Abstract
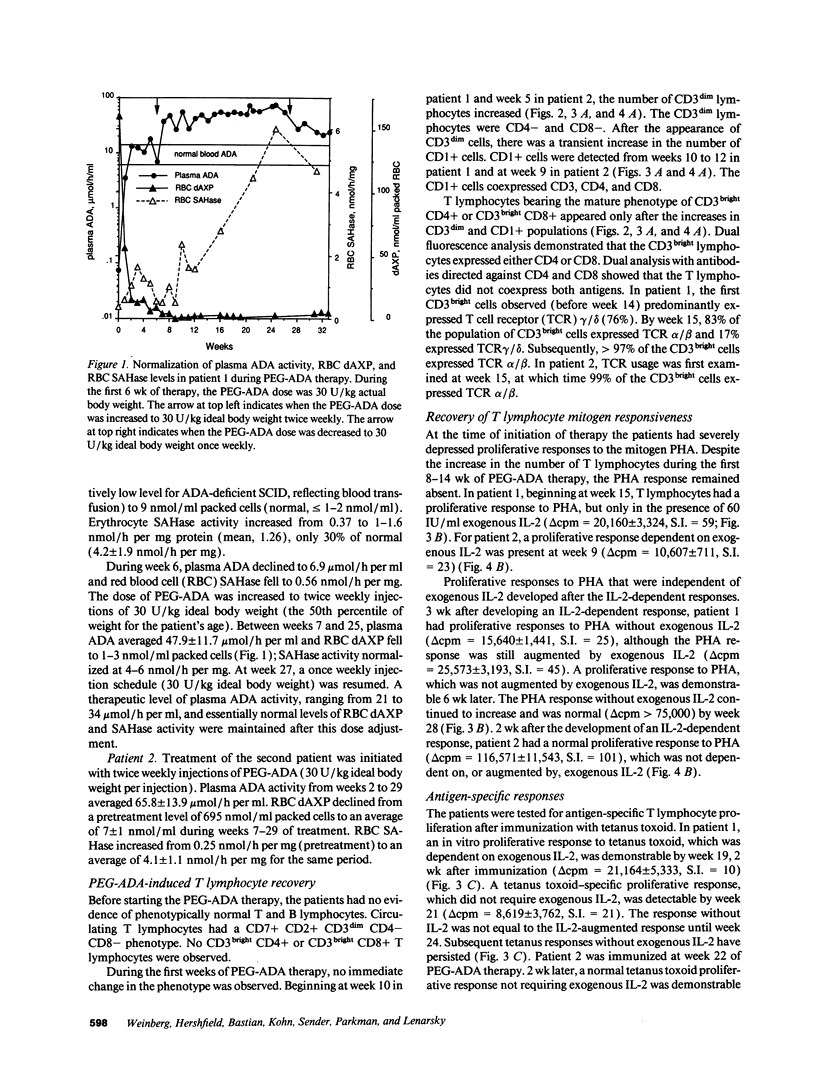
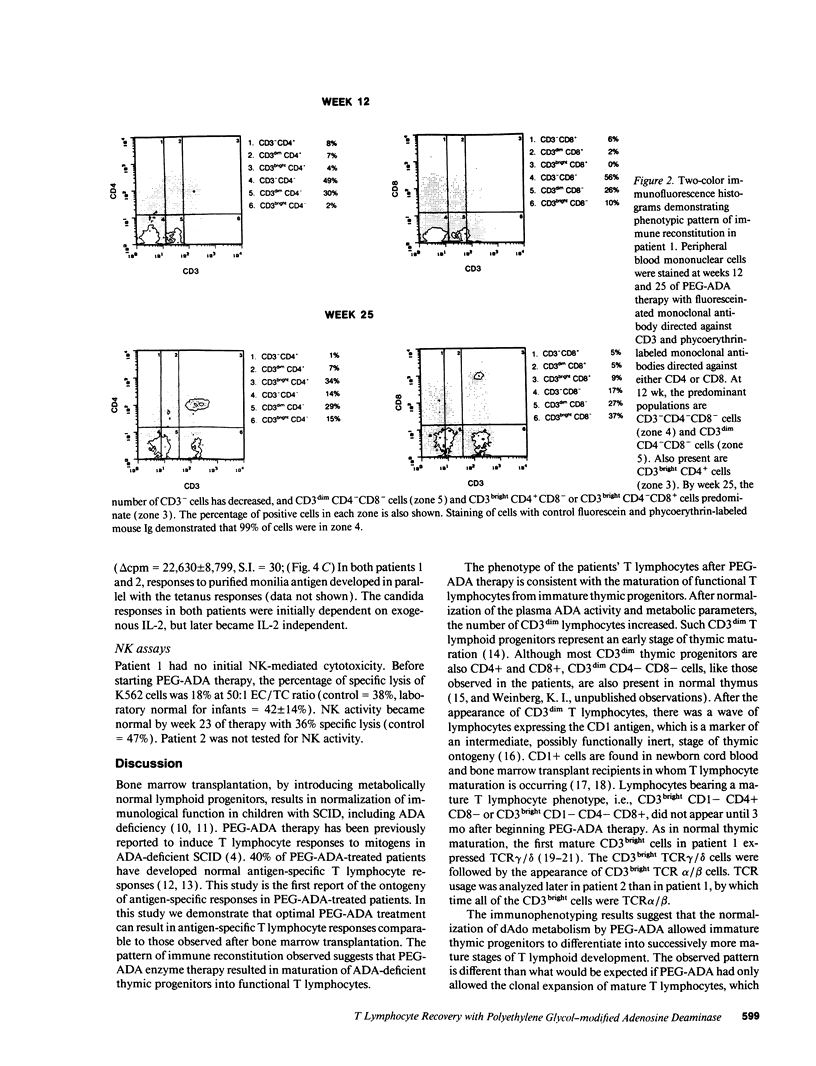
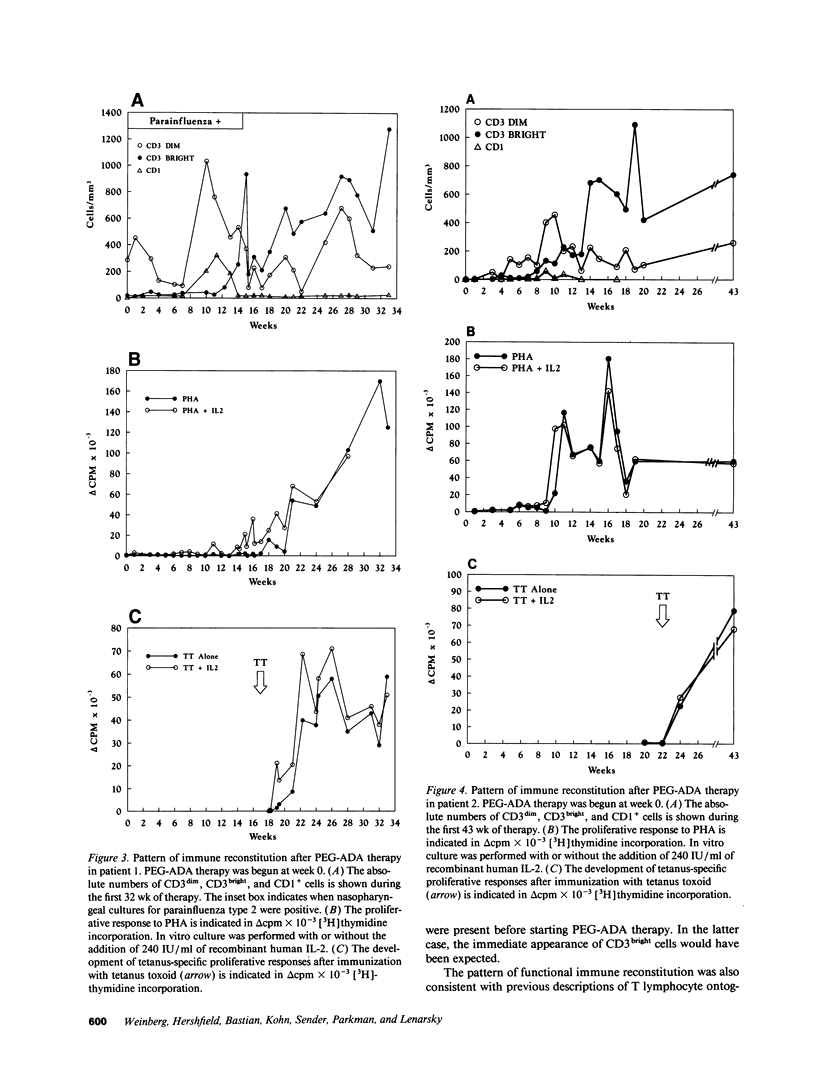
Adenosine deaminase (ADA) deficiency causes severe combined immune deficiency (SCID) by interfering with the metabolism of deoxyadenosine, which is toxic to T lymphocytes at all stages of differentiation. Enzyme replacement with polyethylene glycol-modified ADA (PEG-ADA) has been previously shown to correct deoxyadenosine metabolism and improve mitogen-induced T lymphocyte proliferation. We studied the biochemical and immunologic effects of PEG-ADA in two infants with ADA-deficient SCID. While in a catabolic state, higher doses of PEG-ADA than previously described were required to normalize deoxyadenosine metabolism. After biochemical improvement, the patients recovered immune function in a pattern similar to that observed in normal thymic ontogeny and in patients with immunological reconstitution after bone marrow transplantation. Immune reconstitution was marked by the sequential appearance in the peripheral blood of phenotypic T lymphocytes corresponding to successive stages of thymic differentiation. Functional reconstitution was marked by the successive appearance of mitogen responses dependent on exogenous in vitro IL-2, mitogen responses not requiring exogenous IL-2, antigen-specific responses dependent on exogenous IL-2, and finally, antigen-specific responses not requiring exogenous IL-2. Natural killer function was tested in one patient and normalized with PEG-ADA therapy. Optimal PEG-ADA therapy appears to normalize thymic differentiation in ADA-deficient SCID, resulting in normal antigen-specific immune function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biron C. A., Byron K. S., Sullivan J. L. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989 Jun 29;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Bory C., Boulieu R., Souillet G., Chantin C., Rolland M. O., Mathieu M., Hershfield M. Comparison of red cell transfusion and polyethylene glycol-modified adenosine deaminase therapy in an adenosine deaminase-deficient child: measurement of erythrocyte deoxyadenosine triphosphate as a useful tool. Pediatr Res. 1990 Aug;28(2):127–130. doi: 10.1203/00006450-199008000-00010. [DOI] [PubMed] [Google Scholar]
- Chaffee S., Mary A., Stiehm E. R., Girault D., Fischer A., Hershfield M. S. IgG antibody response to polyethylene glycol-modified adenosine deaminase in patients with adenosine deaminase deficiency. J Clin Invest. 1992 May;89(5):1643–1651. doi: 10.1172/JCI115761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Landais P., Friedrich W., Morgan G., Gerritsen B., Fasth A., Porta F., Griscelli C., Goldman S. F., Levinsky R. European experience of bone-marrow transplantation for severe combined immunodeficiency. Lancet. 1990 Oct 6;336(8719):850–854. doi: 10.1016/0140-6736(90)92348-l. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Dosch H. M. Diagnosis and classification of severe combined immunodeficiency disease. Birth Defects Orig Artic Ser. 1983;19(3):65–72. [PubMed] [Google Scholar]
- Gerli R., Rambotti P., Cernetti C., Velardi A., Spinozzi F., Tabilio A., Martelli M. F., Grignani F., Davis S. A mature thymocyte-like phenotypic pattern on human cord circulating T-lymphoid cells. J Clin Immunol. 1984 Nov;4(6):461–468. doi: 10.1007/BF00916576. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Singer K. H., Denning S. M., Martin M. E. Analysis of expression of CD2, CD3, and T cell antigen receptor molecules during early human fetal thymic development. J Immunol. 