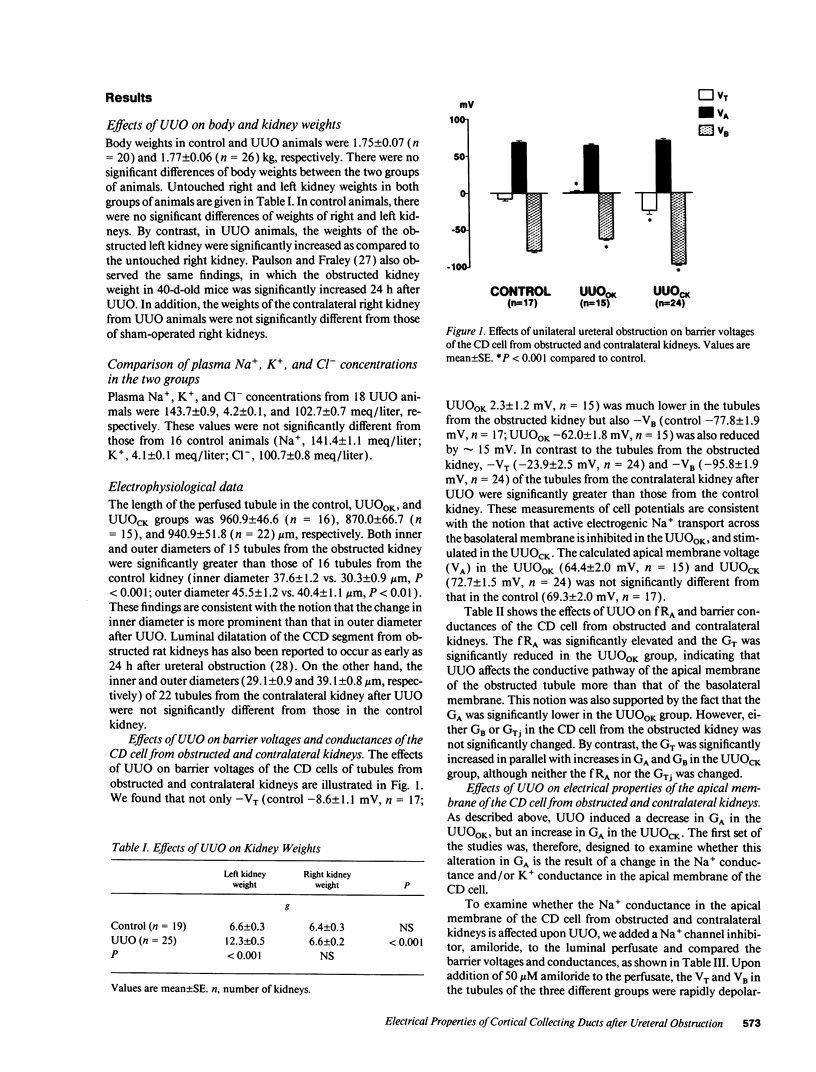
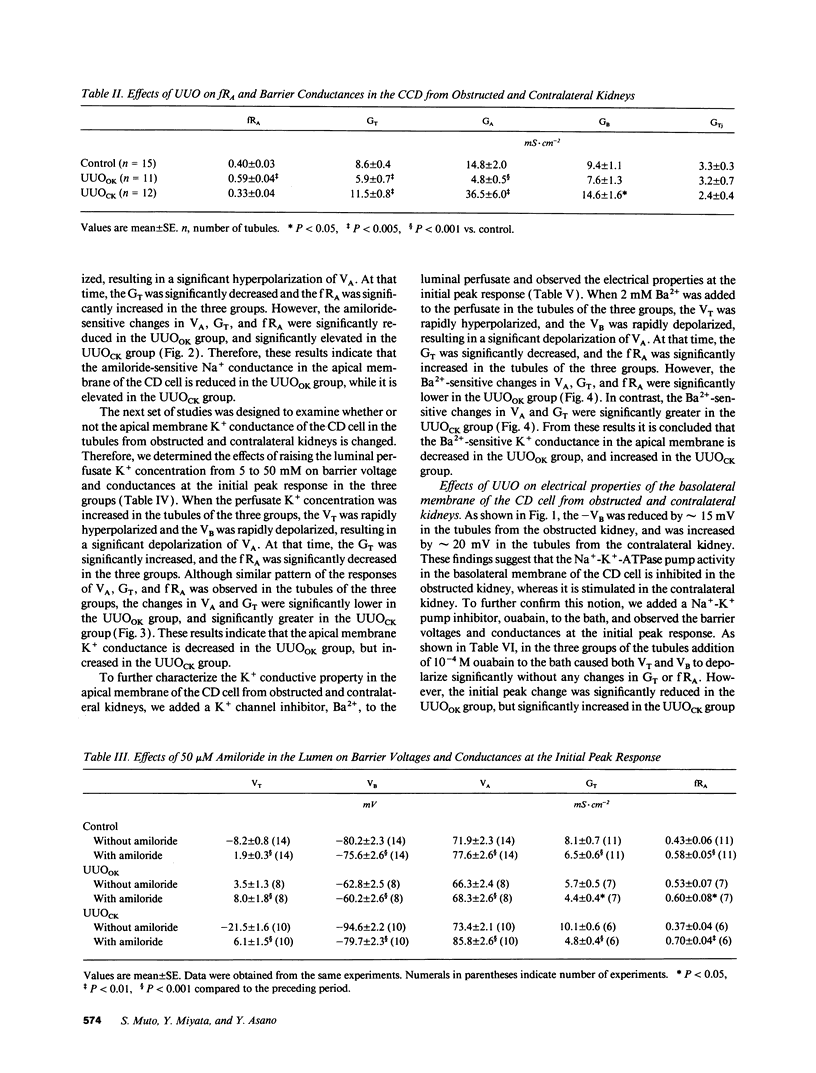
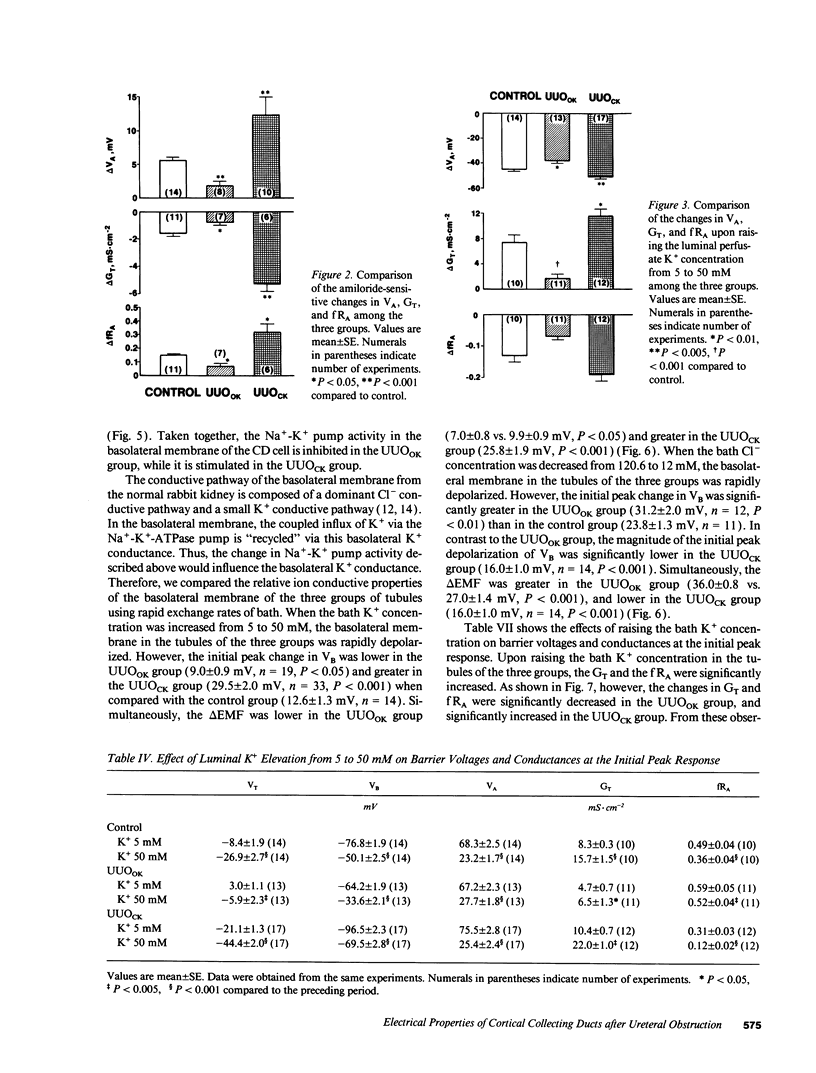
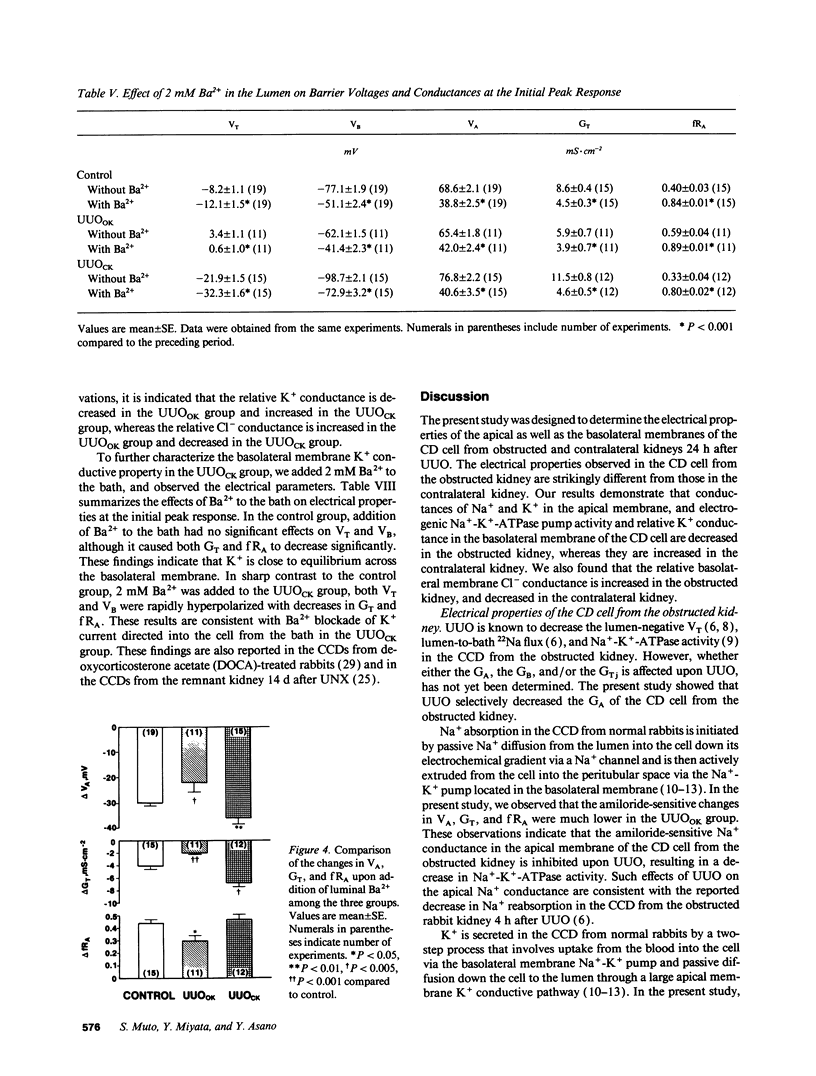
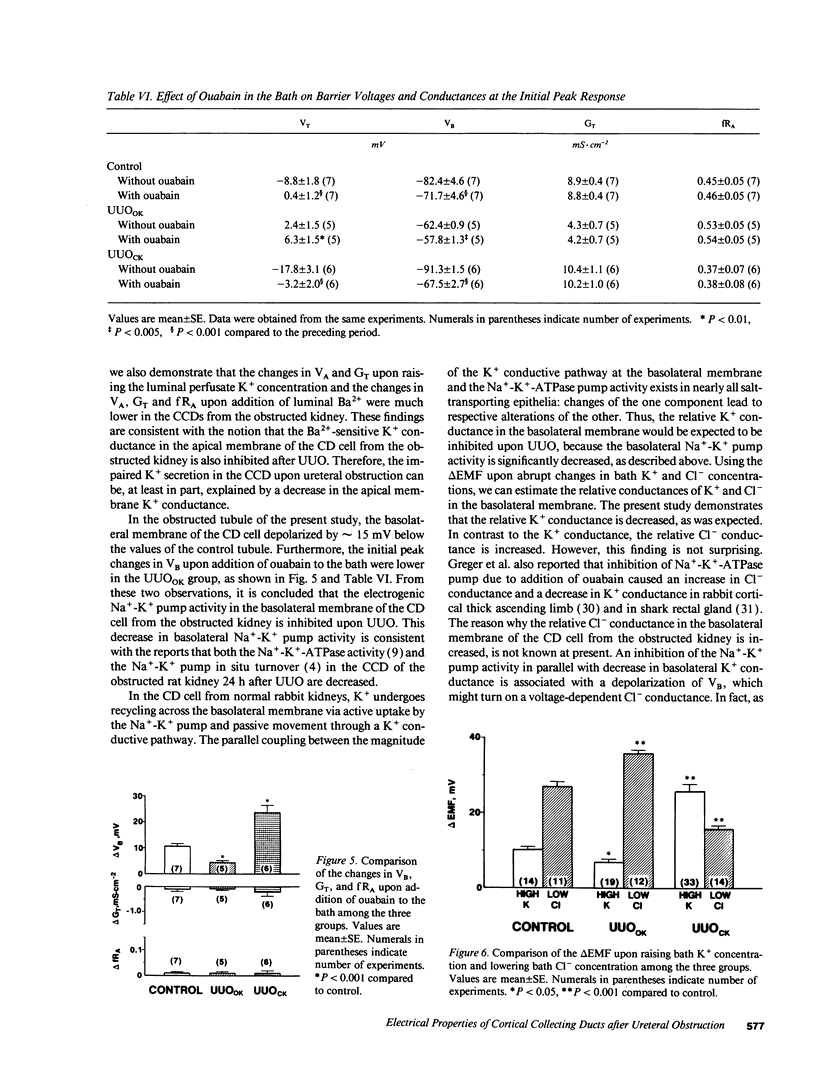
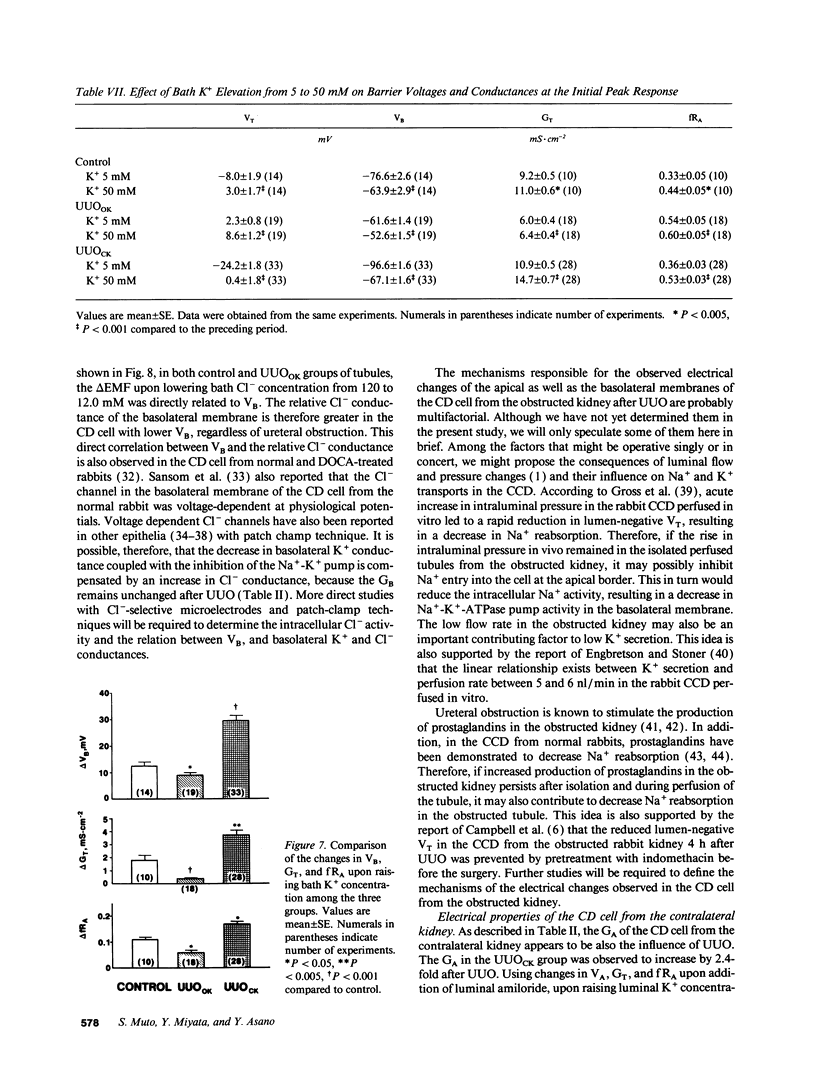
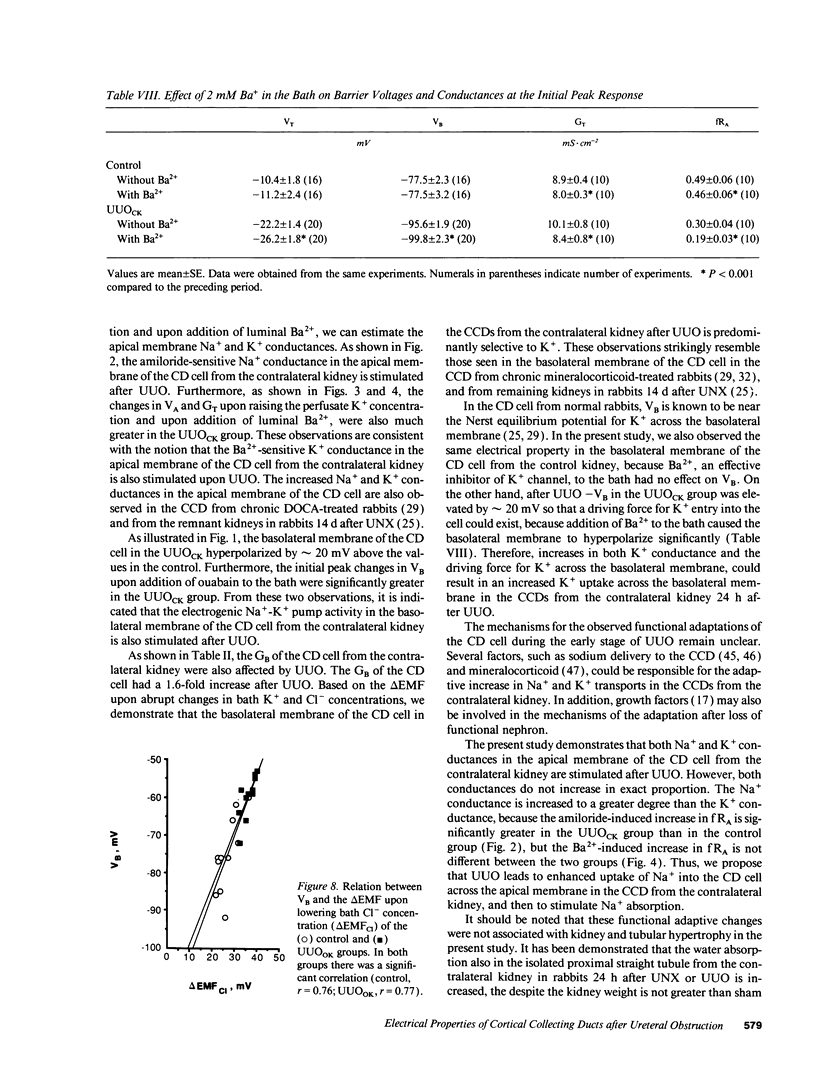
Electrophysiological techniques were used to determine the electrical properties of the collecting duct (CD) cell in the isolated cortical collecting duct from obstructed (UUOOK) and contralateral (UUOCK) kidneys in rabbits 24 h after unilateral ureteral obstruction (UUO); results were compared with those from sham-operated kidneys. The lumen-negative transepithelial voltage and the basolateral membrane voltage (VB) were decreased in the UUOOK, and increased in the UUOCK. The transepithelial conductance (GT) was decreased in parallel with an increase in the fractional apical membrane resistance (fRA) and a decrease in apical membrane conductance in the UUOOK. By contrast, the GT was increased in parallel with increases in apical and basolateral membrane conductances in the UUOCK. The amiloride-sensitive changes in apical membrane voltage (VA), GT and fRA were lower in the UUOOK, but greater in the UUOCK. The changes in VA and GT upon raising the perfusate K+ concentration and upon addition of luminal Ba2+ were decreased in the UUOOK, and increased in the UUOCK. Addition of ouabain to the bath resulted in a smaller depolarization of VB in the UUOOK, but in a greater depolarization in the UUOCK. Upon lowering bath Cl-, the change in basolateral membrane electromotive force (delta EMF) was increased in the UUOOK, and