Abstract
A mutant of tomato (Solanum lycopersicum) with reduced abscisic acid (ABA) production (sitiens) exhibits increased resistance to the necrotrophic fungus Botrytis cinerea. This resistance is correlated with a rapid and strong hydrogen peroxide-driven cell wall fortification response in epidermis cells that is absent in tomato with normal ABA production. Moreover, basal expression of defense genes is higher in the mutant compared with the wild-type tomato. Given the importance of this fast response in sitiens resistance, we investigated cell wall and cuticle properties of the mutant at the chemical, histological, and ultrastructural levels. We demonstrate that ABA deficiency in the mutant leads to increased cuticle permeability, which is positively correlated with disease resistance. Furthermore, perturbation of ABA levels affects pectin composition. sitiens plants have a relatively higher degree of pectin methylesterification and release different oligosaccharides upon inoculation with B. cinerea. These results show that endogenous plant ABA levels affect the composition of the tomato cuticle and cell wall and demonstrate the importance of cuticle and cell wall chemistry in shaping the outcome of this plant-fungus interaction.
Plant defense against pathogens often involves the induction of mechanisms after pathogen recognition, including defense signaling, cell wall strengthening, and localized cell death, but plants also have preformed chemical and structural defense barriers. Fungal pathogens that penetrate the plant tissue directly through the outer surface, rather than via natural plant openings or wounds, must pass through the plant cuticle and epidermal cell wall. Penetration of the host surface happens either by physical means (i.e. by a highly localized pressure in the appressorium) or by chemical means (i.e. by the release of hydrolyzing enzymes). Necrotrophic plant pathogens like Botrytis cinerea typically use the latter strategy. During penetration, they produce cutinases and pectinolytic enzymes such as pectin methylesterases, endopolygalacturonases, and exopolygalacturonases (van Kan, 2006).
The cuticle is a hydrophobic barrier that covers the aerial surfaces of the plant. It is mainly composed of cutin, a polyester matrix, and soluble waxes, a complex mixture of hydrophobic material containing very-long-chain fatty acids and their derivatives, embedded into and deposited onto the cutin matrix. It plays an important role in organ development and protection against water loss (Yephremov et al., 1999; Sieber et al., 2000; Kurata et al., 2003; Jung et al., 2006). The cuticle is generally considered as a mere passive physical barrier against pathogen invasion, but it has also been recognized as a potential source of signaling and elicitor molecules (Jenks et al., 1994; Reina-Pinto and Yephremov, 2009). Plant cutin monomers trigger cutinase secretion in pathogenic fungi (Woloshuk and Kolattukudy, 1986), and cutin and wax components initiate appressorium formation and penetration in appressorium-forming pathogens (Kolattukudy et al., 1995; Francis et al., 1996; Gilbert et al., 1996; Fauth et al., 1998; Dickman et al., 2003). In plants, cutin monomers induce pathogenesis-related gene expression and elicit hydrogen peroxide (H2O2) synthesis (Fauth et al., 1998; Kim et al., 2008; Park et al., 2008). Transgenic tomato (Solanum lycopersicum) plants expressing the yeast Δ-9 desaturase gene had high levels of cutin monomers that inhibited powdery mildew (Erysiphe polygoni) spore germination, leading to enhanced resistance (Wang et al., 2000). Arabidopsis (Arabidopsis thaliana) plants expressing a fungal cutinase or mutants with a defective cuticle, such as long-chain acyl-CoA synthetase2 and bodyguard, are generally more susceptible to bacteria and equally susceptible to biotrophic fungi but are surprisingly resistant to B. cinerea (Bessire et al., 2007; Chassot et al., 2007; Tang et al., 2007). It has been postulated that a defective or thin cuticle encourages these plants to constitutively express defense-related mechanisms and to secrete antifungal compounds to the plant surface, thereby inhibiting B. cinerea growth (Bessire et al., 2007; Chassot et al., 2007). In addition, cuticle metabolic pathways might directly modulate plant-pathogen interactions by interacting with hormonally regulated defense pathways (Fiebig et al., 2000; Garbay et al., 2007; Mang et al., 2009) or with complex lipid signaling pathways leading to hypersensitive cell death (Raffaele et al., 2008).
Once plant pathogens have penetrated the cuticle, they secrete hydrolases that target the plant cell wall (ten Have et al., 1998; Oeser et al., 2002; Vogel et al., 2002; Jakob et al., 2007) that is mainly composed of cellulose, hemicellulose, and pectin (35% of total dry weight). Pectin consists mainly of the polysaccharides homogalacturonan and rhamnogalacturonan I and II. Homogalacturonans are linear chains of α-(1–4)-linked d-GalA residues that can be methylesterified at C-6. Rhamnogalacturonan I and II are more complex, branched polysaccharides. B. cinerea is typically regarded as a pectinolytic pathogen because it possesses an efficient pectinolytic machinery, including a variety of polygalacturonases and pectin methylesterases (PMEs), some of which are important virulence factors (ten Have et al., 1998, 2001; Valette-Collet et al., 2003; Kars et al., 2005). Pectins are a rich source of oligogalacturonides (OGAs), biologically active signaling molecules that can activate plant defense mechanisms (Hahn et al., 1981; Côté and Hahn, 1994; Messiaen and Van Cutsem, 1994; Ridley et al., 2001). The eliciting capacity of the OGAs was shown to depend on their size, which in turn is influenced by the methylesterification pattern of the homogalacturonan fraction (Mathieu et al., 1991; Messiaen and Van Cutsem, 1994). To counteract the activity of fungal pectinases, many plants express polygalacturonase-inhibiting proteins and PME inhibitors, which are localized in the cell wall. The role of these proteins in plant defense against B. cinerea has been extensively demonstrated (Powell et al., 2000; Ferrari et al., 2003; Sicilia et al., 2005; Joubert et al., 2006, 2007; Lionetti et al., 2007). The interaction with the inhibitors not only limits the destructive potential of polygalacturonases but also leads to the accumulation of elicitor-active OGAs (De Lorenzo and Ferrari, 2002). How OGAs are perceived by the plant is still unclear, but in view of the diversity of biological activities and structure requirements, they are thought to be recognized through different proteins, including receptor-like kinases, wall-associated kinases, arabinogalactan proteins, and Pro-rich proteins (Côté and Hahn, 1994; Showalter, 2001; Humphrey et al., 2007).
Over the past years, the role of abscisic acid (ABA) in plant-pathogen interactions has gained increased attention. ABA is mostly negatively correlated with resistance against phytopathogens through down-regulation of defense responses orchestrated by salicylic acid, jasmonic acid, and ethylene (Mohr and Cahill, 2001; Audenaert et al., 2002; Mauch-Mani and Mauch, 2005; Asselbergh et al., 2008). In tomato, the ABA-deficient mutant sitiens has an enhanced resistance to B. cinerea (Audenaert et al., 2002) that depends on a timely, localized oxidative burst leading to rapid epidermal cell wall fortification and a faster and higher induction of defense-related gene expression upon infection compared with the wild type (Asselbergh et al., 2007). Moreover, basal defense gene expression is higher in this mutant than in the wild type. As this early response is of vital importance for the resistant reaction of tomato against B. cinerea, we investigated whether alterations in cuticle and/or cell wall, which form the first barrier to the invading pathogen, affect resistance. We demonstrate that the sitiens cuticle is more permeable and that permeability is positively correlated with resistance to B. cinerea. Furthermore, differences in pectin composition and rate of methylesterification occur. Together, these data hint at an unanticipated role for extracellular matrix components in the resistance of tomato against B. cinerea and thus shed new light on the largely unexplored interrelationship between the extracellular matrix and plant-pathogen interactions.
RESULTS
Different Localization of Pathogen Growth in the Wild Type and sitiens
We previously showed that resistance in sitiens is based on H2O2 accumulation in the epidermal cells starting at 4 h post inoculation (hpi) followed by cell wall fortification starting at 8 hpi, restricting fungal colonization of the underlying tissue (Asselbergh et al., 2007). We here used immunological detection of pectic cell wall components in transverse sections to visualize pectin breakdown upon B. cinerea inoculation (Knox et al., 1990). Using the JIM7 antibody, which binds to methylesterified pectin epitopes, we found that in the wild type, B. cinerea causes degradation of the pectin matrix in all leaf cell layers at 32 hpi, while in sitiens, degradation is restricted to the outer anticlinal and periclinal cell wall of epidermal cells (Fig. 1). The staining pattern of JIM5, which recognizes low to non methylesterified epitopes, was similar to that of JIM7 (Supplemental Fig. S1). Based on these immunological stainings, we could not distinguish quantitative differences in pectin methylesterification between wild-type and sitiens tomato. However, these data confirm the importance of epidermal anticlinal cell wall fortification in obstructing B. cinerea growth and infection, as shown previously (Asselbergh et al., 2007). Moreover, they indicate that in sitiens, defense reactions and cell wall degradation are restricted to the epidermal cell layer, hinting at the epidermis as a source of defense signaling molecules. As the cuticle and cell wall are among the first plant barriers to pathogen ingress, we further investigated whether the fast defense response and resistance in sitiens are caused by changes in cuticle and/or cell wall composition.
