Abstract
Extracellular ATP induces a rise in the level of cytosolic free calcium ([Ca2+]cyt) in plant cells. To expand our knowledge about the function of extracellular nucleotides in plants, the effects of several nucleotide analogs and pharmacological agents on [Ca2+]cyt changes were studied using transgenic Arabidopsis (Arabidopsis thaliana) expressing aequorin or the fluorescence resonance energy transfer-based Ca2+ sensor Yellow Cameleon 3.6. Exogenously applied CTP caused elevations in [Ca2+]cyt that displayed distinct time- and dose-dependent kinetics compared with the purine nucleotides ATP and GTP. The inhibitory effects of antagonists of mammalian P2 receptors and calcium influx inhibitors on nucleotide-induced [Ca2+]cyt elevations were distinct between CTP and purine nucleotides. These results suggest that distinct recognition systems may exist for the respective types of nucleotides. Interestingly, a mutant lacking the heterotrimeric G protein Gβ-subunit exhibited a remarkably higher [Ca2+]cyt elevation in response to all tested nucleotides in comparison with the wild type. These data suggest a role for Gβ in negatively regulating extracellular nucleotide signaling and point to an important role for heterotrimeric G proteins in modulating the cellular effects of extracellular nucleotides. The addition of extracellular nucleotides induced multiple temporal [Ca2+]cyt oscillations, which could be localized to specific root cells. The oscillations were attenuated by a vesicle-trafficking inhibitor, indicating that the oscillations likely require ATP release via exocytotic secretion. The results reveal new molecular details concerning extracellular nucleotide signaling in plants and the importance of fine control of extracellular nucleotide levels to mediate specific plant cell responses.
The calcium ion, Ca2+, is a ubiquitous second messenger that is used to regulate a wide range of cellular processes (Clapham, 2007). A number of plant environmental and developmental responses are encoded to distinct Ca2+ signal patterns with specific frequencies and amplitudes of cytosolic free Ca2+ concentration ([Ca2+]cyt). These signal patterns can take the form of pulsating [Ca2+]cyt spiking/oscillations (Berridge et al., 2003). In plants, such [Ca2+]cyt oscillations occur in various cell types (e.g. stomatal guard cells, pollen tubes, and legume root hairs) and play a critical role in responding to environmental signals (Evans et al., 2001; Oldroyd and Downie, 2008; McAinsh and Pittman, 2009).
ATP is a ubiquitous compound in all living cells; it not only provides the energy to drive many biochemical reactions but also functions in signal transduction as a substrate for kinases, adenylate cyclases, etc. However, ATP was also shown to be an essential signaling agent outside of cells in animals, where it is referred to as extracellular ATP. Extracellular ATP is involved in numerous cellular processes, including neurotransmission, immune responses, cell growth, and cell death (Khakh and Burnstock, 2009). In mammalian cells, plasma membrane-localized receptors, purinoceptors of the P2X and P2Y classes, bind ATP as well as other nucleotides at the cell surface to activate intracellular signaling cascades via second messengers. Binding of extracellular ATP to P2X receptors gates calcium influx, whereas activation of P2Y receptors stimulates the recruitment of heterotrimeric G proteins to trigger cytoplasmic signaling and gene expression. As a common phenomenon, the activated receptors induce the elevation of [Ca2+]cyt, which in turn activates the production of downstream messengers such as nitric oxide and reactive oxygen species (ROS; Shen et al., 2005; Fields and Burnstock, 2006).
A possible physiological role for extracellular ATP in plants was first reported in studies in which exogenously applied ATP was found to stimulate closure of the Venus flytrap (Dionaea muscipula; Jaffe, 1973), to induce the formation of nucleases in excised Avena leaves (Udvardy and Farkas, 1973), and to induce potassium ion uptake into cells of maize (Zea mays) leaf slices (Lüttge et al., 1974). Over the past several years, extracellular ATP was found to be an important signaling compound in plants that induces various plant responses, including root-hair growth (Lew and Dearnaley, 2000; Kim et al., 2006), stress responses (Thomas et al., 2000; Jeter et al., 2004; Song et al., 2006), gravitropism (Tang et al., 2003), cell viability (Chivasa et al., 2005), pathogen responses (Chivasa et al., 2009), and thigmotropism (Weerasinghe et al., 2009). The release of extracellular ATP from root cells was directly imaged by Kim et al. (2006) using a luciferase construct engineered to bind to plant cell wall cellulose. Recently, using this reporter, Weerasinghe et al. (2009) measured the release of ATP from root cells in response to touch. This documentation of the presence of extracellular ATP in plants at levels sufficient to induce cellular responses suggests that extracellular ATP likely plays an important role throughout plant growth and development. However, no P2 receptor homologs have been identified in plants, despite the fact that plants share a number of cellular responses to ATP with animal cells. For example, the addition of exogenous ATP or ADP triggers an increase in [Ca2+]cyt levels in whole seedlings, dissected root tissues, and root epidermal protoplasts of Arabidopsis (Arabidopsis thaliana; Demidchik et al., 2003, 2009; Jeter et al., 2004). The production of ROS in response to ATP addition was detected in various plant tissues (Kim et al., 2006; Song et al., 2006; Wu et al., 2008; Demidchik et al., 2009). More recently, the plasma membrane NADPH oxidase RBOHC (for respiratory burst oxidase homolog C) was shown to be required for extracellular ATP-induced ROS production in Arabidopsis primary roots (Demidchik et al., 2009). Extracellular ATP also stimulates the production of nitric oxide in tomato (Solanum lycopersicum) culture cells and in Salvia miltiorrhiza hairy roots (Foresi et al., 2007; Wu and Wu, 2008). These reports suggest that extracellular ATP signals across the plasma membrane by triggering elevation in [Ca2+]cyt, which activates the production of downstream messengers. Ultimately, these cell responses induce the expression of various genes, such as MAPKs, LOX, and ACS6 (Jeter et al., 2004; Song et al., 2006), and cause physiological responses, as described above.
In animal cells, extracellular ATP-evoked elevations in [Ca2+]cyt are often observed in the form of oscillations that result from the transient opening of Ca2+ channels located either in the plasma membrane or in cytosolic Ca2+ stores. Intracellular calcium release is often mediated through phospholipase C (PLC)-mediated signaling coupled to heterotrimeric G proteins (Mahoney et al., 1992; Visegrady et al., 2000; Hanley et al., 2004). In plants, plasma membrane Ca2+-permeable channels are known to contribute to extracellular ATP-induced [Ca2+]cyt elevation (Demidchik et al., 2009). However, neither the mechanisms underlying extracellular ATP-evoked Ca2+ signaling nor the possible involvement of heterotrimeric G proteins has been characterized in plants.