decreased in the UUOCK. Reversely, upon raising bath K+, the delta EMF was decreased in the UUOOK, and increased in the UUOCK. We conclude: (a) the conductances of Na+ and K+ in the apical membrane, and active Na(+)-K+ pump activity and relative K+ conductance in the basolateral membrane are decreased in the UUOOK, and increased in the UUOCK; (b) the relative basolateral membrane Cl- conductance was increased in the UUOOK, and decreased in the UUOCK.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bander S. J., Buerkert J. E., Martin D., Klahr S. Long-term effects of 24-hr unilateral ureteral obstruction on renal function in the rat. Kidney Int. 1985 Oct;28(4):614–620. doi: 10.1038/ki.1985.173. [DOI] [PubMed] [Google Scholar]
- Batlle D. C., Arruda J. A., Kurtzman N. A. Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med. 1981 Feb 12;304(7):373–380. doi: 10.1056/NEJM198102123040701. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Campbell H. T., Bello-Reuss E., Klahr S. Hydraulic water permeability and transepithelial voltage in the isolated perfused rabbit cortical collecting tubule following acute unilateral ureteral obstruction. J Clin Invest. 1985 Jan;75(1):219–225. doi: 10.1172/JCI111677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier R. L., Gomez R. A., Jones C. E. Developmental determinants of recovery after relief of partial ureteral obstruction. Kidney Int. 1988 Apr;33(4):775–781. doi: 10.1038/ki.1988.66. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Davis B. B., Needleman P. Localization of exaggerated prostaglandin synthesis associated with renal damage. Prostaglandins. 