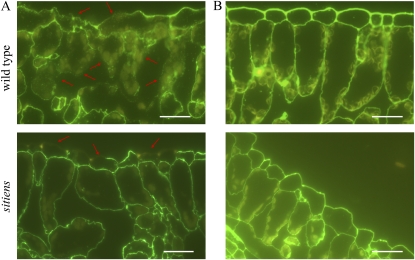
Figure 1.
Pectin degradation during B. cinerea infection in sitiens and wild-type tomato. Degradation of pectin at 32 h after B. cinerea inoculation (A) or mock inoculation (B) was detected with monoclonal antibody JIM7 and secondary labeling with fluorescein isothiocyanate. Some sites with cell wall degradation are indicated with arrows. Bars = 50 μm. At least 10 samples from different plants were examined for the wild type and sitiens, and representative images are shown.
Ultrastructural Differences in Cell Wall and Cuticle of sitiens
Transverse sections of fifth leaves of the wild type and sitiens were examined by transmission electron microscopy (Fig. 2). The cuticle was visible as a dark (electron-dense) layer on top of the cell wall. Abnormalities in the sitiens cuticle were visible as thicker and irregular electron-dense layers, which besides structural differences might indicate a different lipid content in the cuticle of the mutant (Fig. 2, B and D). A distinction could also be made between the cell wall architecture of the wild type and sitiens. In the wild type, the epidermal periclinal cell wall is regular in shape, whereas that of sitiens was generally thicker and less dense. Furthermore, irregular depositions of cell wall material were found in the mutant, giving rise to structural distortions in the epidermal cell walls (Fig. 2E). Complementation of sitiens plants with exogenous ABA restored the wild-type phenotype in the mutant, whereas the control treatment did not (Fig. 2, C and D).
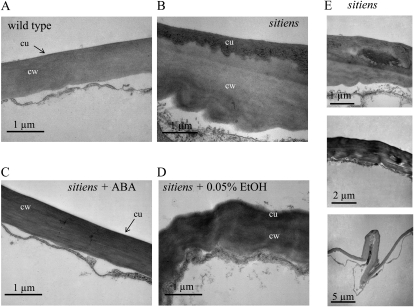
Figure 2.
Transmission electron micrographs of wild-type and sitiens transverse leaf sections. Micrographs show leaf epidermal cell walls of the wild type (A), sitiens (B), sitiens + 100 μm ABA in 0.05% ethanol (C), and sitiens + 0.05% ethanol (D). The cuticle (cu) is visible as the dark (electron-dense) apposition on the cell wall (cw). E, Examples of cell wall abnormalities found in cell wall and cuticle of sitiens leaf. Similar observations were made in sections of three different wild-type and sitiens plants. The experiment was repeated with similar results.
ABA Deficiency Leads to a Decrease in Surface Hydrophobicity and Leaf Trichome Number
Cuticular permeability to watery solutions and hydrophilic compounds is known to be correlated with wax amounts and composition (Hauke and Schreiber, 1998; Popp et al., 2005). Furthermore, wax amounts and the relative composition of the hydrocarbon, alcohol, and aldehyde fractions of the cuticular wax determine the hydrophobicity of the leaf surface (Bringe et al., 2006; Koch and Ensikat, 2007). Therefore, hydrophobicity of wild-type and sitiens leaves was analyzed by the contact angle method, in which contact angles of a droplet of distilled water with the surface are measured (Kasahara et al., 1993). The contact angles for sitiens were smaller than those for the wild-type leaves, indicating a more hydrophilic surface and thus a difference in wax amount or composition (Fig. 3, A and B). Cuticle composition has been shown to also affect trichome development in several Arabidopsis mutants (Yephremov et al., 1999; Wellesen et al., 2001). Moreover, ABA deficiency in plants leads to pleiotropic effects and might thus affect trichome density. As trichome density could influence droplet formation on the tomato leaf surface, we counted the trichomes on wild-type and sitiens leaf discs of 4 mm diameter. Clearly, ABA deficiency in the sitiens tomato mutant leads to a decrease in leaf trichome number (Fig. 3, C and D).
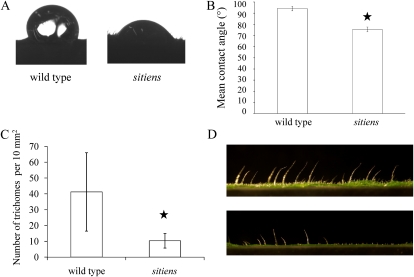
Figure 3.
Leaf surface hydrophobicity and trichome density. A, Images of droplets on wild-type and sitiens leaf surfaces illustrating the differences in surface tension. B, Hydrophobicity determined by measuring the contact angle of a 10-μL droplet of distilled water on the leaf surface by the sessile drop method. Mean contact angles were averages of at least 10 measurements. Fourth leaves of 5-week-old plants (seventh leaf stage) were used. Error bars indicate se. A Student’s t test indicated that differences between the wild type and sitiens were statistically significant, indicated by the star (P < 0.001). C, Number of trichomes per 10 mm2. Error bars indicate se. A Mann-Whitney test revealed a significant difference between the wild type and sitiens, indicated by the star (P < 0.001). D, Representative photographs of leaf sections illustrating differences in trichome density between the wild type (top) and sitiens (bottom). [See online article for color version of this figure.]
ABA Deficiency Leads to Increased Surface Permeability
As a measure for surface permeability, we monitored the chlorophyll efflux rates (Chen et al., 2004). Total chlorophyll was extracted at room temperature with 80% ethanol and sampled at different time points during a 4-h period from equal amounts of intact leaves of wild-type, sitiens, and ABA-complemented sitiens plants. Clearly, the chlorophyll efflux was faster in sitiens (Fig. 4A), and complementation with ABA restored permeability of the sitiens cuticle to the wild-type level (Fig. 4B). However, since the sitiens mutant is known to have a higher stomatal density, which might influence chlorophyll efflux rate (Nagel et al., 1994), we additionally monitored leaf surface permeability to aqueous staining solutions to assess the permeability of the cuticle of sitiens and wild-type plants. We used three histological stains that bind different cell wall epitopes: the pectic carboxyl group-binding dyes ruthenium red and alcian blue and the polychromatic cell wall dye toluidine blue. Based on dye accumulation in leaf discs floating on staining solutions, increased permeability of the sitiens cuticle was demonstrated (Fig. 4C; data shown for toluidine blue, with all dyes giving similar results). Dye accumulation was not linked with stomatal distribution (Fig. 4C; data not shown). Complementation with ABA reduced the permeability to toluidine blue of the sitiens cuticle back to that of the wild type (Fig. 4C).
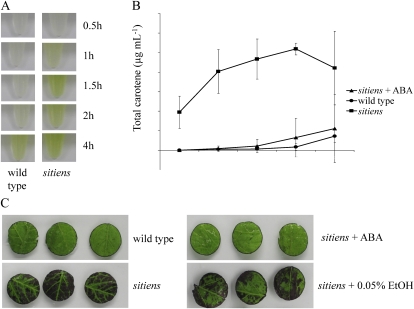
Figure 4.
Cuticle and cell wall permeability of sitiens and the wild type. A, Extraction of chlorophyll from equal amounts of wild-type and sitiens intact leaves for 0.5 to 4 h in 80% ethanol at room temperature. B, Total chlorophyll extracted from leaves of the wild type, sitiens, and sitiens complemented with ABA by spraying 100 μm ABA in 0.05% ethanol until runoff twice per week during their development at different time intervals with sd. C, Leaf discs excised from the fourth leaf of 5-week-old wild-type and sitiens plants floating on their adaxial side on a 0.05% toluidine blue solution in water for 1 h. sitiens plants were complemented with ABA by spraying 100 μm ABA in 0.05% ethanol until runoff twice per week during development. As a control, a mock solution containing 0.05% ethanol was used. Biological replicates of the experiments produced similar results.