1988 Dec 1;141(11):3776–3784. [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hershfield M. S., Buckley R. H., Greenberg M. L., Melton A. L., Schiff R., Hatem C., Kurtzberg J., Markert M. L., Kobayashi R. H., Kobayashi A. L. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med. 1987 Mar 5;316(10):589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Fetter J. E., Small W. C., Bagnara A. S., Williams S. R., Ullman B., Martin D. W., Jr, Wasson D. B., Carson D. A. Effects of mutational loss of adenosine kinase and deoxycytidine kinase on deoxyATP accumulation and deoxyadenosine toxicity in cultured CEM human T-lymphoblastoid cells. J Biol Chem. 1982 Jun 10;257(11):6380–6386. [PubMed] [Google Scholar]
- Hershfield M. S., Kredich N. M. Resistance of an adenosine kinase-deficient human lymphoblastoid cell line to effects of deoxyadenosine on growth, S-adenosylhomocysteine hydrolase inactivation, and dATP accumulation. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4292–4296. doi: 10.1073/pnas.77.7.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keever C. A., Small T. N., Flomenberg N., Heller G., Pekle K., Black P., Pecora A., Gillio A., Kernan N. A., O'Reilly R. J. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989 Apr;73(5):1340–1350. [PubMed] [Google Scholar]
- Nikolic-Zugic J., Moore M. W. T cell receptor expression on immature thymocytes with in vivo and in vitro precursor potential. Eur J Immunol. 1989 Oct;19(10):1957–1960. doi: 10.1002/eji.1830191030. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. J., Kapoor N., Kirkpatrick D., Flomenberg N., Pollack M. S., Dupont B., Good R. A., Reisner Y. Transplantation of hematopoietic cells for lethal congenital immunodeficiencies. Birth Defects Orig Artic Ser. 1983;19(3):129–137. [PubMed] [Google Scholar]
- O'Reilly R. J., Keever C. A., Small T. N., Brochstein J. The use of HLA-non-identical T-cell-depleted marrow transplants for correction of severe combined immunodeficiency disease. Immunodefic Rev. 1989;1(4):273–309. [PubMed] [Google Scholar]