1981 Dec;22(6):933–944. doi: 10.1016/0090-6980(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Dicker S. E., Shirley D. G. Compensatory hypertrophy of the contralateral kidney after unilateral ureteral ligation. J Physiol. 1972 Jan;220(1):199–210. doi: 10.1113/jphysiol.1972.sp009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebata S., Muto S., Asano Y. Effects of uninephrectomy on electrical properties of the cortical collecting duct from rabbit remnant kidneys. J Clin Invest. 1992 Oct;90(4):1547–1557. doi: 10.1172/JCI116023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbretson B. G., Stoner L. C. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol. 1987 Nov;253(5 Pt 2):F896–F903. doi: 10.1152/ajprenal.1987.253.5.F896. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A., Tan Y. P., Trautmann A. Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflugers Arch. 1986 Jan;406(1):65–68. doi: 10.1007/BF00582955. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Badie-Dezfooly B., Lowe A. G., Hamzeh A., Wells J., Salehmoghaddam S. Stimulation of Na+/H+ antiport is an early event in hypertrophy of renal proximal tubular cells. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1736–1740. doi: 10.1073/pnas.82.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Yanagawa N., Schultze R. G., Tuck M., Trizna W. Functional profile of the isolated uremic nephron: potassium adaptation in the rabbit cortical collecting tubule. J Clin Invest. 