Cuticle Permeability Is Directly Linked with Resistance against B. cinerea
Increased resistance to B. cinerea in Arabidopsis mutants with an aberrant cuticle has been shown to be, at least in part, the consequence of the diffusion of antimicrobial compounds from the epidermal cells to the leaf surface, thereby inhibiting the hyphal growth (Bessire et al., 2007; Chassot et al., 2007). To determine whether a similar phenomenon is responsible for the resistant phenotype in sitiens plants, we measured the germination rate of B. cinerea spores in quarter-strength potato dextrose broth that had been incubated for 18 h on wild-type and sitiens leaf surfaces so that any antifungal substances could have diffused into them. Quantification of the spore germination for 5 h revealed no significant difference in the presence of the wild-type or sitiens exudates (Supplemental Fig. S2A). Additionally, microscopic monitoring of hyphal growth on the leaf surface revealed no differences between sitiens and the wild type (Supplemental Fig. S2B). To test whether the cuticular permeability correlates directly with disease resistance against B. cinerea, we tested all leaves of 5-week-old wild-type and sitiens plants for permeability and disease susceptibility (Fig. 5). Disease and permeability indices were calculated for leaf discs of the same leaf, and their correlation was statistically analyzed. The permeability of the leaves was variable at different developmental stages, and a significant negative correlation was observed between permeability and susceptibility in sitiens, as indicated by the Spearman’s rank correlation coefficient (ρ = −0.770, P < 0.01), but not for the wild type (ρ = −0.330), due to the very high susceptibility of all leaves (Fig. 5B).
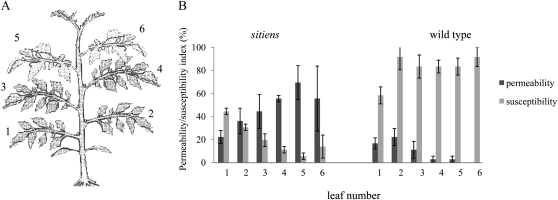
Figure 5.
Correlation between leaf surface permeability and resistance against B. cinerea. A, Schematic representation of a 5-week-old tomato plant (seventh leaf stage) with leaf numbers indicated. B, Permeability index and disease index calculated on four leaf discs of each leaf of 5-week-old (seventh leaf stage) wild-type and sitiens plants. Disease index was evaluated using four scoring categories (0, resistant; 1, slightly spreading lesion; 2, moderately spreading lesion; 3, severely spreading lesion), and permeability index was calculated based on the surface area stained with toluidine blue using four categories (0, 0%–25%; 1, 25%–50%; 2, 50%–75%; 3, 75%–100%). Data represent means ± se of three different plants with four discs per leaf and per plant (n = 12). A significant correlation was observed between permeability and resistance in sitiens, as indicated by the Spearman’s rank correlation coefficient (ρ = −0.770, P < 0.01), but not for the wild type (ρ = −0.330).
Increased Methylesterification Rate of sitiens Homogalacturonan
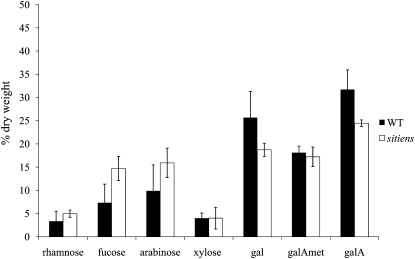
As B. cinerea produces pectinolytic enzymes during infection, we tested whether the wild type and sitiens are differentially sensitive to pectinase treatment. Pectinase or polygalacturonase catalyzes the random hydrolysis of 1,4-α-d-galactosiduronic linkages in pectin and other galacturonans. Methylesterified pectin is not degradable by polygalacturonases and requires the action of PMEs. Wild-type leaf discs floating on pectinase solutions of different concentrations were more susceptible to polygalacturonase degradation after 3 d than sitiens leaf discs, suggesting a lower degree of methylesterification (Fig. 6). Treatment with cellulase at different concentrations resulted in similar degradation patterns in the wild type and sitiens, indicating that both genotypes were equally prone to degradation by cellulase (Supplemental Fig. S3). A more detailed chemical analysis of cell wall sugars was performed on sitiens and wild-type total leaf crude cell wall preparations. Figure 7 presents the monosaccharide composition of the sitiens and wild-type cell walls. A Student’s t test was used to find differences between wild-type and sitiens monosaccharide composition (P < 0.05). The neutral sugar levels, expressed as a percentage of cell wall dry weight, did not differ significantly between mutant and control plants, except for Fuc. Total Gal and GalA contents were significantly lower in sitiens than in the wild type. Further analysis revealed that the decrease is mainly due to a reduction in Gal and unmethylesterified GalA (Fig. 7). In conclusion, sitiens has a relatively higher degree of methylesterification of homogalacturonic residues compared with the wild type.
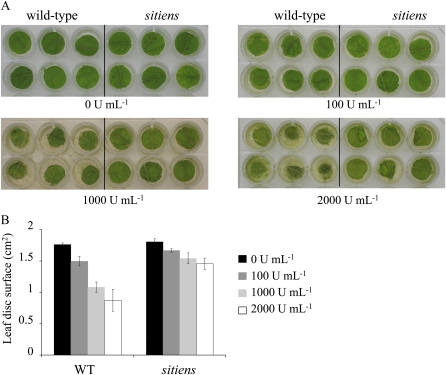
Figure 6.
Pectinase treatments of wild-type and sitiens leaf discs. A, Leaf discs excised from fifth leaves of wild-type and sitiens plants floating at room temperature on 1 mL of pectinase (Pectinex Ultra SPL; Sigma-Aldrich) solutions at different enzyme concentrations (units mL−1). Photographs were taken after 3 d. B, Quantification of the leaf disc surface area with the image-analysis software APS Assess 2.0. Two biological replicates gave similar results. WT, Wild type.
Figure 7.
Cell wall composition of wild-type (WT) and sitiens plants. Monosaccharide composition of the cell walls of fifth leaves of 5-week-old wild-type and sitiens plants. Results are expressed as a percentage of cell wall dry weight. Bars indicate se (n = 4; n = 2 for Gal [gal], GalA [galA], and methylesterified GalA [galAmet]).
Differences in Oligosaccharides Released from sitiens and Wild-Type Epidermal Cell Walls
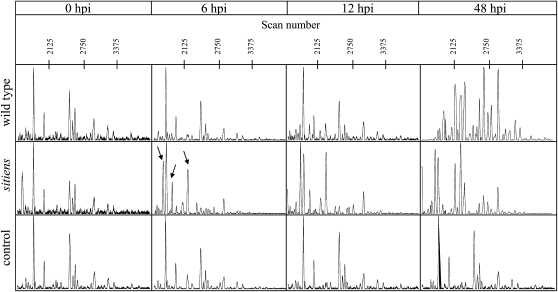
The OGAs released from the cell wall during pathogen infection are known to play a role in disease signaling and pathogen-induced gene expression (Norman et al., 1999). OGAs with a degree of polymerization between 9 and 15 are potent inducers of plant defense responses (Mathieu et al., 1991; Bellincampi et al., 1993; Côté and Hahn 1994), although smaller pectin fragments have some bioactivity (Boudart et al., 1995; Simpson et al., 1998; Moerschbacher et al., 1999). As the degree of methylesterification in epidermal cell walls of sitiens apparently differs from that in the wild type, we hypothesized that different OGAs might be released. We analyzed the carbohydrates released from the wild-type and sitiens leaf surfaces into an infection droplet at 0, 6, 12, and 48 hpi using capillary electrophoresis (Williams et al., 2003; Ström and Williams, 2004; Goubet et al., 2006). As a control, a B. cinerea inoculation solution was incubated for the same periods. Derivatization of the carbohydrates with 8-amino-1,3,6-pyrenetrisulfonic acid, a fluorescent label that readily reacts with the reducing end of oligosaccharides and polysaccharides, allowed detection of the labeled oligosaccharides. Electropherograms of the different samples are presented (Fig. 8). At 0 hpi, no differences can be observed between wild-type, sitiens, and control samples. Peaks observed around scans 1,850, 2,000, 2,500, 2,630, 3,000, and 3,250 are thus originating from the Glc-containing B. cinerea inoculation solution. At 6 hpi, no differences can be observed between wild-type and control samples. However, in sitiens, new peaks with strong fluorescence around scans 1,750 and 2,250 appear, indicating modifications in the saccharide composition in the inoculation droplet at that moment. A similar pattern is observed at 12 hpi. At 48 hpi in the wild type, electrophoretic mobilities drastically shift compared with previous time points, and more peaks are distinguishable. This indicates that the oligosaccharide composition in the wild-type droplet drastically differs from that of the previous time points, which might correlate with total digestion of the plant cell wall upon infection by B. cinerea. Around 48 hpi, B. cinerea infection causes visible symptoms on wild-type leaves that generally appear as macerated lesions (Asselbergh et al., 2007). In sitiens, differences in the electropherogram occur mainly in signal intensities. Infection symptoms in sitiens plants at this time point are restricted to localized necrotizing cells beneath the infection droplet, but without tissue maceration.
Figure 8.
