Abstract
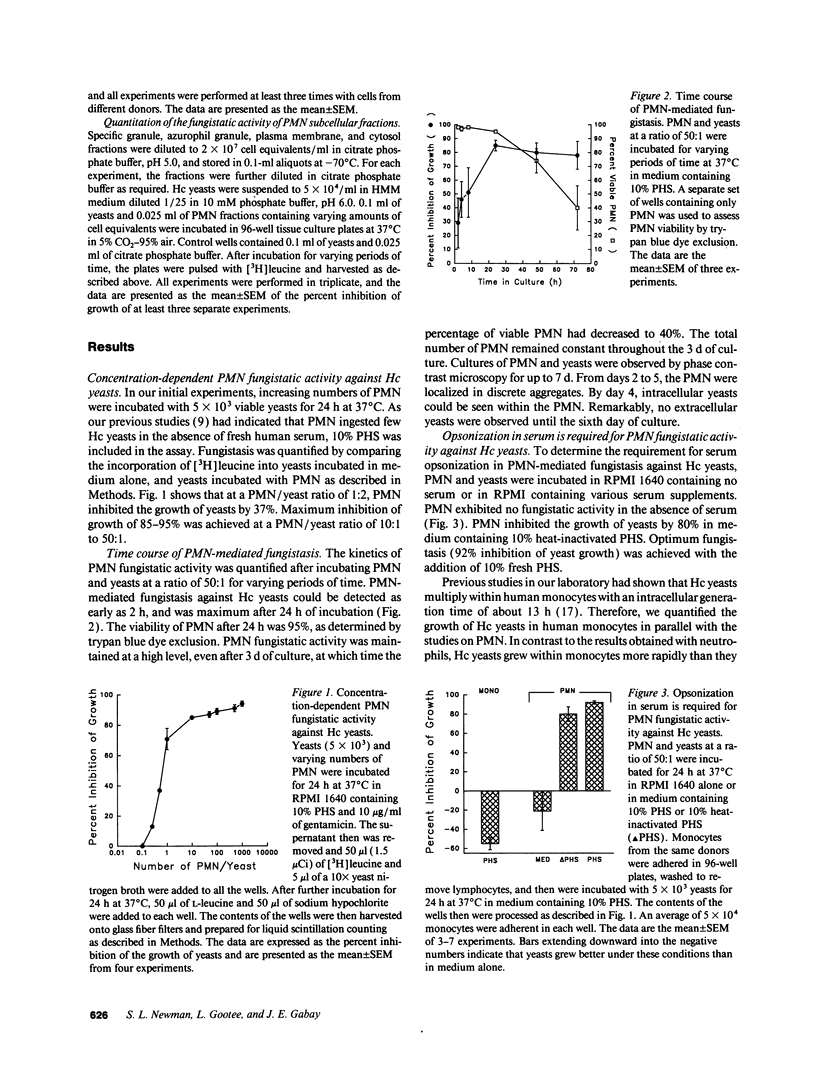
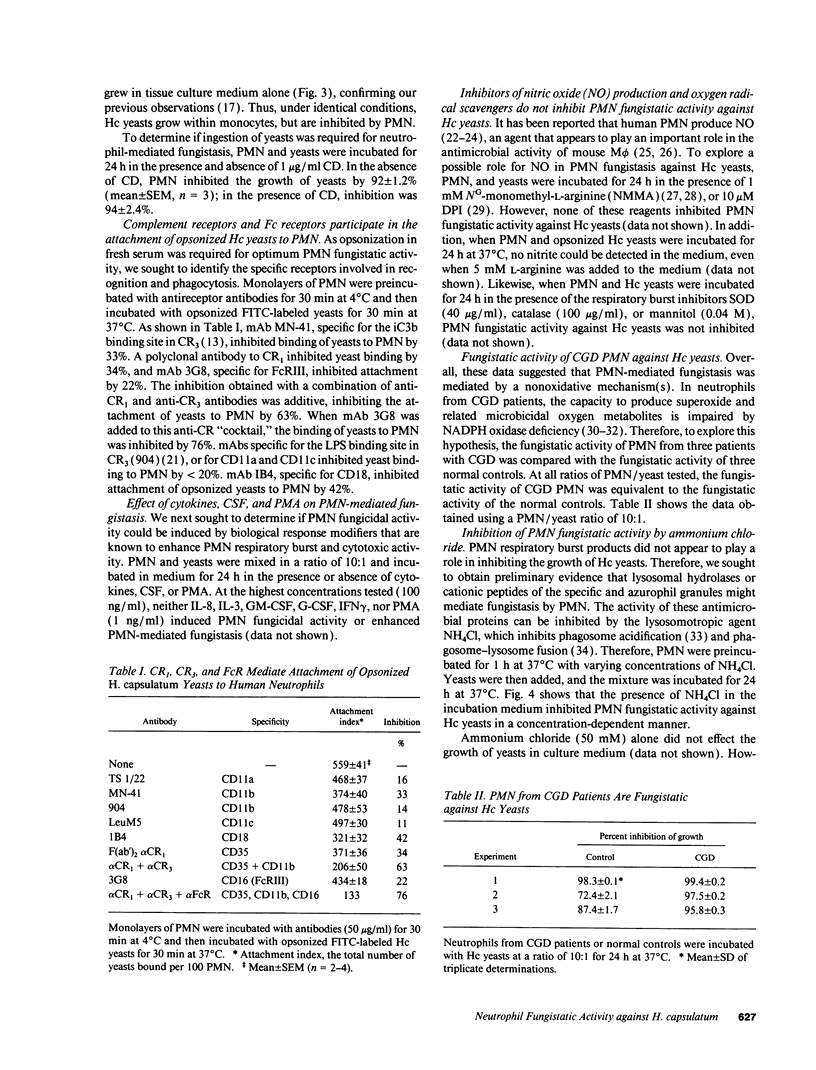
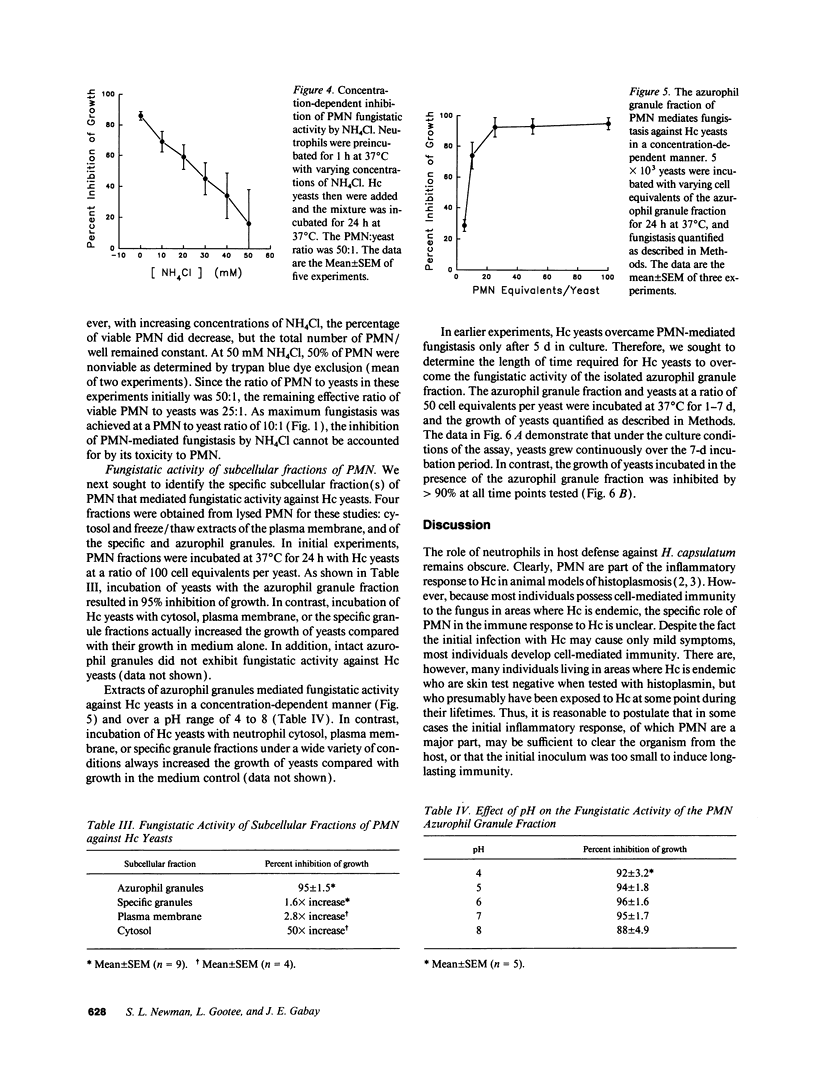
Human neutrophils (PMN) demonstrated potent fungistatic activity against Histoplasma capsulatum (Hc) yeasts in a sensitive microassay that quantifies the growth of yeasts by the incorporation of [3H]leucine. At a PMN:yeast ratio of 1:2, PMN inhibited the growth of yeasts by 37%. Maximum inhibition of 85% to 95% was achieved at a PMN/yeast ratio of 10:1 to 50:1. Opsonization of the yeasts in fresh or heat-inactivated serum was required for PMN-mediated fungistasis, but ingestion of the yeasts was not required. Recognition and phagocytosis of opsonized yeasts was via PMN complement receptor (CR) type 1 (CR1), CR3, and FcRIII (CD16). PMN fungistatic activity was evident by 2 h, was maximum at 24 h, and persisted up to 5 d. In contrast, yeasts multiplied within monocytes to a greater extent than in culture medium alone. PMN from three patients with chronic granulomatous disease (CGD) inhibited the growth of Hc yeasts by an average of 97%, compared with 86% in three normal controls. Furthermore, preincubation of PMN with the lysosomotropic agent NH4Cl inhibited fungistatic activity in a concentration-dependent manner. Finally, experiments with subcellular fractions of PMN demonstrated that the principal component of the fungistatic activity of PMN was localized in the azurophil granules. These data demonstrate that human PMN possess potent fungistatic activity against Hc yeasts and further show that fungistasis is mediated by antimicrobial agents contained in the azurophil granules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazovich K. J., Almeida H. I., Boxer L. A. Recombinant human G-CSF and GM-CSF prime human neutrophils for superoxide production through different signal transduction mechanisms. J Lab Clin Med. 1991 Dec;118(6):576–584. [PubMed] [Google Scholar]
