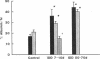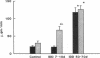Abstract
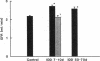
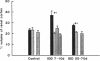
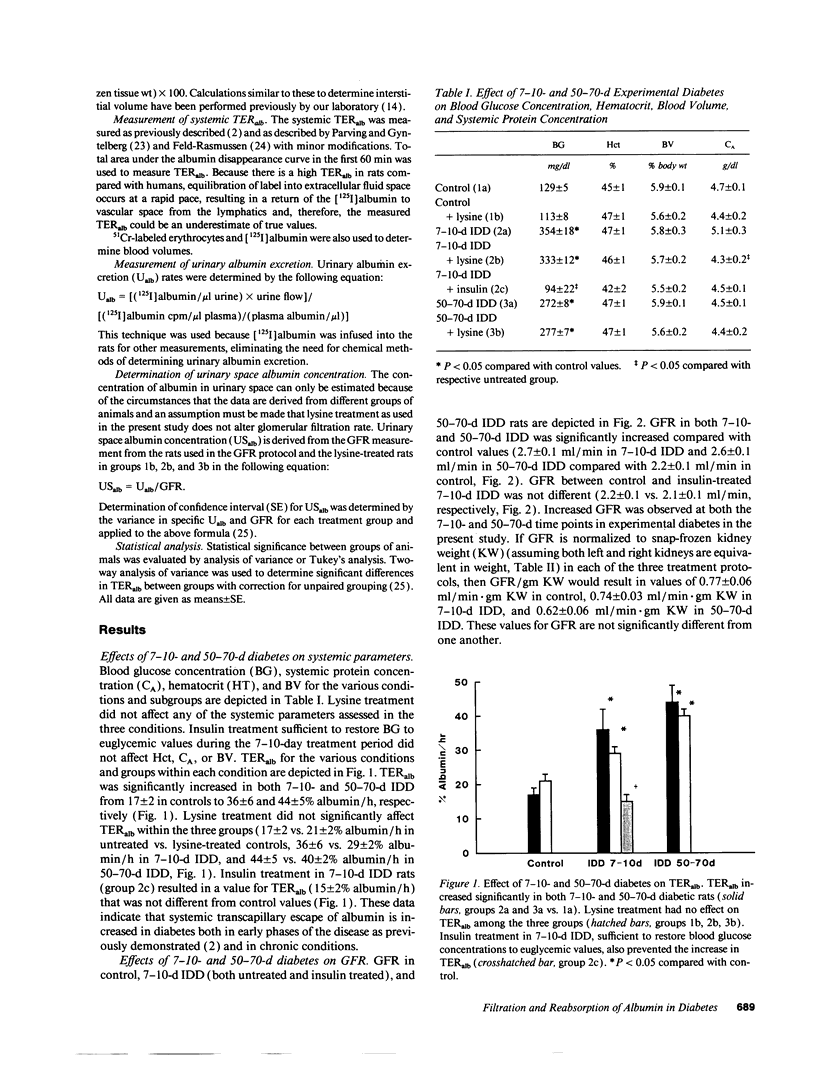
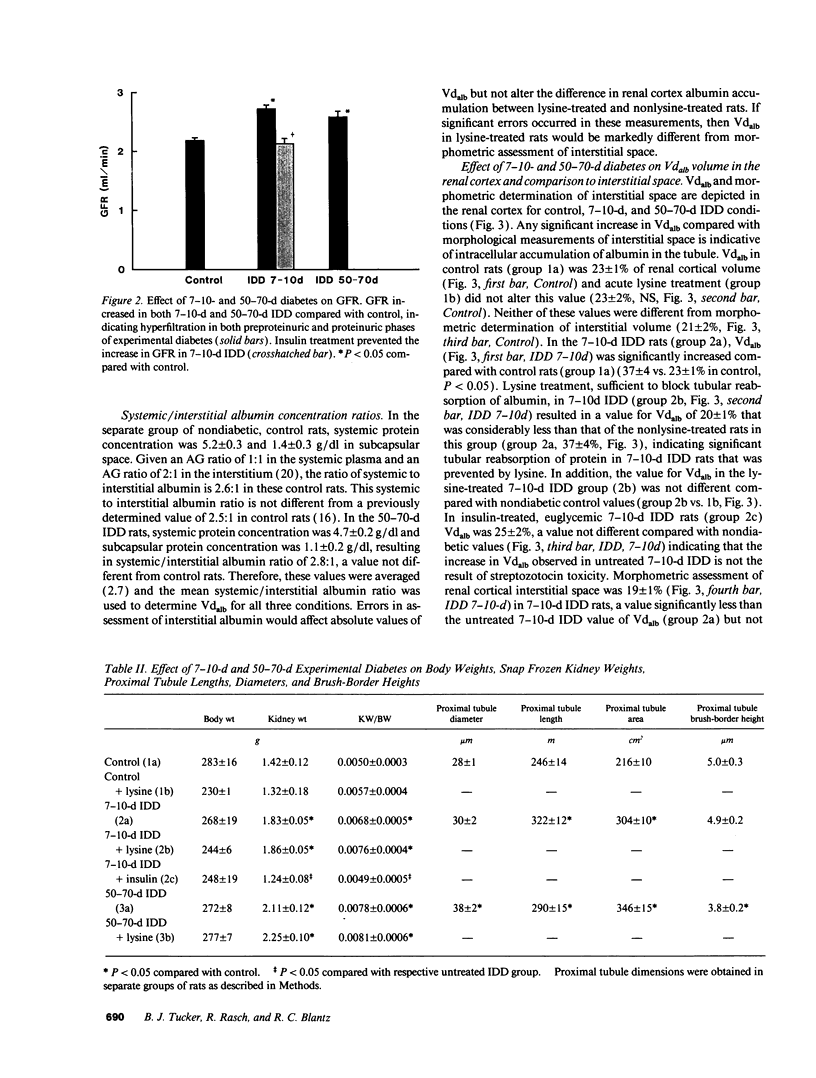
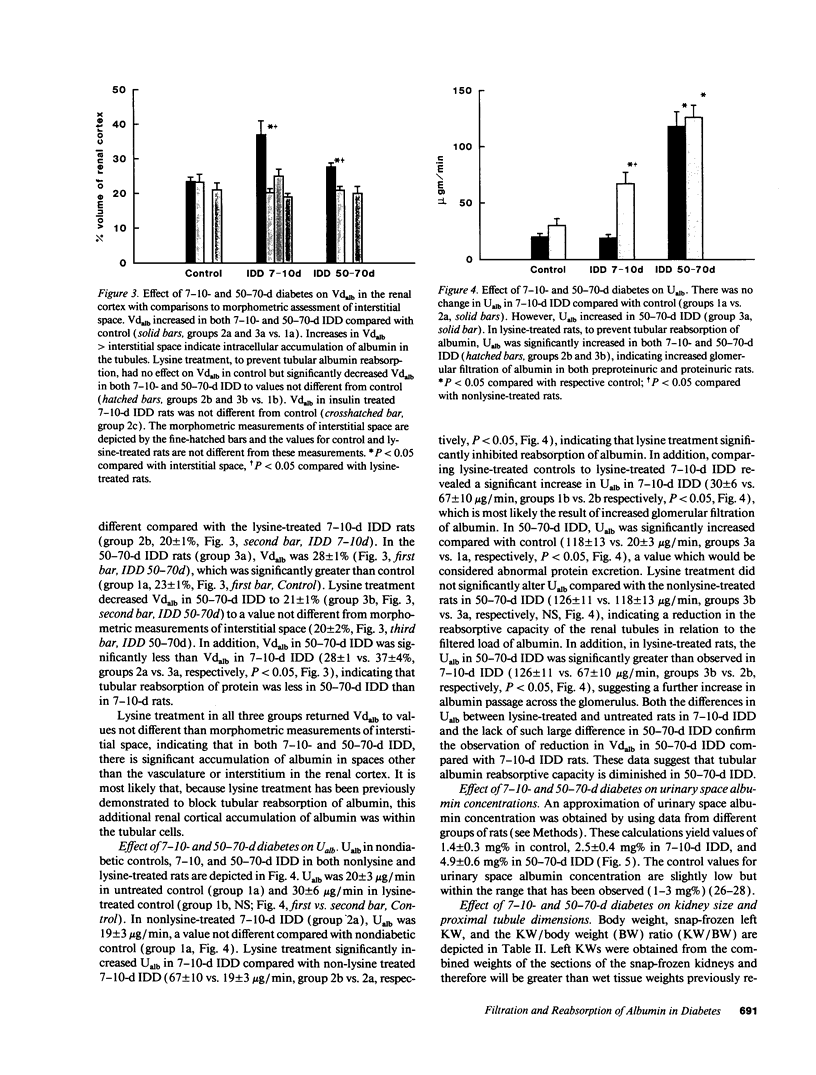
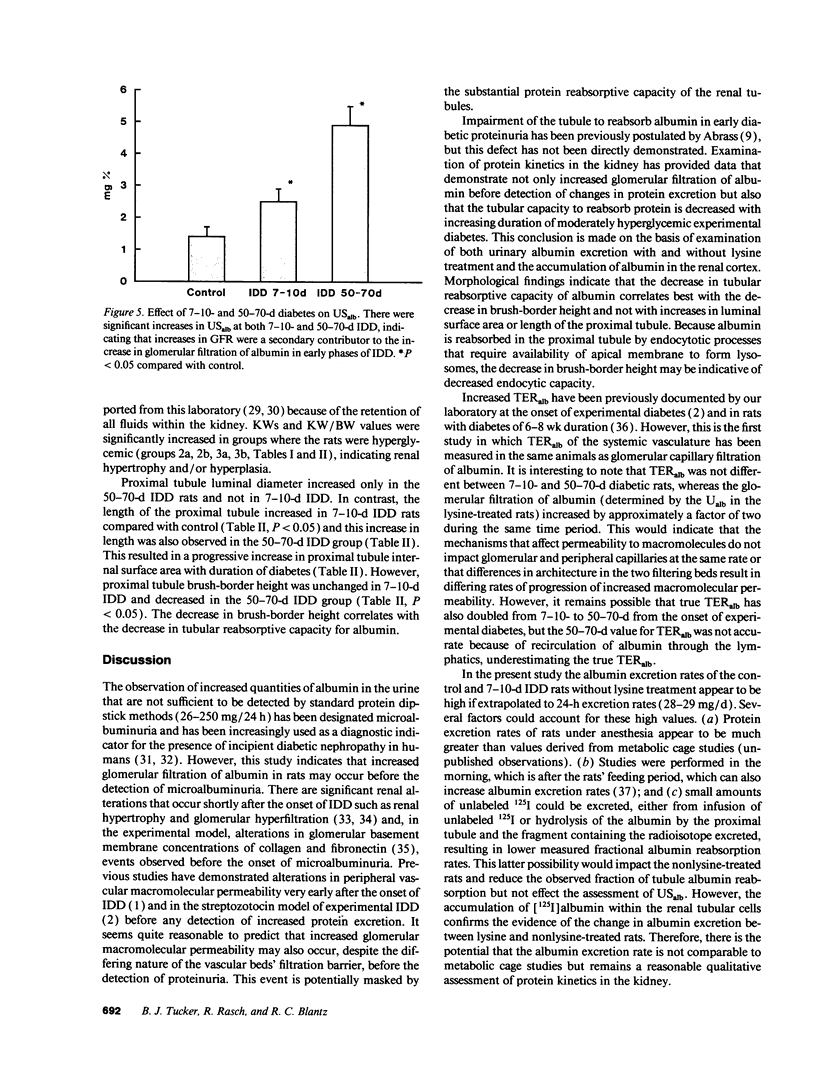
Microalbuminuria (26-250 mg/d) is considered to be an indicator of incipient diabetic nephropathy in humans in insulin-dependent diabetes (IDD). However, before microalbuminuria is observed, glomerular alterations, such as glycosylation of the glomerular basement membrane and glomerular hyperfiltration, in IDD may result in increased filtration of albumin before any observed increase in albumin excretion. Glomerular and tubular albumin kinetics were examined in streptozotocin (65 mg/kg body wt, i.v.) diabetic, Munich-Wistar rats at 7-10 (untreated) and 50-70 d (poorly controlled with small doses of insulin) after the onset of diabetes and compared with nondiabetic controls. Additional rats in each condition received acute lysine treatment to prevent tubular protein reabsorption. Urinary albumin excretion and nonvascular albumin distribution volumes were measured in the renal cortex and compared with morphometric measurements of interstitial space and the proximal tubule to assess intracellular uptake of albumin in the proximal tubule. Urinary albumin excretion under anesthesia was not different in 7-10-d IDD versus controls (19 +/- 3 vs. 20 +/- 3 micrograms/min) but increased in the 50-70-d IDD (118 +/- 13 micrograms/min, P < 0.05). Lysine treatment resulted in increased albumin excretion compared with respective nontreatment in 7-10-d IDD (67 +/- 10 micrograms/min, P < 0.05) but not in controls (30 +/- 6 micrograms/min) or in 50-70-d IDD (126 +/- 11 micrograms/min). Glomerular filtration rate was increased both in 7-10-d IDD (2.7 +/- 0.1 ml/min, P < 0.05) and in 50-70-d IDD (2.6 +/- 0.1 ml/min, P < 0.05) compared with control (2.2 +/- 0.1 