In order to explore their roles as possible ligands of putative nucleotide receptors, the plant [Ca2+]cyt response to six different nucleotides (Fig. 1A) was measured using Arabidopsis seedlings expressing one of two [Ca2+]cyt sensors, either aequorin or the fluorescence resonance energy transfer (FRET)-based Ca2+ sensor Yellow Cameleon 3.6 (YC3.6). The pyrimidine nucleotide CTP as well as the purine nucleotides ATP and GTP induced a strong elevation of [Ca2+]cyt in seedlings. Interestingly, the effects of all the nucleotides on Ca2+ signaling were negatively regulated by a heterotrimeric G protein β-subunit, AGB1. The addition of ATP to aequorin-expressing seedlings induced distinct [Ca2+]cyt oscillations in the presence of the apyrase inhibitor NGXT191. However, in the absence of this inhibitor, such [Ca2+]cyt oscillations could be localized to specific root cell layers using YC3.6 fluorescence. Given the importance of [Ca2+]cyt oscillations in intracellular signaling, the data suggest an important, unexplored role of extracellular ATP in the plant signaling pathways.
Figure 1.
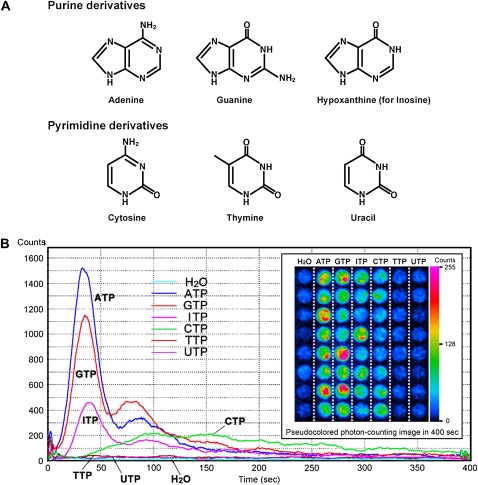
NTPs increase bioluminescence in aequorin-expressing transgenic Arabidopsis seedlings. A, Chemical structures of purine and pyrimidine derivatives. B, Individual 5-d-old aequorin seedlings were transferred to individual wells of a 96-well microplate and incubated overnight in reconstitution buffer containing coelenterazine. Each NTP was then applied at a final concentration of 100 μm. The line graph shows time-dependent changes in photon counts from representative wells of each treatment (bin size = 50 frames, 1 s, 20 bin smoothing). The inset shows a pseudocolored photon-counting image integrated over 400 s after nucleotide treatment calibrated to the inset scale.
RESULTS
Extracellular Nucleoside Triphosphates Increase [Ca2+]cyt in Arabidopsis Seedlings
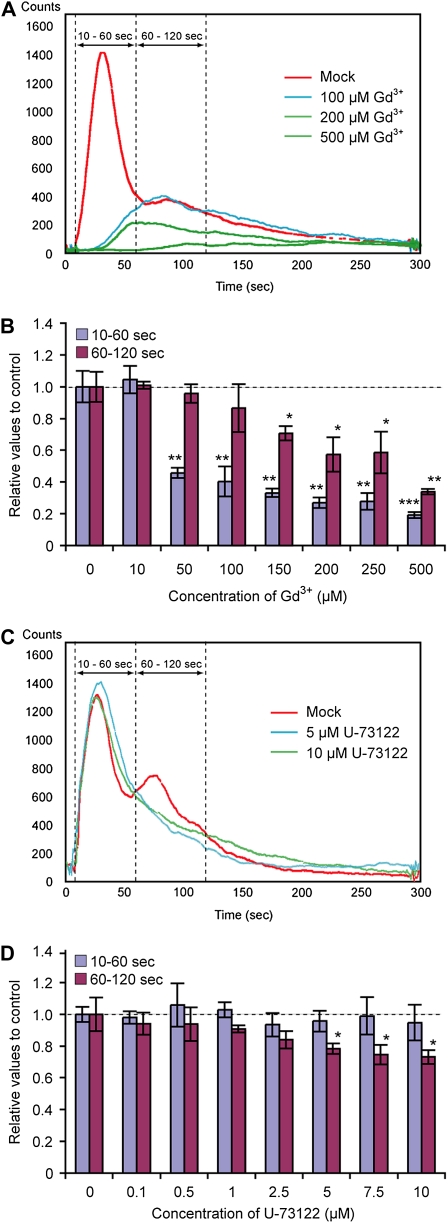
In animal cells, different P2 receptor subtypes have distinct agonist specificities for extracellular nucleotides. In order to explore extracellular nucleotide specificity in plants, we characterized responses to various nucleoside triphosphates (NTPs). The effect of nucleotide addition was measured by characterizing the [Ca2+]cyt changes that each elicited in Arabidopsis seedlings expressing the calcium reporter protein aequorin (Knight et al., 1996). The six NTPs, ATP, GTP, ITP, CTP, TTP, and UTP (their nitrogenous bases are shown in Fig. 1A), were applied to 5-d-old aequorin transgenic seedlings, and the bioluminescence signals were detected with a photon-counting CCD camera (Fig. 1B). The purine-based nucleotides, ATP, GTP, and ITP, caused strong transient elevations in bioluminescence signals, which comprised two distinguishable peaks; the first peak occurred at 30 to 40 s and the second at 80 to 90 s after nucleotide application. This result led us to further examine the nature of this biphasic [Ca2+]cyt response. The plant [Ca2+]cyt response was analyzed in the presence of various inhibitors, Gd3+, La3+ (Ca2+ channel inhibitors), and U-73122 (a PLC inhibitor), with measurements pooled for 10 to 60 s (the initial peak) and 60 to 120 s (the second elevation in [Ca2+]cyt) after ATP addition. The first peak of ATP-induced [Ca2+]cyt elevation was significantly inhibited by 50 to 100 μm Gd3+, whereas the second peak was only inhibited at higher concentrations (150 μm or higher). The ATP effect on [Ca2+]cyt was nullified at 500 μm Gd3+ (Fig. 2, A and B). A similar result was obtained with La3+ treatment (data not shown). In contrast, in the presence of 5 to 10 μm U-73122, a slight inhibition (approximately 20%) was observed in the second peak but not in the first peak (Fig. 2, C and D). An inactive analog of U-73122, U-73343, had no effect on the ATP-induced [Ca2+]cyt response (data not shown). Based on these results, we conclude that the first peak of ATP-induced [Ca2+]cyt elevation is likely due to external Ca2+ entry, whereas the second peak likely corresponds to both the external Ca2+ entry and release of Ca2+ from interior stores.