- Ochs H. D., Buckley R. H., Kobayashi R. H., Kobayashi A. L., Sorensen R. U., Douglas S. D., Hamilton B. L., Hershfield M. S. Antibody responses to bacteriophage phi X174 in patients with adenosine deaminase deficiency. Blood. 1992 Sep 1;80(5):1163–1171. [PubMed] [Google Scholar]
- Rappeport J. M., Dunn M. J., Parkman R. Immature T lymphocytes in the peripheral blood of bone marrow transplant recipients. Transplantation. 1983 Dec;36(6):674–680. doi: 10.1097/00007890-198336060-00018. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Mendelsohn J., Seegmiller J. E. Adenosine metabolism in phytohemagglutinin-stimulated human lymphocytes. J Clin Invest. 1976 Sep;58(3):654–666. doi: 10.1172/JCI108512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H. Human T cell receptor gamma delta + T cells. Semin Immunol. 1991 Mar;3(2):119–129. [PubMed] [Google Scholar]
- Toribio M. L., Martinez C., Marcos M. A., Marquez C., Cabrero E., de la Hera A. A role for T3+4-6-8- transitional thymocytes in the differentiation of mature and functional T cells from human prothymocytes. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6985–6988. doi: 10.1073/pnas.83.18.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Ciobanu N., Moore M. A., Gulati S., O'Reilly R. J., Mertelsmann R. Defective interleukin 2 production in patients after bone marrow transplantation and in vitro restoration of defective T lymphocyte proliferation by highly purified interleukin 2. Blood. 1984 Aug;64(2):380–385. [PubMed] [Google Scholar]
- de la Hera A., Toribio M. L., Martinez C. Delineation of human thymocytes with or without functional potential by CD1-specific antibodies. Int Immunol. 1989;1(5):496–502. doi: 10.1093/intimm/1.5.496. [DOI] [PubMed] [Google Scholar]