Electropherograms obtained from capillary electrophoresis of wild-type and sitiens oligosaccharides released into B. cinerea inoculation droplets. Oligosaccharides released into B. cinerea inoculation droplets on fifth leaves of 5-week-old plants were sampled at 0, 6, 12, and 48 hpi and labeled with 8-amino-1,3,6-pyrenetrisulfonic acid. Signal intensity as relative fluorescence units is given in the ordinate, and scan numbers are given in the abscissa. For each sample, three biological replicates were analyzed, of which one is represented here.
DISCUSSION
The ABA-deficient sitiens tomato mutant has previously been shown to be more resistant to the necrotrophic fungus B. cinerea (Audenaert et al., 2002). A timely induction of defense reactions, including H2O2 production and cell wall fortification in epidermal cells, was shown to be important for the increased resistance in this mutant (Asselbergh et al., 2007). Additionally, basal defense gene expression is higher in the sitiens mutant compared with the wild type (Asselbergh et al., 2007). In this study, we show that ABA deficiency in tomato leads to alterations in leaf surface structure and composition, which might shed some new light on the resistance mechanism of this mutant to B. cinerea.
Using immunological staining of leaf sections with antibodies that bind to pectin epitopes, we showed that pectin breakdown in wild-type tomato 32 h after B. cinerea inoculation occurs in epidermal cells as well as in the mesophyll. In sitiens, pectin breakdown was restricted to the outer periclinal and anticlinal cell walls at 32 hpi. This is in accordance with previous findings that rapid induction of H2O2-fueled cell wall fortification prevents spreading of the fungus in the mutant. Together, these data suggest that the outer surface layer plays a vital role in the resistance mechanism against B. cinerea.
Ultrastructural analysis of leaf sections using transmission electron microscopy revealed deformation of epidermal cell walls and irregular deposition of electron-opaque material, which represents cutin, in the sitiens mutant. As the cuticle is a modification of the epidermal cell wall, with the cutin matrix linked to the cell wall constituents, it is likely that changes in the epidermal cell wall metabolism may affect the cuticle and vice versa. A difference in cuticle composition was also suggested by the lower leaf surface hydrophobicity observed in sitiens as measured by the contact angle method. Differences in contact angle, however, might have been influenced by the lower trichome density in sitiens. Intriguingly, Arabidopsis fiddlehead-1 and lacerata mutants, which have altered cuticle composition, also have dramatically lowered trichome numbers (Yephremov et al., 1999; Wellesen et al., 2001). Overexpression of SHN, encoding an AP2/EREBP transcription factor involved in regulating the metabolism of lipid and/or cell wall components, resulted in altered wax composition and a decrease in leaf trichome number in Arabidopsis (Aharoni et al., 2004). Similarly, several eceriferum (cer) mutants of barley (Hordeum vulgare) and Arabidopsis originally isolated as wax deficient exhibit increases in stomata number (Zeiger and Stebbins, 1972; Gray et al., 2000). It has been suggested that the cuticle composition affects epidermal cell differentiation (Bird and Gray, 2003). As indicated by our data on trichome numbers and the higher stomatal density in the sitiens mutant (Nagel et al., 1994), a similar connection between cuticle composition and epidermal cell differentiation might exist in tomato, although mere pleiotropic effects of ABA deficiency cannot be ruled out at this point.
Structural and/or compositional differences in the sitiens cuticle were associated with higher cuticular permeability, which was shown to be positively correlated with higher resistance to B. cinerea. Similar observations have been made in Arabidopsis cuticle mutants (Bessire et al., 2007; Chassot et al., 2007; Tang et al., 2007). Several scenarios have been proposed to explain the increased resistance to B. cinerea. In some studies, resistance (partially) depended on enhanced export of antifungal compounds to the leaf surface (Bessire et al., 2007; Chassot et al., 2007). As germination of B. cinerea spores was not different in leaf exudates from sitiens or wild-type plants and no differences in germination and penetration on wild-type or sitiens surfaces have been observed (Asselbergh et al., 2007; this study), this mechanism probably does not (significantly) contribute to the resistance observed in sitiens. Additionally, it rules out an effect of leaf surface structure or composition in the early infection stages of B. cinerea.
Another scenario considers the increased permeability of the cuticle to elicitors from B. cinerea, allowing a faster induction of defense mechanisms (Chassot et al., 2007). Furthermore, it has been speculated that cutin monomers, which are released onto the surface of Arabidopsis cuticle mutants, or the action of cutinase/hydrolase might be relevant for the resistance to B. cinerea (Xiao et al., 2004; Chassot and Métraux, 2005; Chassot et al., 2007). Monomers released from the cuticular layer are able to induce plant defense reactions (Schweizer et al., 1996a, 1996b; Chassot and Métraux, 2005; Kim et al., 2008). In etiolated hypocotyls of cucumber (Cucumis sativus), cutin monomers from hydrolysates of cucumber, apple (Malus domesticus), and tomato elicited H2O2 production (Fauth et al., 1998), further supporting the notion that plants have the potential to recognize breakdown products of the cuticle and activate defense-related mechanisms.
We suggest that plant defense elicitors released by B. cinerea, or hydrolytic enzymes producing cell wall-derived elicitors, may diffuse faster through the more permeable sitiens cuticle. Alternatively, monomers released from a defective cuticle might induce signaling events leading to the activation of plant defense mechanisms. In favor of this hypothesis, pathogenesis-related gene expression is higher in sitiens plants under noninfected conditions than that of the wild type (Asselbergh et al., 2007). Although no sensor for cuticle integrity was known until now, a mechanism involving a sensor in the extracellular matrix has been described. The Arabidopsis plasma membrane-bound receptor-like kinase THESEUS1 has been found to act as a cell wall-integrity sensor that positively regulates the expression of genes related to defense, oxidative stress, and cell wall metabolism (Hématy et al., 2007).
It is interesting that, in contrast to the link between cuticle permeability and B. cinerea resistance in Arabidopsis and tomato leaves, in tomato fruits a less permeable cuticle leads to B. cinerea resistance. Fruits of the tomato mutant delayed fruit deterioration have a denser cutin matrix and higher wax and cutin contents than the wild-type cv Ailsa Craig and are highly resistant to B. cinerea, unless the cuticle is damaged (Saladié et al., 2007). This indicates that there is a different defense mechanism against B. cinerea operating in tomato leaves as compared with fruits, which have a 10-fold thicker cuticle.
As ABA plays an important role in drought stress regulation and the cuticle protects plants against drought, it is likely that ABA might regulate the biosynthesis of this waxy layer, although strong evidence for direct involvement of basal endogenous ABA in the cuticle biosynthesis process is scarce. Some genes involved in cuticle biosynthesis (AtMYB41, AtCER6, AtWBC11, and LpLTP1-3) are induced by exogenously applied or stress-induced ABA, and the CER6 gene involved in long-chain fatty acid biosynthesis contains a cis-ABA-responsive element in its promoter (Treviño and O’Connell, 1998; Hooker et al., 2002; Luo et al., 2007; Cominelli et al., 2008). More recently, it has been shown that water deficiency and ABA treatment induce an increase in cuticular wax load in Arabidopsis, and water deficit increases total cutin monomer amount and alters the proportional amounts of cutin monomers (Kosma et al., 2009). Our results clearly indicate a role for ABA in the regulation of cuticle biosynthesis. Accordingly, transcript levels of a lipid transfer protein are low in sitiens compared with the wild type (Asselbergh et al., 2007). Besides their role in plant defense, lipid transfer proteins are involved in the transport of lipids through the extracellular matrix to form cuticular wax (Cameron et al., 2006).
We also observed an effect of ABA deficiency on the rate of homogalacturonan methylesterification, which is controlled by PMEs (Wolf et al., 2009). Contradictory roles for ABA in PME regulation have been reported (Micheli, 2001). PME activity is inhibited by ABA during yellow cedar (Chamaecyparis nootkatensis) and Medicago truncatula seed germination (Ren and Kermode, 2000; Gimeno-Gilles et al., 2009). In tomato seed germination, ABA enhances PME activity (Downie et al., 1998). In drought-stressed Arabidopsis, PME genes are down-regulated (Bray, 2004), but in ripening banana (Musa acuminata) fruit, ABA increases PME activity (Lohani et al., 2004). Although immunological staining was not sensitive enough to reveal putative differences in pectin methylesterification rate between the wild type and sitiens (Fig. 1; Supplemental Fig. S1), pectinase treatment and gas chromatography-mass spectrometry (GC-MS) data indicated a higher degree of methylesterification in the sitiens mutant. Our data show a correlation between low endogenous ABA content and higher pectin methylesterification in tomato leaves, indicating that ABA plays a role in the regulation of PMEs that are expressed in the leaves.