ml/min). Calculated urinary space albumin concentrations increased early in IDD with 2.5 +/- 0.4 mg% in 7-10-d IDD and 4.9 +/- 0.6 mg% in 50-70-d IDD compared with control (1.4 +/- 0.3 mg%). The increase in filtration of albumin is in excess of that attributable to hyperfiltration before increased albumin excretion early in diabetes. In 50-70-d IDD, absolute tubular reabsorption of albumin is decreased, correlating to the decrease in brush border height of the proximal tubule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K. Diabetic proteinuria. Glomerular or tubular in origin? Am J Nephrol. 1984;4(6):337–346. doi: 10.1159/000166849. [DOI] [PubMed] [Google Scholar]
- Abrass C. K., Peterson C. V., Raugi G. J. Phenotypic expression of collagen types in mesangial matrix of diabetic and nondiabetic rats. Diabetes. 1988 Dec;37(12):1695–1702. doi: 10.2337/diab.37.12.1695. [DOI] [PubMed] [Google Scholar]
- Bertolatus J. A., Hunsicker L. G. Glomerular sieving of anionic and neutral bovine albumins in proteinuric rats. Kidney Int. 1985 Sep;28(3):467–476. doi: 10.1038/ki.1985.153. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B. S., Hauser E. B. Glycation of albumin, not glomerular basement membrane, alters permeability in an in vitro model. Diabetes. 1992 Nov;41(11):1415–1421. doi: 10.2337/diab.41.11.1415. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen B. Increased transcapillary escape rate of albumin in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1986 May;29(5):282–286. doi: 10.1007/BF00452063. [DOI] [PubMed] [Google Scholar]
- Fioretto P., Trevisan R., Valerio A., Avogaro A., Borsato M., Doria A., Semplicini A., Sacerdoti D., Jones S., Bognetti E. Impaired renal response to a meat meal in insulin-dependent diabetes: role of glucagon and prostaglandins. Am J Physiol. 1990 Mar;258(3 Pt 2):F675–F683. doi: 10.1152/ajprenal.1990.258.3.F675. [DOI] [PubMed] [Google Scholar]