1979 Oct;64(4):1033–1043. doi: 10.1172/JCI109540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). II. Effects of inhibitors. Pflugers Arch. 1984 Dec;402(4):364–375. doi: 10.1007/BF00583937. [DOI] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G. R., Schoumacher R. A., Frizzell R. A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988 Apr;254(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Isolated nephron segments from rabbit models of obstructive nephropathy. J Clin Invest. 1982 Jan;69(1):165–174. doi: 10.1172/JCI110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett J. P. Functional adaptation to reduction in renal mass. Physiol Rev. 1979 Jan;59(1):137–164. doi: 10.1152/physrev.1979.59.1.137. [DOI] [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. I. Depression of sodium transport. Am J Physiol. 1981 Oct;241(4):F452–F460. doi: 10.1152/ajprenal.1981.241.4.F452. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Bachmann S., Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol. 1985 Mar;248(3 Pt 2):F374–F381. doi: 10.1152/ajprenal.1985.248.3.F374. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Le Hir M. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. I. Structural changes. Cell Tissue Res. 1982;224(3):469–492. doi: 10.1007/BF00213746. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Stanton B. A. Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am J Physiol. 1988 Dec;255(6 Pt 2):F1256–F1268. doi: 10.1152/ajprenal.1988.255.6.F1256. [DOI] [PubMed] [Google Scholar]
- Kimura H., Mujais S. K. Cortical collecting duct Na-K pump in obstructive nephropathy. Am J Physiol. 1990 May;258(5 Pt 2):F1320–F1327. doi: 10.1152/ajprenal.1990.258.5.F1320. [DOI] [PubMed] [Google Scholar]