Figure 2.
Effects of a PLC inhibitor and a Ca2+ channel inhibitor on ATP-induced [Ca2+]cyt response in Arabidopsis seedlings. Arabidopsis seedlings harboring reconstituted aequorin were preincubated for 30 min with Gd3+ (a Ca2+ channel inhibitor; A and B) or U-73122 (a PLC inhibitor; C and D) at the indicated concentrations; 100 μm ATP was then applied. The remaining aequorin in the tissues was then discharged for [Ca2+]cyt calculation (see “Materials and Methods”). A and C, Kinetic differences of ATP-induced bioluminescence in the presence or absence of Gd3+ (A) or U-73122 (C). B and D, Data were calculated as integrated [Ca2+]cyt values over 10 to 60 s or 60 to 120 s and then converted into a relative value to mock treatment. Asterisks indicate statistically significant differences compared with the mock treatment control: * P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001.
CTP (a pyrimidine-based nucleotide) also elicited a significant elevation in [Ca2+]cyt, but neither TTP nor UTP caused a significant response at up to 100 μm (Fig. 1B). There was a notable difference in the kinetics of the [Ca2+]cyt response between CTP and purine-based NTPs. The kinetics of the bioluminescence signal induced by CTP exhibited a broad peak at 100 to 150 s after application, whereas, as noted above, the purine-based NTPs induced a sharp and transient increase in Ca2+ levels. Moreover, the CTP-induced [Ca2+]cyt elevation was inhibited only by Gd3+ and La3+ but not by U-73122 (Supplemental Fig. S1), tentatively suggesting that this [Ca2+]cyt elevation is primarily generated by Ca2+ influx that is likely distinct from PLC-mediated Ca2+ mobilization.
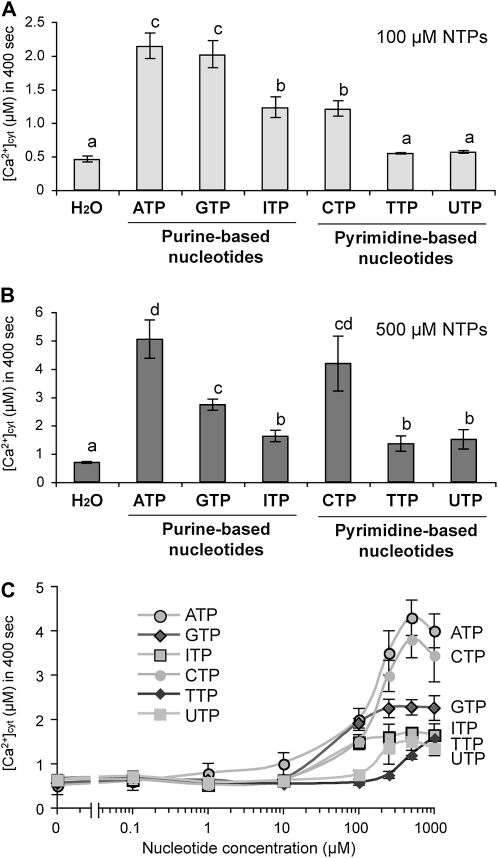
To compare the magnitude of the effects of CTP and other NTPs, the response over 400 s was integrated to calculate overall [Ca2+]cyt levels. Figure 3A shows that the [Ca2+]cyt levels induced by the NTPs were induced in the following descending order at 100 μm: ATP = GTP > ITP = CTP >> TTP, UTP. However, this order was changed at higher nucleotide concentrations (500 μm) as follows: ATP = CTP ≥ GTP > ITP, TTP, UTP (Fig. 3B). Closer examination revealed that the calcium response with 100 μm ATP was confined primarily to root tissues, while at the higher concentration (500 μm), leaf tissues also responded (data not shown). This may account for the relative differences seen between these treatments.
Figure 3.
Effects of NTPs on [Ca2+]cyt response in Arabidopsis seedlings. Each integrated [Ca2+]cyt was obtained from luminescence data recorded over 400 s after treatment and discharging of the remaining aequorin signal (see “Materials and Methods”). A and B, Histograms show means with se (n = 8) as integrated [Ca2+]cyt values that were recorded after treatment with 100 μm (A) or 500 μm (B) NTPs. Different letters indicate statistically significant differences at P < 0.05. C, Dose-dependent curves of NTP-induced [Ca2+]cyt responses.
To better characterize the effects of NTPs on the [Ca2+]cyt response, we applied NTPs over a range of concentrations from 0.1 to 1,000 μm (Fig. 3C). The effect of ATP on [Ca2+]cyt was stronger than any of the other nucleotides and was easily detectable at 10 μm (P < 0.05) and saturated at 250 μm. The GTP effect was comparable to the ATP effect at 100 μm and reached a plateau level of 50% of that seen with ATP after 250 μm, whereas ITP exhibited a similar dose-dependent curve to GTP. The effect of CTP was comparable to that of ATP at higher concentrations (greater than 250 μm), although moderate effects were found at lower concentrations (e.g. 60% of the ATP effect at 100 μm). TTP and UTP were only effective at concentrations greater than 250 μm. Because all of the nucleotides were applied as sodium salts, we assessed the effect of NaCl on the [Ca2+]cyt response. NaCl up to 5 mm caused no significant [Ca2+]cyt elevation (P > 0.4 versus water treatment control; data not shown). Thus, the effects of NTPs on [Ca2+]cyt elevation are specific, even at high concentrations (up to 1,000 μm). Taken together, these results suggest that plants indeed exhibit cellular responses with specificities that depend on the nitrogenous base structures of the nucleotides (Fig. 1A), which may reflect the specificity of individual receptor mechanisms.