- Fukui M. [Altered glomerular mRNA expression of basement membrane components and type I collagen in diabetic rats treated with or without insulin therapy]. Nihon Jinzo Gakkai Shi. 1991 Mar;33(3):275–281. [PubMed] [Google Scholar]
- GRAY S. J., STERLING K. The tagging of red cells and plasma proteins with radioactive chromium. J Clin Invest. 1950 Dec;29(12):1604–1613. doi: 10.1172/JCI102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargens A. R., Tucker B. J., Blantz R. C. Renal lymph protein in the rat. Am J Physiol. 1977 Oct;233(4):F269–F273. doi: 10.1152/ajprenal.1977.233.4.F269. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H. Pathogenesis of diabetic glomerulopathy: hemodynamic considerations. Semin Nephrol. 1990 May;10(3):219–227. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. II. Effects of varying osmolality, ionic strength, buffer system and fixative concentration of glutaraldehyde solutions. J Ultrastruct Res. 1966 Jun;15(3):283–309. doi: 10.1016/s0022-5320(66)80110-7. [DOI] [PubMed] [Google Scholar]
- Meyer T. W. Mechanisms of proteinuria in diabetic renal disease. Semin Nephrol. 1990 May;10(3):194–202. [PubMed] [Google Scholar]
- Mogensen C. E., Christensen C. K., Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983 May;32 (Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E., Sølling Studies on renal tubular protein reabsorption: partial and near complete inhibition by certain amino acids. Scand J Clin Lab Invest. 1977 Oct;37(6):477–486. doi: 10.3109/00365517709101835. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Mende C. W. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int. 1972;1(1):3–11. doi: 10.1038/ki.1972.2. [DOI] [PubMed] [Google Scholar]
- Park C. H. Time course and vectorial nature of albumin metabolism in isolated perfused rabbit PCT. Am J Physiol. 1988 Sep;255(3 Pt 2):F520–F528. doi: 10.1152/ajprenal.1988.255.3.F520. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Noer I., Deckert T., Evrin P. E., Nielsen S. L., Lyngsoe J., Mogensen C. E., Rorth M., Svendsen P. A., Trap-Jensen J. The effect of metabolic regulation on microvascular permeability to small and large molecules in short-term juvenile diabetics. Diabetologia. 1976 May;12(2):161–166. doi: 10.1007/BF00428983. [DOI] [PubMed] [Google Scholar]