- Klahr S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983 Feb;23(2):414–426. doi: 10.1038/ki.1983.36. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Light D. B., Schwiebert E. M., Fejes-Toth G., Naray-Fejes-Toth A., Karlson K. H., McCann F. V., Stanton B. A. Chloride channels in the apical membrane of cortical collecting duct cells. Am J Physiol. 1990 Feb;258(2 Pt 2):F273–F280. doi: 10.1152/ajprenal.1990.258.2.F273. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Muto S., Furuya H., Tabei K., Asano Y. Site and mechanism of action of epidermal growth factor in rabbit cortical collecting duct. Am J Physiol. 1991 Feb;260(2 Pt 2):F163–F169. doi: 10.1152/ajprenal.1991.260.2.F163. [DOI] [PubMed] [Google Scholar]
- Muto S., Giebisch G., Sansom S. Effects of adrenalectomy on CCD: evidence for differential response of two cell types. Am J Physiol. 1987 Oct;253(4 Pt 2):F742–F752. doi: 10.1152/ajprenal.1987.253.4.F742. [DOI] [PubMed] [Google Scholar]
- Muto S., Sansom S., Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest. 1988 Feb;81(2):376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Yasoshima K., Yoshitomi K., Imai M., Asano Y. Electrophysiological identification of alpha- and beta-intercalated cells and their distribution along the rabbit distal nephron segments. J Clin Invest. 1990 Dec;86(6):1829–1839. doi: 10.1172/JCI114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., Tang J. M., Palmer L. G. Single-channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol. 1984;80(1):81–89. doi: 10.1007/BF01868692. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Sansom S. C. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol. 1984;82(3):281–295. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- Paulson D. F., Fraley E. E. Chemical evidence for early but unsustained growth in the obstructed mouse kidney. Am J Physiol. 1970 Oct;219(4):872–875. doi: 10.1152/ajplegacy.1970.219.4.872. [DOI] [PubMed] [Google Scholar]
- Paulson D. F., Fraley E. E. Compensatory renal growth after unilateral ureteral obstruction. Kidney Int. 1973 Jul;4(1):22–27. doi: 10.1038/ki.1973.76. [DOI] [PubMed] [Google Scholar]
- Ribeiro C., Suki W. N. Acidification in the medullary collecting duct following ureteral obstruction. Kidney Int. 1986 Jun;29(6):1167–1171. doi: 10.1038/ki.1986.123. [DOI] [PubMed] [Google Scholar]
- Sabatini S., Kurtzman N. A. Enzyme activity in obstructive uropathy: basis for salt wastage and the acidification defect. Kidney Int. 1990 Jan;37(1):79–84. doi: 10.1038/ki.1990.11. [DOI] [PubMed] [Google Scholar]
- Salehmoghaddam S., Bradley T., Mikhail N., Badie-Dezfooly B., Nord E. P., Trizna W., Kheyfets R., Fine L. G. Hypertrophy of basolateral Na-K pump activity in the proximal tubule of the remnant kidney. Lab Invest. 1985 Oct;53(4):443–452. [PubMed] [Google Scholar]
- Salihagić A., Macković M., Banfić H., Sabolić I. Short-term and long-term stimulation of Na+-H+ exchange in cortical brush-border membranes during compensatory growth of the rat kidney. Pflugers Arch. 1988 Dec;413(2):190–196. doi: 10.1007/BF00582530. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., Agulian S., Muto S., Illig V., Giebisch G. K activity of CCD principal cells from normal and DOCA-treated rabbits. Am J Physiol. 