Mammalian P2 Receptor Antagonists Inhibit Extracellular NTP-Induced [Ca2+]cyt Responses in Arabidopsis Seedlings
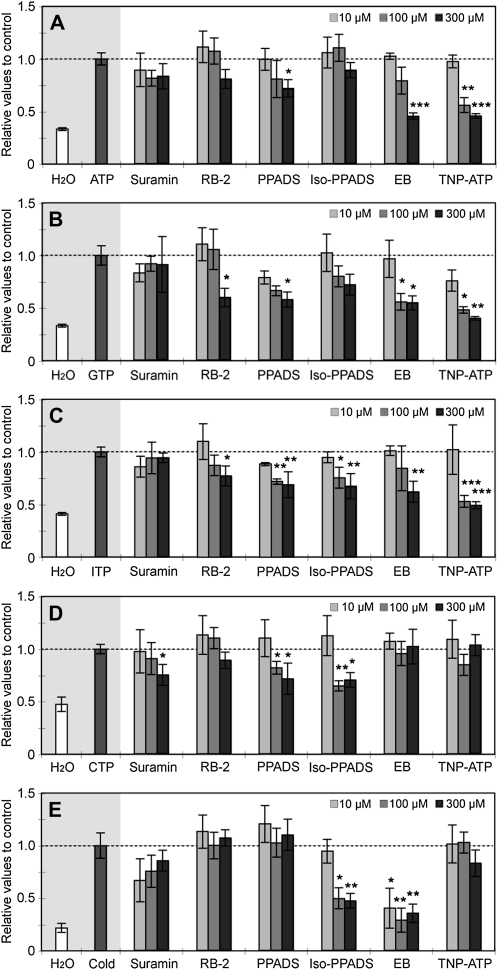
A variety of P2 receptor antagonists have been characterized based on their ability to inhibit specific mammalian P2 receptor subtypes (Ralevic and Burnstock, 1998). While these compounds have been used in plant studies, due to the lack of well-characterized plant P2 receptors, their activity against plant extracellular ATP receptors is assumed but not proven. For example, the nonselective P2 receptor antagonists, suramin and pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid (PPADS), inhibited extracellular ATP-induced [Ca2+]cyt response in excised Arabidopsis roots (Demidchik et al., 2003). In theory, it may be possible to distinguish distinct plant NTP recognition mechanisms by their differing sensitivity to these various antagonists. These methods are well established for animal studies (Ralevic and Burnstock, 1998; González et al., 2005). Hence, we expanded upon earlier studies by including additional antagonists (i.e. reactive blue-2, iso-PPADS, Evans blue, and TNP-ATP) in addition to suramin and PPADS.
As shown in Figure 4, PPADS inhibited the ATP-induced [Ca2+]cyt elevation, which is consistent with previous reports (Demidchik et al., 2003). PPADS also inhibited the [Ca2+]cyt elevations induced by the other NTPs. In contrast to earlier studies in which dissected roots or root protoplasts were used (Demidchik et al., 2003, 2009), suramin showed no significant effect on purine nucleotide-induced responses in our experiments (Fig. 4, A–C). These contrasting results might be attributed to the tissue-specific sensitivity to suramin or differences of tissue conditions between intact seedlings and protoplasts or dissected roots. However, suramin caused significant inhibition of CTP-induced [Ca2+]cyt elevation (Fig. 4D). This result suggests that CTP recognition by plant cells may be distinct from the recognition of purine-based NTPs. Interestingly, only the effects of purine NTPs were significantly inhibited (by more than 60%) by Evans blue and TNP-ATP (Fig. 4, A–C). This result again suggests distinct recognition systems for purine nucleotides versus CTP. We also verified the inhibitory effects of the antagonists on the biphasic kinetics of the purine nucleotide-induced [Ca2+]cyt responses (data not shown). Our results showed that the antagonists reduced both peaks equally. However, we did notice that only the treatment with TNP-ATP appeared to more strongly inhibit the first Ca2+ peak, relative to that of the second. Given that the first peak is most likely due to the influx of external Ca2+ and the second peak is composed of Ca2+ both from exterior and interior stores, these results suggest that the antagonists, except for TNT-ATP, may inhibit the putative receptors involved in the regulation of Ca2+ flux both from exterior and interior stores.
Figure 4.
Effects of antagonists of mammalian P2 receptors on nucleotide-induced [Ca2+]cyt response in Arabidopsis seedlings. Arabidopsis seedlings harboring reconstituted aequorin were preincubated for 30 min with antagonists for mammalian P2 receptors at the indicated concentrations (10–300 μm). The indicated nucleotide was then applied: ATP (A), GTP (B), ITP (C), and CTP (D). Ice-cold water was applied as a negative control (E). The integrated [Ca2+]cyt values were calculated from normalized data of luminescence signal recorded over 400 s after treatment and discharging of the remaining aequorin signal (see “Materials and Methods”). Histograms show means with se as relative values to a single treatment of nucleotide. Nonselective P2 receptor antagonists are suramin, reactive blue-2 (RB2), and PPADS. Selective P2X antagonists are iso-PPADS, Evans blue (EB), and TNP-ATP. Mean [Ca2+]cyt values of single nucleotide treatment controls were as follows: ATP, 1.9 ± 0.19 μm; GTP, 1.8 ± 0.15 μm; ITP, 1.3 ± 0.06 μm; CTP, 1.2 ± 0.04 μm; cold treatment, 2.6 ± 0.55 μm. Asterisks indicate statistically significant differences compared with a single application of nucleotides: * P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001.
To examine the specificity of the antagonists to NTP-induced responses, we applied another stimulus, cold treatment, known to cause elevation in Ca2+ levels but not thought to act through ATP signaling (Fig. 4E). The [Ca2+]cyt elevation induced by the addition of ice-cold water was not inhibited by most of the antagonists used, except iso-PPADS and Evans blue. Based on these results, iso-PPADS and Evans blue might be considered to have nonspecific effects on NTP-induced responses.
However, all antagonists used in this study, with the exception of suramin, exhibited some side effects at higher concentrations (greater than 300 μm) that reduced the bioluminescence signal even with respect to discharging of all the remaining aequorin in the tissues (data not shown), implying that these antagonists might inhibit the enzymatic activity of aequorin and/or the luminescence signal itself. Therefore, we were unable to pursue experiments with higher concentrations.