- Parving H. P., Gyntelberg F. Transcapillary escape rate of albumin and plasma volume in essential hypertension. Circ Res. 1973 May;32(5):643–651. doi: 10.1161/01.res.32.5.643. [DOI] [PubMed] [Google Scholar]
- Provost S. B., Tucker B. J. Effect of 14 day head-down tilt on renal function and vascular and extracellular fluid volumes in the conscious rat. Physiologist. 1992 Feb;35(1 Suppl):S105–S106. [PubMed] [Google Scholar]
- Rasch R., Mogensen C. E. Urinary excretion of albumin and total protein in normal and streptozotocin diabetic rats. Acta Endocrinol (Copenh) 1980 Nov;95(3):376–381. doi: 10.1530/acta.0.0950376. [DOI] [PubMed] [Google Scholar]
- Rasch R., Osterby R. No influence of an aldose reductase inhibitor on glycogen deposition in tubules from streptozotocin diabetic rats. J Diabet Complications. 1989 Oct-Dec;3(4):198–201. doi: 10.1016/0891-6632(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Rasch R. Tubular lesions in streptozotocin-diabetic rats. Diabetologia. 1984 Jul;27(1):32–37. doi: 10.1007/BF00253498. [DOI] [PubMed] [Google Scholar]
- STERLING K. Radioactive chromium technic for circulating red cell volume (CRCV). Methods Med Res. 1960;8:69–76. [PubMed] [Google Scholar]
- Scandling J. D., Myers B. D. Glomerular size-selectivity and microalbuminuria in early diabetic glomerular disease. Kidney Int. 1992 Apr;41(4):840–846. doi: 10.1038/ki.1992.129. [DOI] [PubMed] [Google Scholar]
- Sølling K., Mogensen C. E. Studies on the mechanism of renal tubular protein reabsorption. Proc Eur Dial Transplant Assoc. 1977;14:543–549. [PubMed] [Google Scholar]