One explanation for the high resistance of sitiens might be that the pectin layer is less degradable by hydrolase activities that are secreted by B. cinerea, due to the higher degree of methylesterification. However, sitiens is more resistant to several other (biotrophic) pathogens that do not secrete pectinases for penetration and/or tissue colonization (Thaler and Bostock, 2004; Achuo et al., 2006). Moreover, B. cinerea secretes PMEs during leaf penetration, and it is not clear whether PME activity is required for the virulence of the pathogen (Valette-Collet et al., 2003; Kars et al., 2005). Rather, we hypothesize that in sitiens, the methylesterification pattern and degree account for the release of more active elicitors. OGAs with a polymerization degree between 9 and 15 are known to be most bioactive (Mathieu et al., 1991; Messiaen and Van Cutsem, 1993). Additionally, methylesterification rate was shown to have an effect on elicitation capacity of OGAs against B. cinerea in strawberry (Fragaria vesca; Osorio et al., 2008). Our hypothesis is strengthened by the observation that different carbohydrates accumulated in infection droplets incubated on sitiens leaves than on those of the wild type. Thus, our results indicate that the higher degree of pectin methylesterification in sitiens might result in the release of different and more bioactive OGAs upon pectin breakdown by B. cinerea. The identity of the carbohydrates as well as their potential in vivo elicitor activity are the subjects of further investigation.
A synergistic effect of the alterations in sitiens cuticle and pectin is conceivable. A model of this hypothesis is presented (Fig. 9). Dimers of homogalacturonide chains bridged by Ca2+ ions form an egg-box conformation that is necessary for OGAs to be biologically most active (Decreux and Messiaen, 2005). This egg-box conformation induced a significant increase in the binding of OGAs to the transmembrane receptor-like wall-associated kinase WAK1 (Cabrera et al., 2008). Moreover, when fungi invade plant tissue, chitin oligosaccharides and de-N-acetylated chitosan oligosaccharides are produced, which elicit defense responses in various plants (Hahn, 1996). Chitosan oligomers were recently shown to stabilize the OGA egg boxes, leading to enhanced elicitor capacity in vitro (Cabrera et al., 2010). Previous results showed that in sitiens, a chitinase is highly expressed prior to infection (Asselbergh et al., 2007). A high activity of this enzyme in the extracellular space could induce the release of chitin and chitosan oligomers from B. cinerea and subsequently associate with OGAs released from the cell wall of epidermal cells. As stabilization of the elicitor-active egg-box conformation is time dependent (Cabrera et al., 2008), one could speculate that the higher permeability of the sitiens cuticle permits faster elicitor maturation, resulting in faster induction of defense reactions. Our observation of a clear correlation between leaf surface permeability and resistance is in favor of this hypothesis.
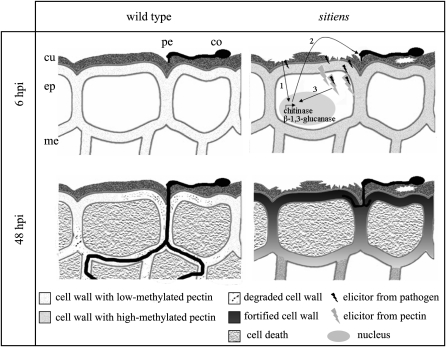
Figure 9.
Schematic representation of the putative oligosaccharide signaling in the wild type and sitiens. In sitiens, the perception by the plant of a defective cuticle might lead to the constitutive expression of chitinases and glucan endo-1,3-β-glucosidases (1), releasing elicitor-active molecules from the fungal cell wall (2). The higher degree of methylesterification in the sitiens cell wall delivers more active host elicitors. Both types of elicitors can have a synergistic effect or even interact with each other to form even more elicitor-active complexes (3). Together with the higher permeability of the sitiens cuticle, the signaling would be faster and more effective with defense gene expression and the resistant phenotype that typifies the mutant as a consequence. In the wild type, basal expression of chitinases and glucan endo-1,3-β-glucosidases is not high enough to release elicitors from the germinating B. cinerea, and expression is induced only upon infection. The cuticle is not permeable, which delays the diffusion of signaling molecules/complexes into the cytosol of epidermal cells. Because of the absence of early signaling reactions, defense is delayed, resulting in cell wall breakdown, cell death, and fungal spreading. co, Conidium; cu, cuticle; ep, epidermal cell layer; me, mesophyll cell layer; pe, point of penetration.
There has been a long-standing controversy over whether or not hydrolase activity enhances fungal infection (Schäfer, 1993; Valette-Collet et al., 2003; Kars et al., 2005). Our results indicate that the composition of the epidermal leaf surface itself has an influence on the end products of hydrolase activity and may influence the role of these enzymes as a virulence factor.
In conclusion, we have shown that endogenous ABA levels play a decisive role in (the regulation of) cuticle and cell wall biosynthesis in tomato leaves and consequently determine the structure and composition of the outer surface layers. ABA deficiency resulted in a more permeable cuticle and a higher degree of pectin methylesterifcation. We furthermore demonstrated that the more permeable cuticle of the ABA-deficient sitiens mutant is directly correlated with enhanced resistance to B. cinerea. We suggested that the higher degree of pectin methylesterification in the mutant results in more bioactive OGAs. Together with the higher permeability that allows faster diffusion of elicitors, this results in a faster induction of defense responses in sitiens, which renders these plants more resistant to B. cinerea than the wild type. A model was presented in which synergistic effects of the alterations in cell wall and cuticle composition contribute to the resistant interaction of sitiens with B. cinerea. Further studies will be needed to shed light on the exact role of the cuticle and pectin composition in the resistance of tomato against B. cinerea and in plant-pathogen interactions in general.
MATERIALS AND METHODS
Plant Material and Exogenous ABA Treatment
The tomato (Solanum lycopersicum) wild-type “Moneymaker” and its sitiens mutant (a kind gift of Prof. M. Koornneef, Wageningen University) were grown in potting compost soil (Substrat 4; Klasmann-Deilmann) in a growth chamber at 22°C with 75% relative humidity under a 16-h-light/8-h-dark regime. For the GC-MS experiment, plants were grown in a greenhouse with approximately 65% relative humidity. ABA complementation was as described by Achuo et al. (2006). Plants were sprayed until runoff with 100 μm ABA in 0.05% ethanol every 5 d during their development. Controls were fed with 0.05% ethanol.
Fungal Material and Infection Method
Conidia of Botrytis cinerea strain R16 (Faretra and Pollastro, 1991) were obtained as described (Audenaert et al., 2002). An inoculation suspension was prepared containing 5 × 105 spores mL−1 in 0.01 m KH2PO4 and 6.67 mm Glc. Conidia were pregerminated for 2 h in the inoculation suspension at 22°C. Fifth leaves were used for B. cinerea inoculation with 10-μL droplets. Incubation was at 22°C under dark conditions. Symptoms were evaluated after 3 to 4 d. For mock inoculation, the inoculation suspension was prepared omitting the spores.
Tissue Fixation and Immunological Staining
Transverse sections of tomato leaves embedded in LR White (LRW; Sigma-Aldrich, Fluka) resin were used for immunological staining. Leaf segments (2 mm × 8 mm) were fixed in 4% paraformaldehyde and 1% glutaric dialdehyde (Acros Organics) in 0.05 m PIPES (Sigma-Aldrich) for 2.5 h after 30 min of vacuum infiltration. After dehydration in a graded series of ethanol, LRW was gradually added to the samples: one-third LRW + two-thirds ethanol for 40 min; one-half LRW + one-half ethanol for 40 min; and two-thirds LRW + one-third ethanol for 40 min. Next, three successive incubations of 2 h in pure LRW were done. The samples were transferred to electron microscopy-purpose beam microembedding capsules (Laborimpex) filled with LRW and incubated for 24 h at 60°C for polymerization. Semithin sections (2 μm) were cut with a motorized rotary microtome (Leica RM2265; Leica Microsystems). After blocking the sections using 3.33% normal rabbit serum (Gentaur) in TBSB-T (Tris-buffered saline solution, pH 7.4, + 0.1% bovine serum albumin + 0.01% Tween 20), primary antibody incubation (JIM5 and JIM7 [PlantProbes], 20 times diluted in TBSB-T) was performed overnight at 4°C. An anti-rat IgG-fluorescein isothiocyanate conjugate (Sigma-Aldrich) was used as a secondary antibody. Samples were washed several times, mounted in Vectashield Hardset mounting medium (VWR), and examined with an Olympus BX51 epifluorescence microscope with U-MWB2 filter cube (450- to 480-nm excitation filter, DM500 dichroic beam splitter, and BA515 long-pass filter).