The Extracellular NTP-Induced [Ca2+]cyt Response Is Enhanced in Heterotrimeric G Protein Mutants
Because P2Y receptor-dependent [Ca2+]cyt responses in mammalian cells are exclusively mediated through the functions of heterotrimeric G proteins, we wondered whether plant heterotrimeric G proteins are involved in extracellular NTP-induced Ca2+ signaling. Since the Gα-subunit (GPA1) and Gβ-subunit (AGB1) are encoded by single-copy genes in the Arabidopsis genome, a genetic approach was used to study their involvement in the NTP-induced Ca2+ response. The single G protein mutants gpa1-4 and agb1-2 and the double mutant gpa1-4;agb1-2 were generated in the aequorin transgenic background by cross-pollination (see “Materials and Methods”). The individual mutants and the double mutant were then subjected to assays for the ATP-induced [Ca2+]cyt response.
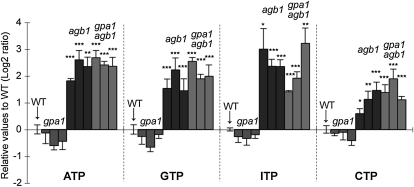
Upon addition of ATP to the mutants gpa1-4 and agb1-2, the latter mutant exhibited at least a 3.5-fold stronger [Ca2+]cyt response, whereas the response of the gpa1-4 mutant was similar to that of the wild type (Fig. 5). These data suggest that extracellular ATP-induced Ca2+ signaling is negatively regulated by the Gβ-subunit AGB1 or the Gβγ complex. The [Ca2+]cyt response in the gpa1-4;agb1-2 double mutant was similar to that seen in agb1-2 but not in gpa1-4. These results indicate that Gβ(γ), but not the Gα-subunit GPA1, is involved in nucleotide-induced Ca2+ signaling, rather than AGB1 acting through epistatic effects on GPA1. Similar results to those observed upon ATP addition were obtained with the addition of GTP, ITP, or CTP (Fig. 5). These data imply that these nucleotides, after being recognized on the plasma membrane, may share a common intracellular signaling pathway to the triggering of Ca2+ increase that is negatively regulated by AGB1.
Figure 5.
Effects of nucleotides on [Ca2+]cyt response in heterotrimeric G protein mutant backgrounds. NTPs (100 μm) were applied to three independent aequorin lines in gpa1-4, agb1-2, and gpa1-4;agb1-2 mutant backgrounds. Histograms show means with se as relative values (log2 ratio) to the wild-type (WT) background control. Note that aequorin seedlings in agb1-2 and gpa1-4;agb1-2 backgrounds exhibited hyperresponsiveness to each of the nucleotides used. Mean [Ca2+]cyt values of wild-type controls were as follows: ATP, 2.1 ± 0.36 μm; GTP, 2.0 ± 0.22 μm; ITP, 1.3 ± 0.06 μm; CTP, 1.2 ± 0.06 μm. Asterisks indicate statistically significant differences compared with the wild-type aequorin control: * P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001.
We also observed similar hypersensitivity of agb1-2 and gpa1-4;agb1-2 mutants to the poorly hydrolyzable analogs of ATP and ADP, ATPγS and ADPβS (data not shown). Again, this result suggests that AGB1 is regulating nucleotide signaling following receptor recognition and not via the regulation of apoplastic ATP (e.g. by changing ectoapyrase activity).
Poorly Hydrolyzable Nucleotide Analogs Induce [Ca2+]cyt Oscillations: Inhibition of Ectoapyrase Increases the Number of [Ca2+]cyt Oscillations Induced by Extracellular Nucleotides
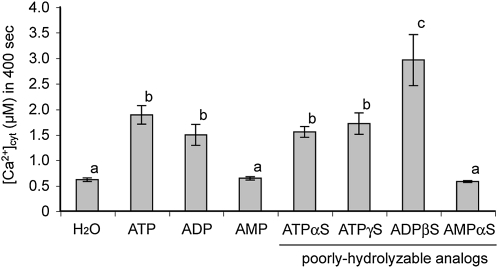
In order to ensure that the responses seen were to the nucleotides and not the result of hydrolysis, we examined the [Ca2+]cyt response to poorly hydrolyzable (thiol-containing) analogs of adenine-based nucleotides (i.e. ATPγS and ADPβS), which are relatively less susceptible to hydrolysis (Fig. 6). ATP, ADP, and their poorly hydrolyzable analogs induced significant increases in [Ca2+]cyt, whereas AMP and its poorly hydrolyzable analog had no significant effect. We also confirmed that adenosine, adenine, and adenine derivatives (e.g. zeatin, kinetin, benzyl adenine), as well as inorganic phosphate, had no significant effects compared with mock-treated controls. These data suggest that the increased [Ca2+]cyt is a specific response to nucleoside triphosphate and diphosphate. Intriguingly, the effect of ADPβS (poorly hydrolyzable ADP) was significantly stronger than all adenine nucleotides tested; the response was 1.5- to 2-fold higher than that of ATP, ATPγS, or ADP (P < 0.04, P < 0.03, and P < 0.01, respectively, versus ADPβS). The fact that poorly hydrolyzable analogs of both ATP and ADP have activities with different magnitudes could indicate the existence of multiple and distinct receptor mechanisms for specific nucleotides.
Figure 6.
Effects of hydrolyzable and poorly hydrolyzable nucleotide analogs on [Ca2+]cyt response in Arabidopsis seedlings. Native nucleotides or poorly hydrolyzable analogs were applied at a concentration of 100 μm. Experimental and analytical procedures were identical to those used in Figure 3. Different letters denote statistically significant differences (P < 0.05).