- Baughman R. P., Kim C. K., Vinegar A., Hendricks D. E., Schmidt D. J., Bullock W. E. The pathogenesis of experimental pulmonary histoplasmosis. Correlative studies of histopathology, bronchoalveolar lavage, and respiratory function. Am Rev Respir Dis. 1986 Oct;134(4):771–776. doi: 10.1164/arrd.1986.134.4.771. [DOI] [PubMed] [Google Scholar]
- Brummer E., Kurita N., Yosihida S., Nishimura K., Miyaji M. Fungistatic activity of human neutrophils against Histoplasma capsulatum: correlation with phagocytosis. J Infect Dis. 1991 Jul;164(1):158–162. doi: 10.1093/infdis/164.1.158. [DOI] [PubMed] [Google Scholar]
- Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990 Mar;85(3):904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Dobson N. J., Lambris J. D., Ross G. D. Characteristics of isolated erythrocyte complement receptor type one (CR1, C4b-C3b receptor) and CR1-specific antibodies. J Immunol. 1981 Feb;126(2):693–698. [PubMed] [Google Scholar]
- Eddy A., Newman S. L., Cosio F., LeBien T., Michael A. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984 Jun;31(3):371–389. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Heiple J. M., Cohn Z. A., Nathan C. F. Subcellular location and properties of bactericidal factors from human neutrophils. J Exp Med. 1986 Nov 1;164(5):1407–1421. doi: 10.1084/jem.164.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Green S. J., Mellouk S., Hoffman S. L., Meltzer M. S., Nacy C. A. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990 Aug;25(1-3):15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- Howard D. H. Fate of Histoplasma capsulatum in guinea pig polymorphonuclear leukocytes. Infect Immun. 1973 Sep;8(3):412–419. doi: 10.1128/iai.8.3.412-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita N., Brummer E., Yoshida S., Nishimura K., Miyaji M. Antifungal activity of murine polymorphonuclear neutrophils against Histoplasma capsulatum. J Med Vet Mycol. 1991;29(3):133–143. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Tewari R. P. Determination of viability of Histoplasma capsulatum yeast cells grown in vitro: comparison between dye and colony count methods. J Med Vet Mycol. 1987 Apr;25(2):107–114. [PubMed] [Google Scholar]