Electron Microscopy
For transmission electron microscopy, tomato leaf sections (2 × 2 μm) were excised from fourth leaves of 5-week-old plants (seventh leaf stage), briefly immersed in dextran 15% to 20%, and frozen immediately in a high-pressure freezer (EM Pact; Leica Microsystems). Over a period of 4 d, tissues were freeze substituted in dry acetone containing 1% OsO4 and 0.1% glutaraldehyde as follows: −90°C for 26 h, 2°C h−1 temperature increase over 15 h, −60°C for 8 h, 2°C h−1 temperature increase over 15 h, −30°C for 8 h, with rinsing three times with acetone in an Automatic Freeze Substitution System (Leica Microsystems). At the end, samples were slowly warmed up to 4°C and infiltrated at 4°C stepwise in Spurr’s resin over 3 d. Ultrathin sections of gold interference color were cut with an ultramicrotome (Ultracut E; Leica Microsystems) and collected on formvar-coated copper slot grids. Sections were poststained in an LKB ultrastainer (http://www.lkb.com.au/) for 40 min in uranyl acetate at 40°C and for 10 min in lead stain at 20°C and then transferred to grids. Grids were viewed with a JEOL 1010 transmission electron microscope operating at 80 kV.
Leaf Surface Hydrophobicity Measurement
Contact angles of 10-μL droplets of distilled water on wild-type and sitiens fourth leaf surfaces were measured according to the sessile drop contact angle method with the EasyDrop tensiometer and Droplet Shape Analysis software (Krüss) using the L-Y circle-fitting method. Measurements were done at room temperature. The values of the contact angles were averages of at least 10 measurements. For each experiment, the fourth leaves of at least five different plants were used. A two-sided Student’s t test was applied for statistical analysis. The experiment was repeated with similar results.
Trichome Density
Trichome density on the adaxial leaf side was measured by counting the trichomes on leaf discs of 4 mm diameter. At least 10 replicates of the surface of the fourth leaf of three different wild-type and sitiens plants were examined. Data were statistically analyzed using a Mann-Whitney test.
Toluidine Blue, Alcian Blue, and Ruthenium Red Staining for Assessment of Cuticular Permeability
Leaf discs from fourth leaves of 5-week-old plants (seventh leaf stage) floating on the adaxial surface were placed in a 0.05% aqueous dye solution for 1 h (Sterling, 1970; Tanaka et al., 2004).
Chlorophyll Efflux Rate Measurement
To measure the chlorophyll efflux rate, equally sized leaflets from fourth leaves from three 5-week-old plants were used as described (Chen et al., 2004). Three replicates of mutant and wild-type leaves were immersed in an 80% ethanol solution in 50-mL tubes with covers. Tubes were agitated gently on a shaker platform. Aliquots of 1 mL were removed at 0, 0.5, 1, 1.5, 2, and 4 h after initial immersion. The amount of chlorophyll extracted into the solution was quantified with a UV-160A spectrophotometer and calculated from UV light absorption at 480, 645, and 663 nm.
In Vitro Effect of Leaf Diffusate on B. cinerea Germination
Ten-microliter droplets of quarter-strength potato dextrose broth (Difco) were incubated for 18 h on three wild-type and sitiens leaves. Potential leaf diffusates were collected directly into these droplets and mixed with B. cinerea spores in water to a final concentration of 5 × 104 spores mL−1. Spore germination was observed with the microscope for 5 h.
In Vivo Assessment of Hyphal Growth
For in vivo assessment of hyphal growth, leaf discs of fifth leaves were inoculated with B. cinerea as described above and sampled at 2, 4, 6, 8, and 10 hpi by clearing and fixing in 100% ethanol. Hyphae were visualized using 0.01% trypan blue in 10% acetic acid. Bright-field microscopy was performed as described above, and hyphae length was measured using the software package CELL-F (Olympus Soft Imaging Solutions).
Assessment of Correlation between Permeability and Susceptibility
To study the correlation between permeability and susceptibility in wild-type and sitiens plants, both traits were analyzed on the same leaf. Leaf discs were cut from every leaf (leaves 1–6) of the seventh leaf stage of wild-type and sitiens plants. Per leaf, four discs were inoculated with B. cinerea for disease evaluation as described above, and four leaf discs were used for toluidine blue permeability assay. A disease index was calculated with four different scores (0, resistant; 1, slightly spreading lesion; 2, moderately spreading lesion; 3, severely spreading lesion) using the formula [(0 × a) + (1 × b) + (2 × c) + (3 × d)/(a + b + c + d)] × 100/3 where a, b, c, and d are the number of discs examined with scores 0, 1, 2, and 3, respectively. For each experiment, leaves of three different plants were used (n = 12). A permeability index was determined based on the percentage of leaf disc area covered by toluidine blue after overnight staining (0, 0%–25%; 1, 25%–50%; 2, 50%–75%; 3, 75%–100%). The correlation between both traits was analyzed using Spearman’s rank correlation test.
Pectinase Treatment
Leaf discs were excised from fifth wild-type and sitiens leaves and floated at room temperature for 3 d on 24-well plates on pectinase solutions (Pectinex Ultra SPL; Sigma, P2611) containing 0, 100, 1,000, and 2,000 units mL−1. Degradation was quantified by measuring leaf disc surface area (percentage coverage) with the APS Assess 2.0 software. As a control, leaf discs were floated on cellulase solutions (Sigma, C2730).
Chemical Analysis of Cell Walls Using GC-MS
Fifth leaves of four 5-week-old wild-type and sitiens plants were washed four times with 70% ethanol at 70°C and subsequently lyophilized overnight, ground in a potter homogenizer, and washed in acetone and methanol. The remaining homogenate was considered as representative of the cell walls. Five milligrams of this cell wall preparation of each sample was used for further analysis. The prepared cell wall was resuspended in 1.25 mL of 1 m imidazole and reduced with 3 × 0.25 mL per 100 mg mL−1 NaBD4 for 2 h according to Kim and Carpita (1992). Samples were neutralized with 3 × 50 μL of acetic acid and dialyzed overnight. Upon lyophilization, samples were dissolved in 0.25 mL of water. To distinguish Gal, GalA, and methylesterified GalA by GC-MS, the sample was split in two and a second reduction was done overnight with NaBH4 (to obtain the proportion of esterified over total uronic acids) or NaBD4 (to obtain the total uronic acids), followed by neutralization with acetic acid. After dialysis and freeze drying, carboxyl-reduced wall material was hydrolyzed with 2.5 m trifluoroacetic acid for 2 h at 100°C. Alditol acetate derivatives were prepared as described (Albersheim et al., 1967). Derivatized samples were redissolved in 20 μL of dichloromethane, and 1-μL aliquots were analyzed by GC-MS with a high-polarity 25-m × 0.22-mm (i.d.) BPX70 column (SGE). Myoinositol was added to the samples as an internal standard. Identification of the derivatives was based on published mass spectra and the elution order in relation to standards. The degree of esterification of uronic acid groups was calculated by the relative proportions of diagnostic fragments in the NaBH4- and NaBD4-reduced samples (mass-to-charge ratios of 217 and 219 for hexosyl derivatives).
Oligosaccharide Sampling
Ten-microliter B. cinerea inoculation droplets containing 5.25 × 104 conidia mL−1 in 15.7 mm KH2PO4 and 25 mm Glc were inoculated on fifth leaves of 5-week-old wild-type and sitiens leaves and incubated at 22°C for 0, 6, 12, and 48 h. As a control, B. cinerea inoculation suspension was incubated under the same conditions. After inoculation, droplets were collected and centrifuged for 2 h at 22,000g to remove B. cinerea spores. One hundred microliters of the supernatant was vacuum dried and used for further analysis.
Electrophoretic Separation of Labeled Oligosaccharides
The samples were labeled overnight by incubation at 37°C in 1 μL of a 1:1 mixture of 20 mm 8-amino-1,3,6-pyrenetrisulfonic acid in 1.2 m citric acid and 1 m NaCNBH3 in dimethyl sulfoxide. Samples were injected in the capillaries of an ABI 3130 DNA sequencer (Applied Biosystems) and analyzed with Genescan 3.1 software (Applied Biosystems) as described (Laroy et al., 2006).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Pectin degradation during B. cinerea infection in sitiens and wild-type tomato.
Supplemental Figure S2. Evaluation of spore germination and hyphal growth.
Supplemental Figure S3. Cellulase treatment of wild-type and sitiens leaf discs.