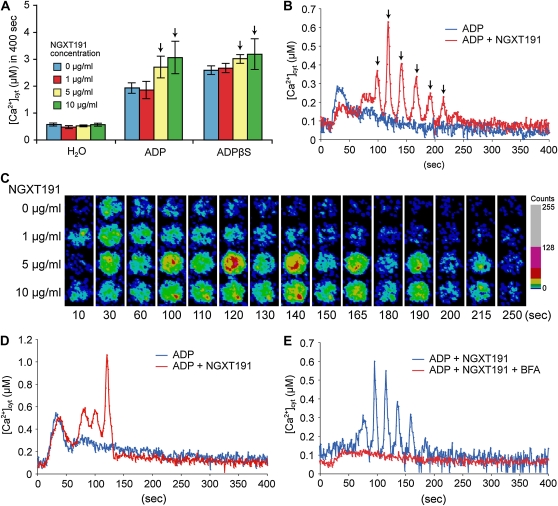
A number of reports suggest that plant extracellular ATP levels could be modulated through the action of ectoapyrases (Steinebrunner et al., 2003; Wolf et al., 2007; Wu et al., 2007; Govindarajulu et al., 2009); that is, membrane-associated nucleoside triphosphohydrolases and diphosphohydrolases with the predicted catalytic domain in the apoplast. Hence, it is possible that the differential response to ADP and ADPβS was due to the hydrolysis of nucleotides by ectoapyrase. Previously, Windsor et al. (2003) identified a plant apyrase inhibitor, NGXT191. Application of NGXT191 alone had no effect on [Ca2+]cyt (data not shown). However, consistent with the hydrolysis of ADP by ectoapyrase, in the presence of NGXT191, seedlings responded to ADP addition to a level equivalent to that seen with the addition of ADPβS alone (Fig. 7A). Most notably, in the presence of NGXT191, ADP induced temporal [Ca2+]cyt oscillations that lasted for 150 s with approximately 20-s periodicity for four to six peaks (Fig. 7, B and C; Supplemental Movie S1). Interestingly, the [Ca2+]cyt oscillations were also induced by other nucleotides, such as ADPβS and ATPγS, in the presence of NGXT191 (Supplemental Table S1). Similar [Ca2+]cyt oscillation responses were also observed in the G protein mutants, although the number of the oscillations was slightly reduced in the agb1-2 and gpa1-4;agb1-2 mutants (Fig. 7D; Supplemental Table S2). Note that the initial peak before the start of the ADP-induced oscillations appears to be reduced by NGXT191 treatment in Figure 7B. However, this observation was not significant, since it did not occur in all the experiments conducted.
Figure 7.
NGXT191 elicited nucleotide-induced [Ca2+]cyt oscillations. Arabidopsis seedlings harboring reconstituted aequorin were treated with 100 μm nucleotides after 30 min of preincubation with NGXT191. A, Histogram represents each integrated [Ca2+]cyt response obtained from data recorded over 400 s upon nucleotide treatments. Arrows indicate the NGXT191 concentration that evoked nucleotide-induced [Ca2+]cyt oscillations. B, ADP-induced [Ca2+]cyt oscillations in the wild type. Arrows indicate amplitude peaks in the oscillations. C, Time-lapse movie of wild-type aequorin transgenic seedlings treated with ADP after preincubation with NGXT191 (see Supplemental Movie S1). Frames from the recording at the indicated times (s) are shown in a pseudocolored photon-counting image. D, ADP-induced [Ca2+]cyt oscillations in the agb1-2 mutant background. E, Effect of the vesicle-trafficking inhibitor BFA on ADP-induced [Ca2+]cyt oscillations. Ten micromolar BFA was applied together with NGXT191 for preincubation.
To characterize the [Ca2+]cyt oscillations, we assessed the effects of the Ca2+ channel blockers (Gd3+ and verapamil) and a PLC inhibitor (U-73122) on nucleotide responses in the presence of NGXT191. All of these chemicals completely abolished the [Ca2+]cyt oscillations induced by ATP and ADP in the presence of NGXT191 (Table I), indicating that the [Ca2+]cyt oscillations are likely orchestrated by Ca2+ influxes from exterior and interior stores.
Table I. Effects of inhibitors on ADP- or ATP-induced [Ca2+]cyt oscillations in Arabidopsis.
Aequorin seedlings were treated with 100 μm nucleotides after simultaneous preincubation with 10 μg mL−1 NGXT191 and the indicated inhibitors.
| Nucleotide | Pharmaceutical | No. of Oscillationsa | Frequency of [Ca2+]cyt Oscillation Occurrenceb |
| ADP | Mock (water) | 4.6 ± 0.2 | 5:6 |
| Mock (dimethyl sulfoxide) | 4.3 ± 0.3 | 6:6 | |
| Gd3+ (50 μm) | — | 0:6 | |
| Verapamil (100 μm) | — | 0:9 | |
| U-73122 (1 μm) | — | 0:6 | |
| NPA (50 μm) | 3.9 ± 0.4 | 8:9 | |
| BFA (10 μm) | 2.8 ± 0.4 | 2:12 | |
| ATP | Mock (water) | 4.4 ± 0.6 | 6:6 |
| Mock (dimethyl sulfoxide) | 4.0 ± 0.6 | 6:6 | |
| Gd3+ (50 μm) | — | 0:6 | |
| Verapamil (100 μm) | — | 0:9 | |
| U-73122 (1 μm) | — | 0:6 | |
| NPA (50 μm) | 4.0 ± 0.4 | 8:9 | |
| BFA (10 μm) | 3.0 ± 2.0 | 3:12 |
Samples that did not exhibit oscillations were omitted from the calculations.
Frequency of [Ca2+]cyt oscillation occurrence is presented as the ratio number of occurrences:total number of samples tested.
We initially presumed from the observed [Ca2+]cyt oscillations that exogenously applied nucleotides undergo immediate degradation by ectoapyrases, and also that a continuous nucleotide stimulus causes the [Ca2+]cyt oscillation response only in the absence of ectoapyrase activity. In order to investigate the possibility that the relative instability of ATP and ADP affected the [Ca2+]cyt response kinetics, we tested the effect of the poorly hydrolyzable nucleotides ATPγS and ADPβS (Fig. 6). One would presume that these compounds would effectively result in a more continuous and stable stimulus of nucleotides at the cell surface. The kinetics of the [Ca2+]cyt response seen with ATPγS or ADPβS was very similar to that found with the native nucleotides (data not shown); that is, oscillations were only seen in the presence of NGXT191 (Fig. 7B; Supplemental Table S1).