1989 Jan;256(1 Pt 2):F136–F142. doi: 10.1152/ajprenal.1989.256.1.F136. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., La B. Q., Carosi S. L. Double-barreled chloride channels of collecting duct basolateral membrane. Am J Physiol. 1990 Jul;259(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1990.259.1.F46. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., O'Neil R. G. Effects of mineralocorticoids on transport properties of cortical collecting duct basolateral membrane. Am J Physiol. 1986 Oct;251(4 Pt 2):F743–F757. doi: 10.1152/ajprenal.1986.251.4.F743. [DOI] [PubMed] [Google Scholar]
- Scherzer P., Wald H., Czaczkes J. W. Na-K-ATPase in isolated rabbit tubules after unilateral nephrectomy and Na+ loading. Am J Physiol. 1985 Apr;248(4 Pt 2):F565–F573. doi: 10.1152/ajprenal.1985.248.4.F565. [DOI] [PubMed] [Google Scholar]
- Shimamura T., Kissane J. M., Györkey F. Experimental hydroneophrosis. Nephron dissection and electron microscopy of the kidney following obstruction of the ureter and in recovery from obstruction. Lab Invest. 1966 Mar;15(3):629–640. [PubMed] [Google Scholar]
- Shirley D. G., Walter S. J. Acute and chronic changes in renal function following unilateral nephrectomy. Kidney Int. 1991 Jul;40(1):62–68. doi: 10.1038/ki.1991.180. [DOI] [PubMed] [Google Scholar]
- Stanton B. A., Kaissling B. Adaptation of distal tubule and collecting duct to increased Na delivery. II. Na+ and K+ transport. Am J Physiol. 1988 Dec;255(6 Pt 2):F1269–F1275. doi: 10.1152/ajprenal.1988.255.6.F1269. [DOI] [PubMed] [Google Scholar]