Supplementary Material
References
- Achuo EA, Prinsen E, Höfte M. (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol 55: 178–186 [Google Scholar]
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A. (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16: 2463–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albersheim P, Nevins DJ, English PD, Karr A. (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr Res 5: 340–345 [Google Scholar]
- Asselbergh B, Curvers K, França SC, Audenaert K, Vuylsteke M, Van Breusegem F, Höfte M. (2007) Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol 144: 1863–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh B, De Vleesschauwer D, Höfte M. (2008) Global switches and fine-tuning: ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21: 709–719 [DOI] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Höfte MM. (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128: 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Salvi G, De Lorenzo G, Cervone F, Marfà V, Eberhard S, Darvill A, Albersheim P. (1993) Oligogalacturonides inhibit the formation of roots on tobacco explants. Plant J 4: 207–213 [Google Scholar]
- Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, MacDonald-Comber Petétot J, Métraux JP, Nawrath C. (2007) A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J 26: 2158–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird SM, Gray JE. (2003) Signals from the cuticle affect epidermal cell differentiation. New Phytol 157: 9–23 [DOI] [PubMed] [Google Scholar]
- Boudart G, Dechamp-Guillaume G, Lafitte C, Ricart G, Barthe JP, Mazau D, Esquerré-Tugayé MT. (1995) Elicitors and suppressors of hydroxyproline-rich glycoprotein accumulation are solubilized from plant cell walls by endopolygalacturonase. Eur J Biochem 232: 449–457 [DOI] [PubMed] [Google Scholar]
- Bray EA. (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J Exp Bot 55: 2331–2341 [DOI] [PubMed] [Google Scholar]
- Bringe K, Schumacher CFA, Schmitz-Eiberger M, Steiner U, Oerke EC. (2006) Ontogenetic variation in chemical and physical characteristics of adaxial apple leaf surfaces. Phytochemistry 67: 161–170 [DOI] [PubMed] [Google Scholar]
- Cabrera JC, Boland A, Cambier P, Frettinger P, Van Cutsem P. (2010) Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 20: 775–786 [DOI] [PubMed] [Google Scholar]
- Cabrera JC, Boland A, Messiaen J, Cambier P, Van Cutsem P. (2008) Egg box conformation of oligogalacturonides: the time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology 18: 473–482 [DOI] [PubMed] [Google Scholar]
- Cameron KD, Teece MA, Smart LB. (2006) Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol 140: 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot C, Métraux JP. (2005) The cuticle as source of signals for plant defense. Plant Biosyst 139: 28–31 [Google Scholar]
- Chassot C, Nawrath C, Métraux JP. (2007) Cuticular defects lead to full immunity to a major plant pathogen. Plant J 49: 972–980 [DOI] [PubMed] [Google Scholar]
- Chen G, Sagi M, Weining S, Krugman T, Fahima T, Korol AB, Nevo E. (2004) Wild barley eibi1 mutation identifies a gene essential for leaf water conservation. Planta 219: 684–693 [DOI] [PubMed] [Google Scholar]
- Cominelli E, Sala T, Calvi D, Gusmaroli G, Tonelli C. (2008) Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J 53: 53–64 [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG. (1994) Oligosaccharins: structures and signal transduction. Plant Mol Biol 26: 1379–1411 [DOI] [PubMed] [Google Scholar]
- Decreux A, Messiaen J. (2005) Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol 46: 268–278 [DOI] [PubMed] [Google Scholar]
- De Lorenzo G, Ferrari S. (2002) Polygalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Curr Opin Plant Biol 5: 295–299 [DOI] [PubMed] [Google Scholar]
- Dickman MB, Ha YS, Yang Z, Adams B, Huang C. (2003) A protein kinase from Colletotrichum trifolii is induced by plant cutin and is required for appressorium formation. Mol Plant Microbe Interact 16: 411–421 [DOI] [PubMed] [Google Scholar]
- Downie B, Dirk LMA, Hadfield KA, Wilkins TA, Bennett AB, Bradford KJ. (1998) A gel diffusion assay for quantification of pectin methylesterase activity. Anal Biochem 264: 149–157 [DOI] [PubMed] [Google Scholar]
- Faretra F, Pollastro S. (1991) Genetic basis of resistance to benzimidazole and dicarboximide fungicides in Botryotinia fuckeliana (Botrytis cinerea). Mycol Res 95: 943–951 [Google Scholar]
- Fauth M, Schweizer P, Buchala A, Markstädter C, Riederer M, Kato T, Kauss H. (1998) Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2 elicitors. Plant Physiol 117: 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Vairo D, Ausubel FM, Cervone F, De Lorenzo G. (2003) Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15: 93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D. (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12: 2001–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SA, Dewey FM, Gurr SJ. (1996) The role of cutinase in germling development and infection by Erysiphe graminis f.sp. hordei. Physiol Mol Plant Pathol 49: 201–211 [Google Scholar]
- Garbay B, Tautu MT, Costaglioli P. (2007) Low level of pathogenesis-related protein 1 mRNA expression in 15-day-old Arabidopsis cer6-2 and cer2 eceriferum mutants. Plant Sci 172: 299–305 [Google Scholar]
- Gilbert RD, Johnson AM, Dean RA. (1996) Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea. Physiol Mol Plant Pathol 48: 335–346 [Google Scholar]
- Gimeno-Gilles C, Lelièvre E, Viau L, Malik-Ghulam M, Ricoult C, Niebel A, Leduc N, Limami AM. (2009) ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture modifying enzymes and structural proteins in Medicago truncatula embryo axis. Mol Plant 2: 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubet F, Ström A, Quéméner B, Stephens E, Williams MAK, Dupree P. (2006) Resolution of the structural isomers of partially methylesterified oligogalacturonides by polysaccharide analysis using carbohydrate gel electrophoresis. Glycobiology 16: 29–35 [DOI] [PubMed] [Google Scholar]
- Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM. (2000) The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713–716 [DOI] [PubMed] [Google Scholar]
- Hahn MG. (1996) Microbial elicitors and their receptors in plants. Annu Rev Phytopathol 34: 387–412 [DOI] [PubMed] [Google Scholar]
- Hahn MG, Darvill AG, Albersheim P. (1981) Host-pathogen interactions. XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol 68: 1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauke V, Schreiber L. (1998) Ontogenetic and seasonal development of wax composition and cuticular transpiration of ivy (Hedera helix L.) sun and shade leaves. Planta 207: 67–75 [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H. (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Hooker TS, Millar AA, Kunst L. (2002) Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol 129: 1568–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR. (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytol 176: 7–21 [DOI] [PubMed] [Google Scholar]
- Jakob K, Kniskern JM, Bergelson J. (2007) The role of pectate lyase and the jasmonic acid defense response in Pseudomonas viridiflava virulence. Mol Plant Microbe Interact 20: 146–158 [DOI] [PubMed] [Google Scholar]
- Jenks MA, Joly RJ, Peters PJ, Rich PJ, Axtell JD, Ashworth EN. (1994) Chemically induced cuticle mutation affecting epidermal conductance to water vapor and disease susceptibility in Sorghum bicolor (L.) Moench. Plant Physiol 105: 1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert DA, Kars I, Wagemakers L, Bergmann C, Kemp G, Vivier MA, van Kan JAL. (2007) A polygalacturonase-inhibiting protein from grapevine reduces the symptoms of the endopolygalacturonase BcPG2 from Botrytis cinerea in Nicotiana benthamiana leaves without any evidence for in vitro interaction. Mol Plant Microbe Interact 20: 392–402 [DOI] [PubMed] [Google Scholar]
- Joubert DA, Slaughter AR, Kemp G, Becker JVW, Krooshof GH, Bergmann C, Benen J, Pretorius IS, Vivier MA. (2006) The grapevine polygalacturonase-inhibiting protein (VvPGIP1) reduces Botrytis cinerea susceptibility in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Res 15: 687–702 [DOI] [PubMed] [Google Scholar]
- Jung KH, Han MJ, Lee DY, Lee YS, Schreiber L, Franke R, Faust A, Yephremov A, Saedler H, Kim YW, et al. (2006) Wax-deficient anther1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 18: 3015–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kars I, McCalman M, Wagemakers L, van Kan JAL. (2005) Functional analysis of Botrytis cinerea pectin methylesterase genes by PCR-based targeted mutagenesis: Bcpme1 and Bcpme2 are dispensable for virulence of strain B05.10. Mol Plant Pathol 6: 641–652 [DOI] [PubMed] [Google Scholar]
- Kasahara A, Morisaki H, Hattori T. (1993) Hydrophobicity of the cells of fast- and slow-growing bacteria isolated from a grassland soil. J Gen Appl Microbiol 39: 381–388 [Google Scholar]
- Kim JB, Carpita NC. (1992) Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiol 98: 646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Park JH, Kim MC, Cho SH. (2008) Cutin monomer induces expression of the rice OsLTP5 lipid transfer protein gene. J Plant Physiol 165: 345–349 [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Koch K, Ensikat HJ. (2007) The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39: 759–772 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Rogers LM, Li D, Hwang CS, Flaishman MA. (1995) Surface signaling in pathogenesis. Proc Natl Acad Sci USA 92: 4080–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü S, Joubès J, Jenks MA. (2009) The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol 151: 1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata T, Kawabata-Awai C, Sakuradani E, Shimizu S, Okada K, Wada T. (2003) The YORE-YORE gene regulates multiple aspects of epidermal cell differentiation in Arabidopsis. Plant J 36: 55–66 [DOI] [PubMed] [Google Scholar]