Thus, it is difficult to reconcile how ectoapyrase inhibition increases the number of nucleotide-induced [Ca2+]cyt oscillations. An alternative hypothesis is that the initial addition of ATP or ADP triggers serial release of endogenous ATP that, under normal conditions, is rapidly degraded by ectoapyrases before generating the serial [Ca2+]cyt oscillations (see “Discussion”). This would explain why the ectoapyrase inhibitor NGXT191 was required for [Ca2+]cyt oscillations even when poorly hydrolyzable nucleotides were added. It was reported that extracellular ATP is released from plant cells by plasma membrane ATP-binding cassette (ABC) transporters and exocytosis (Thomas et al., 2000; Kim et al., 2006). Therefore, to determine which secretion system is responsible for the phenomenon, we treated plants with brefeldin A (BFA), which is a vesicle-trafficking inhibitor, and 1-naphthylphthalamic acid (NPA), which is an inhibitor of the ABC transporters belonging to the multidrug resistance-like P-glycoprotein subclass (MDR/PGPs), especially PGP1 and PGP19 (Murphy et al., 2002; Geisler et al., 2003). The number of oscillations and the frequency of occurrence of the [Ca2+]cyt oscillations were remarkably diminished in presence of BFA but not NPA (Fig. 7E; Table I). These results imply that the [Ca2+]cyt oscillations are likely to be induced by released ATP (or some other compound[s]) secreted by vesicle trafficking.
NTP-Induced [Ca2+]cyt Responses Show Cell Type-Specific Kinetics
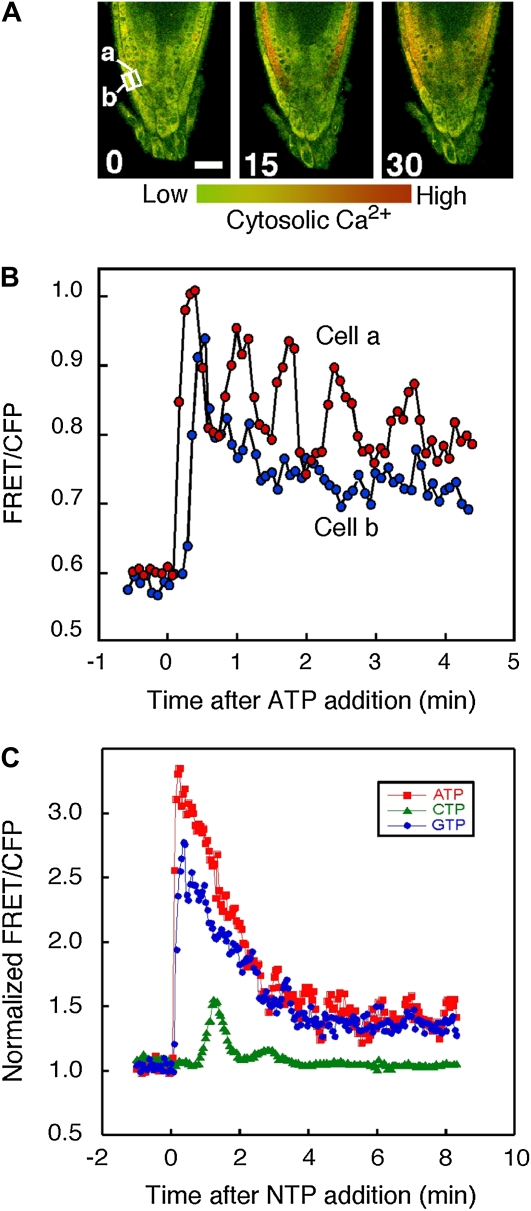
The specificity of the kinetics of [Ca2+]cyt response to each nucleotide led us to ask whether these changes reflected different NTP responses being exhibited by different cell types and tissues or whether individual cells could respond to all NTP types but with different parameters. The experiments using aequorin transgenic seedlings indicated that the majority of the [Ca2+]cyt response to nucleotides (100 μm or less) was confined to the root tissues (data not shown). Hence, the response of roots to NTP addition was examined utilizing plants expressing the GFP-based Cameleon sensor. This sensor was originally developed for use in mammalian cells (Miyawaki et al., 1997) and was first applied to plants in guard cells (Allen et al., 1999). We applied a version with enhanced FRET signal, YC3.6 (Nagai et al., 2004), and confocal ratio imaging of plant roots (Monshausen et al., 2008) to follow the dynamics of the NTP-induced [Ca2+]cyt response with cellular resolution.
Supplemental Figure S2 shows that when the response was averaged over the root tip, ATP caused rapid changes in [Ca2+]cyt consistent with the kinetics monitored by aequorin. Moreover, the cellular analysis possible with this confocal approach revealed complex cell type specificity in the details of these kinetics (Fig. 8); lateral root cap cells responded more slowly than the subtending cell layer to exogenous ATP, even though these cells would have experienced extracellular ATP before those in the underlying cell layers. Interestingly, the subtending layer was capable of exhibiting strong oscillations in [Ca2+]cyt not seen in the overlying cell layer (Figs. 8, A and B; Supplemental Fig. S3), although the occurrence and magnitude of these oscillations were variable (oscillations seen in 57% of cells; n = 14). These changes suggest that there are cell type differences in response to ATP, possibly reflecting a variety of perception and/or response machinery in each cell type. Such differences did not reflect generic differences in the [Ca2+]cyt responsiveness of different cell layers; for example, cold shock treatment also increased [Ca2+]cyt but led to a very different pattern of cell type-selective response and recovery of [Ca2+]cyt levels (Supplemental Fig. S3; Supplemental Movies S2 and S3).
Figure 8.
Effects of NTPs on [Ca2+]cyt response in Arabidopsis seedlings monitored using YC3.6. Arabidopsis seedlings expressing YC3.6 were treated with 10 μm ATP, CTP, or GTP as indicated. Changes in [Ca2+]cyt were monitored at 5-s (A and B) or 3-s (C) intervals using confocal ratio imaging as described in “Materials and Methods.” [Ca2+]cyt has been pseudocolor coded according to the inset scale. Representative of five or more roots per treatment are shown. A, ATP-induced changes in [Ca2+]cyt imaged using YC3.6. Panels show time points in seconds following ATP treatment. Bar = 20 μm. B, ATP-induced changes in [Ca2+]cyt monitored in individual cells shown in A. C, NTP-induced changes in [Ca2+]cyt monitored in individual cell type a from A. CFP, Cyan fluorescent protein.
Aequorin measurements in the presence of BFA suggested that secretion plays a role in nucleotide release. Therefore, the effect of BFA was tested while monitoring [Ca2+]cyt with YC3.6 in root cells. Similar to the effects seen using the aequorin-expressing plants, the observed [Ca2+]cyt changes at the single cell level following ATP treatment were diminished following a preincubation in 10 μm BFA (data not shown). Consistent with the fact that NGXT191 treatment was not essential to see calcium oscillations in root cells using YC3.6 fluorescence, preincubation of root for 30 min with 10 mg mL−1 NGXT191 did not affect the calcium response seen upon the addition of ATP (data not shown).