- Stanton B. A., Kaissling B. Regulation of renal ion transport and cell growth by sodium. Am J Physiol. 1989 Jul;257(1 Pt 2):F1–10. doi: 10.1152/ajprenal.1989.257.1.F1. [DOI] [PubMed] [Google Scholar]
- Tabei K., Levenson D. J., Brenner B. M. Early enhancement of fluid transport in rabbit proximal straight tubules after loss of contralateral renal excretory function. J Clin Invest. 1983 Sep;72(3):871–881. doi: 10.1172/JCI111058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirakomen K., Kozlov N., Arruda J. A., Kurtzman N. A. Renal hydrogen ion secretion after release of unilateral ureteral obstruction. Am J Physiol. 1976 Oct;231(4):1233–1239. doi: 10.1152/ajplegacy.1976.231.4.1233. [DOI] [PubMed] [Google Scholar]
- Vehaskari V. M., Hering-Smith K. S., Klahr S., Hamm L. L. Increased sodium transport by cortical collecting tubules from remnant kidneys. Kidney Int. 1989 Jul;36(1):89–95. doi: 10.1038/ki.1989.165. [DOI] [PubMed] [Google Scholar]
- Vehaskari V. M., Herndon J. Role of mineralocorticoids in adaptation of rabbit cortical collecting duct after loss of renal mass. Am J Physiol. 1991 Jun;260(6 Pt 2):F793–F799. doi: 10.1152/ajprenal.1991.260.6.F793. [DOI] [PubMed] [Google Scholar]
- Whinnery M. A., Shaw J. O., Beck N. Thromboxane B2 and prostaglandin E2 in the rat kidney with unilateral ureteral obstruction. Am J Physiol. 1982 Mar;242(3):F220–F225. doi: 10.1152/ajprenal.1982.242.3.F220. [DOI] [PubMed] [Google Scholar]
- Zalups R. K., Stanton B. A., Wade J. B., Giebisch G. Structural adaptation in initial collecting tubule following reduction in renal mass. Kidney Int. 1985 Apr;27(4):636–642. doi: 10.1038/ki.1985.58. [DOI] [PubMed] [Google Scholar]