- Lanier L. L., Arnaout M. A., Schwarting R., Warner N. L., Ross G. D. p150/95, Third member of the LFA-1/CR3 polypeptide family identified by anti-Leu M5 monoclonal antibody. Eur J Immunol. 1985 Jul;15(7):713–718. doi: 10.1002/eji.1830150714. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Gee J. B., Bensch K. G. Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J Biol Med. 1971 Dec;44(3):286–300. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Respiratory burst in adherent human neutrophils: triggering by colony-stimulating factors CSF-GM and CSF-G. Blood. 1989 Jan;73(1):301–306. [PubMed] [Google Scholar]
- Newman S. L., Bucher C., Rhodes J., Bullock W. E. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest. 1990 Jan;85(1):223–230. doi: 10.1172/JCI114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Gootee L., Bucher C., Bullock W. E. Inhibition of intracellular growth of Histoplasma capsulatum yeast cells by cytokine-activated human monocytes and macrophages. Infect Immun. 1991 Feb;59(2):737–741. doi: 10.1128/iai.59.2.737-741.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Gootee L. Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts. Infect Immun. 1992 Nov;60(11):4593–4597. doi: 10.1128/iai.60.11.4593-4597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K., Tucci M. A. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol. 1991 Feb 1;146(3):967–974. [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olken N. M., Rusche K. M., Richards M. K., Marletta M. A. Inactivation of macrophage nitric oxide synthase activity by NG-methyl-L-arginine. Biochem Biophys Res Commun. 1991 Jun 14;177(2):828–833. doi: 10.1016/0006-291x(91)91864-9. [DOI] [PubMed] [Google Scholar]