- Laroy W, Contreras R, Callewaert N. (2006) Glycome mapping on DNA sequencing equipment. Nat Protoc 1: 397–405 [DOI] [PubMed] [Google Scholar]
- Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D. (2007) Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol 143: 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohani S, Trivedi PK, Nath P. (2004) Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol 31: 119–126 [Google Scholar]
- Luo B, Xue XY, Hu WL, Wang LJ, Chen XY. (2007) An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol 48: 1790–1802 [DOI] [PubMed] [Google Scholar]
- Mang HG, Laluk KA, Parsons EP, Kosma DK, Cooper BR, Park HC, AbuQamar S, Boccongelli C, Miyazaki S, Consiglio F, et al. (2009) The Arabidopsis RESURRECTION1 gene regulates a novel antagonistic interaction in plant defense to biotrophs and necrotrophs. Plant Physiol 151: 290–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu Y, Kurkdjian A, Xia H, Guern J, Koller A, Spiro MD, O’Neill M, Albersheim P, Darvill A. (1991) Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J 1: 333–343 [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B, Mauch F. (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8: 409–414 [DOI] [PubMed] [Google Scholar]
- Messiaen J, Van Cutsem P. (1993) Defense gene transcription in carrot cells treated with oligogalacturonides. Plant Cell Physiol 34: 1117–1123 [Google Scholar]
- Messiaen J, Van Cutsem P. (1994) Pectic signal transduction in carrot cells: membrane, cytosolic and nuclear responses induced by oligogalacturonides. Plant Cell Physiol 35: 677–689 [Google Scholar]
- Micheli F. (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Moerschbacher BM, Mierau M, Graeßner B, Noll U, Mort AJ. (1999) Small oligomers of galacturonic acid are endogenous suppressors of disease resistance reactions in wheat leaves. J Exp Bot 50: 605–612 [Google Scholar]
- Mohr PG, Cahill DM. (2001) Relative roles of glyceollin, lignin and the hypersensitive response and the influence of ABA in compatible and incompatible interactions of soybeans with Phytophthora sojae. Physiol Mol Plant Pathol 48: 31–41 [Google Scholar]
- Nagel OW, Konings H, Lambers H. (1994) Growth rate, plant development and water relations of the ABA-deficient tomato mutant sitiens. Physiol Plant 92: 102–108 [Google Scholar]
- Norman C, Vidal S, Palva ET. (1999) Oligogalacturonide-mediated induction of a gene involved in jasmonic acid synthesis in response to the cell-wall-degrading enzymes of the plant pathogen Erwinia carotovora. Mol Plant Microbe Interact 12: 640–644 [DOI] [PubMed] [Google Scholar]
- Oeser B, Heidrich PM, Müller U, Tudzynski P, Tenberge KB. (2002) Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet Biol 36: 176–186 [DOI] [PubMed] [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V. (2008) Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Park JH, Suh MC, Kim TH, Kim MC, Cho SH. (2008) Expression of glycine-rich protein genes, AtGRP5 and AtGRP23, induced by the cutin monomer 16-hydroxypalmitic acid in Arabidopsis thaliana. Plant Physiol Biochem 46: 1015–1018 [DOI] [PubMed] [Google Scholar]
- Popp C, Burghardt M, Friedmann A, Riederer M. (2005) Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: permeation of water and uncharged organic compounds. J Exp Bot 56: 2797–2806 [DOI] [PubMed] [Google Scholar]
- Powell ALT, van Kan J, ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM. (2000) Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol Plant Microbe Interact 13: 942–950 [DOI] [PubMed] [Google Scholar]
- Raffaele S, Vailleay F, Léger A, Joubès J, Miersch O, Huard C, Blée E, Mongrand S, Domergue F, Roby D. (2008) A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis. Plant Cell 20: 752–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Pinto JJ, Yephremov A. (2009) Surface lipids and plant defenses. Plant Physiol Biochem 47: 540–549 [DOI] [PubMed] [Google Scholar]
- Ren C, Kermode AR. (2000) An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol 124: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley BL, O’Neill MA, Mohnen D. (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57: 929–967 [DOI] [PubMed] [Google Scholar]
- Saladié M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM, Niklas KJ, Xiaolin R, Labavitch JM, Shackel KA, Fernie AR, et al. (2007) A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol 144: 1012–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W. (1993) The role of cutinase in fungal pathogenicity. Trends Microbiol 1: 69–71 [DOI] [PubMed] [Google Scholar]
- Schweizer P, Felix G, Buchala A, Müller C, Métraux JP. (1996a) Perception of free cutin monomers by plant cells. Plant J 10: 331–341 [Google Scholar]
- Schweizer P, Jeanguenat A, Whitacre D, Métraux JP, Mösinger E. (1996b) Induction of resistance in barley against Erysiphe graminis f. sp. hordei by free cutin monomers. Physiol Mol Plant Pathol 49: 103–120 [Google Scholar]
- Showalter AM. (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58: 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicilia F, Fernandez-Recio J, Caprari C, De Lorenzo D, Tsernoglou D, Cervone F, Federici L. (2005) The polygalacturonase-inhibiting protein PGIP2 of Phaseolus vulgaris has evolved a mixed mode of inhibition of endopolygalacturonase PG1 of Botrytis cinerea. Plant Physiol 139: 1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Métraux JP, Nawrath C. (2000) Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12: 721–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SD, Ashford DA, Harvey DJ, Bowles DJ. (1998) Short chain oligogalacturonides induce ethylene production and expression of the gene encoding aminocyclopropane 1-carboxylic acid oxidase in tomato plants. Glycobiology 8: 579–583 [DOI] [PubMed] [Google Scholar]
- Sterling C. (1970) Crystal structure of ruthenium red and stereochemistry of its pectic stain. Am J Bot 57: 172–175 [Google Scholar]
- Ström A, Williams MAK. (2004) On the separation, detection and quantification of pectin derived oligosaccharides by capillary electrophoresis. Carbohydr Res 339: 1711–1716 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y. (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37: 139–146 [DOI] [PubMed] [Google Scholar]
- Tang D, Simonich MT, Innes RW. (2007) Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol 144: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Have A, Breuil WO, Wubben JP, Visser J, van Kan JAL. (2001) Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet Biol 33: 97–105 [DOI] [PubMed] [Google Scholar]
- ten Have A, Mulder W, Visser J, van Kan JAL. (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11: 1009–1016 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Bostock RM. (2004) Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85: 48–58 [Google Scholar]
- Treviño MB, O’Connell MA. (1998) Three drought-responsive members of the nonspecific lipid-transfer protein gene family in Lycopersicon pennellii show different developmental patterns of expression. Plant Physiol 116: 1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valette-Collet O, Cimerman A, Reignault P, Levis C, Boccara M. (2003) Disruption of Botrytis cinerea pectin methylesterase gene Bcpme1 reduces virulence on several host plants. Mol Plant Microbe Interact 16: 360–367 [DOI] [PubMed] [Google Scholar]
- van Kan JAL. (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11: 247–253 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC. (2002) PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14: 2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chin CK, Gianfagna T. (2000) Relationship between cutin monomers and tomato resistance to powdery mildew infection. Physiol Mol Plant Pathol 57: 55–61 [Google Scholar]
- Wellesen K, Durst F, Pino F, Benveniste I, Nettesheim K, Wisman E, Steiner-Lange S, Saedler H, Yephremov A. (2001) Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different roles of fatty acid α-hydroxylation in development. Proc Natl Acad Sci USA 98: 9694–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MAK, Foster TJ, Schols HA. (2003) Elucidation of pectin methylester distributions by capillary electrophoresis. J Agric Food Chem 51: 1777–1781 [DOI] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J. (2009) Homogalacturonan methylesterification and plant development. Mol Plant 2: 851–860 [DOI] [PubMed] [Google Scholar]
- Woloshuk CP, Kolattukudy PE. (1986) Mechanism by which contact with plant cuticle triggers cutinase gene expression in the spores of Fusarium solani f. sp. pisi. Proc Natl Acad Sci USA 83: 1704–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Goodwin SM, Xiao Y, Sun Z, Baker D, Tang X, Jenks MA, Zhou JM. (2004) Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J 23: 2903–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H. (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11: 2187–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Stebbins L. (1972) Developmental genetics in barley: a mutant for stomatal development. Am J Bot 59: 143–148 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.