- Tilton R. G., Baier L. D., Harlow J. E., Smith S. R., Ostrow E., Williamson J. R. Diabetes-induced glomerular dysfunction: links to a more reduced cytosolic ratio of NADH/NAD+. Kidney Int. 1992 Apr;41(4):778–788. doi: 10.1038/ki.1992.121. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Chang K., Pugliese G., Eades D. M., Province M. A., Sherman W. R., Kilo C., Williamson J. R. Prevention of hemodynamic and vascular albumin filtration changes in diabetic rats by aldose reductase inhibitors. Diabetes. 1989 Oct;38(10):1258–1270. doi: 10.2337/diab.38.10.1258. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Anderson C. M., Thies R. S., Collins R. C., Blantz R. C. Glomerular hemodynamic alterations during acute hyperinsulinemia in normal and diabetic rats. Kidney Int. 1992 Nov;42(5):1160–1168. doi: 10.1038/ki.1992.400. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Effect of furosemide administration on glomerular and tubular dynamics in the rat. Kidney Int. 1984 Aug;26(2):112–121. doi: 10.1038/ki.1984.144. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Collins R. C., Ziegler M. G., Blantz R. C. Disassociation between glomerular hyperfiltration and extracellular volume in diabetic rats. Kidney Int. 1991 Jun;39(6):1176–1183. doi: 10.1038/ki.1991.149. [DOI] [PubMed] [Google Scholar]
- Tucker B. J. Early onset of increased transcapillary albumin escape in awake diabetic rats. Diabetes. 1990 Aug;39(8):919–923. doi: 10.2337/diab.39.8.919. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Mendonca M. M., Blantz R. C. Contrasting effects of acute insulin infusion on renal function in awake nondiabetic and diabetic rats. J Am Soc Nephrol. 1993 Apr;3(10):1686–1693. doi: 10.1681/ASN.V3101686. [DOI] [PubMed] [Google Scholar]
- Viberti G., Keen H. The patterns of proteinuria in diabetes mellitus. Relevance to pathogenesis and prevention of diabetic nephropathy. Diabetes. 1984 Jul;33(7):686–692. doi: 10.2337/diab.33.7.686. [DOI] [PubMed] [Google Scholar]
- Vittinghus E., Mogensen C. E. Graded exercise and protein excretion in diabetic man and the effect of insulin treatment. Kidney Int. 1982 May;21(5):725–729. doi: 10.1038/ki.1982.89. [DOI] [PubMed] [Google Scholar]