- PROCKNOW J. J., PAGE M. I., LOOSLI C. G. Early pathogenesis of experimental histoplasmosis. Arch Pathol. 1960 Apr;69:413–426. [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Puszkin E., Puszkin S., Lo L. W., Tanenbaum S. W. Binding of cytochalasin D to platelet and muscle myosin. J Biol Chem. 1973 Nov 25;248(22):7754–7761. [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Davis C. E., Schaffner T., Markert M., Douglas H., Braude A. I. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986 Aug;78(2):511–524. doi: 10.1172/JCI112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Seifert R., Böhme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989 Feb 27;244(2):357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- Schnur R. A., Newman S. L. The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J Immunol. 1990 Jun 15;144(12):4765–4772. [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Spitznagel J. K. Antibiotic proteins of human neutrophils. J Clin Invest. 1990 Nov;86(5):1381–1386. doi: 10.1172/JCI114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck M. J., Roth J. A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989 Jul-Aug;11(4):549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Fasehun O. A., Kwon N. S., Gross S. S., Gonzalez J. A., Levi R., Nathan C. F. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991 Jan;5(1):98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Worsham P. L., Goldman W. E. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988 Jun;26(3):137–143. [PubMed] [Google Scholar]
- Wright C. D., Mülsch A., Busse R., Osswald H. Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun. 1989 Apr 28;160(2):813–819. doi: 10.1016/0006-291x(89)92506-0. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Levin S. M., Jong M. T., Chad Z., Kabbash L. G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989 Jan 1;169(1):175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]