Differences in the kinetics of the [Ca2+]cyt response of individual cells were also observed in response to other nucleotides such as GTP and CTP (Fig. 8C; Supplemental Movies S4 and S5). Analysis of the [Ca2+]cyt increases in response to this range of NTPs in a single cell type (Fig. 8C) suggests that a single cell is capable of perceiving all of the NTPs tested and translating these into a cellular Ca2+ signal. However, the differences in the kinetics of the Ca2+ change could carry information regarding the NTP triggering the response. Thus, the different Ca2+ dynamics seen in response to different NTPs monitored using aequorin are unlikely to simply reflect different cell types responding to a specific NTP but suggest that one cell can respond to different NTPs, possibly through multiple receptor systems.
DISCUSSION
Intracellular calcium is a ubiquitous signaling component in plant cells that is increased in response to many physiological stimuli, such as light, touch, pathogenic elicitors, plant hormones, and abiotic stresses (Rudd and Franklin-Tong, 2001). Extracellular ATP also alters [Ca2+]cyt levels presumably mediated through receptors on the plasma membrane (Demidchik et al., 2009). Our new data begin to reveal additional complexity of the plant cell response to extracellular ATP.
It is worth discussing whether pyrimidine and purine nucleotides, other than ATP, can function as extracellular ligands in plants. Although animal P2 receptors were originally identified as receptors for purine nucleotides, they have diverse ligand specificities. For example, there are pyrimidine-recognizing or -preferring P2Y receptors, such as P2Y2, P2Y4, and P2Y6 (Ralevic and Burnstock, 1998; Burnstock, 2007), suggesting that different P2 receptors display specificity for particular nucleotides. In plants, little information exists on the activity of various nucleotides and associated plant cell responses. For instance, early studies demonstrated that ATP and CTP, but not GTP or UTP, are involved in maintaining the water permeability of potato (Solanum tuberosum) storage tissue (Stuart, 1973). UTP in addition to purine NTPs causes potassium ion uptake into the cells of maize leaf slices (Lüttge et al., 1974). Recently, GTP, CTP, and UTP (as well as ATP) were found to induce superoxide production in Arabidopsis leaves (Song et al., 2006). These NTPs prevented cell death induced by the toxin fumonisin B1 in Arabidopsis (Chivasa et al., 2005), whereas GTP and CTP, but not UTP, had similar attenuating effects as ATP on systemic acquired resistance induced by Tobacco mosaic virus (Chivasa et al., 2009). Some authors suggested that the effects of non-ATP nucleotides are probably the result of an artifact caused by the protection of endogenous extracellular ATP from degradation (Ostrom et al., 2000; Lazarowski et al., 2003; Chivasa et al., 2005). An alternative view would be that these differences might reflect differing nucleotide affinities of a single receptor protein. However, our results raise the possibility that individual plant cells are in fact competent to recognize nucleotides in distinctly different manners. In particular, CTP caused a [Ca2+]cyt response that was quite distinct from that of the other nucleotides whether monitored at the whole plant (i.e. with aequorin; Fig. 1) or single cell (i.e. with YC3.6; Fig. 8). For example, the time- and dose-dependent kinetics of [Ca2+]cyt response upon CTP addition were distinct in shape from those resulting from purine nucleotides (Figs. 1, 2, and 8). The CTP-induced [Ca2+]cyt changes were composed of Ca2+ influx from the extracellular space, whereas ATP-induced [Ca2+]cyt increases were dependent on Ca2+ influx and the release of calcium from intracellular stores (as judged by our pharmacological studies; Fig. 2; Supplemental Fig. S1). Additionally, the profiles of the inhibitory effects of mammalian P2 receptor antagonists were distinct for the purine and CTP-induced [Ca2+]cyt responses (Fig. 4). Finally, our imaging data indicate that a single cell type is capable of responding to a range of nucleotides (Fig. 8C), suggesting that these differences in characteristics do not simply reflect differential response kinetics of different tissue types each responding to only a single NTP. Thus, our observations, together with previous studies, suggest that multiple receptors or signaling mechanisms likely exist in plant cells to mediate responses activated by extracellular nucleotides.
Two classes of P2 receptors in mammals mediate extracellular ATP signaling; the ionotropic receptor is responsible for fast cell response, and the metabotropic receptor is involved in the response that has a longer latency after stimuli in comparison with ionotropic signaling (Lechner and Boehm, 2004). In mammals, these two activities show tissue specificity (Burnstock, 2007); occasionally, either or both ionotropic and metabotropic receptors exist together even in the same cell type (e.g. in rat spiral ganglion neurons; Ito and Dulon, 2002). We observed biphasic kinetics of [Ca2+]cyt induced by ATP using whole seedlings of Arabidopsis (Fig. 2), suggesting that plants do possess two types of signal transduction in response to extracellular ATP, each of which may be activated by ionotropic or metabotropic extracellular ATP receptors. Demidchik et al. (2009) reported that Ca2+ mobilization via metabotropic extracellular ATP signaling activates a plasma membrane ionic conductance in protoplasts isolated from the mature root epidermis. This study and that of Demidchik et al. (2009) use different plant material and, therefore, may not be directly comparable. However, we are tempted to speculate that either or both classes of ATP receptors may mediate extracellular ATP signaling depending on the plant cell type. Such questions cannot be adequately addressed until the putative plant ATP receptors are identified and their cell-specific activities can be studied.
In metabotropic P2 receptor signaling, heterotrimeric G proteins are typically required to stimulate PLC-mediated Ca2+ release from the endoplasmic reticulum (ER; Ralevic and Burnstock, 1998; Burnstock, 2007). The PLC pathway is also tentatively implicated in plant cell ATP signaling, as evidenced by the fact that U-73122 (a PLC inhibitor) partially inhibited ATP-induced [Ca2+]cyt responses (Fig. 2, C and D). However, the involvement of G proteins in the extracellular ATP-induced calcium signaling in plant cells is unlikely to be identical to that seen in animals. Most notably, the elevation of [Ca2+]cyt upon ATP addition in the agb1-2 mutant was significantly stronger than that seen in the wild type or gpa1-4 mutant, suggesting that the Gβ-subunit AGB1 negatively regulates extracellular nucleotide signaling. Furthermore, we detected no significant differences in the ratio of the first peak of elevated [Ca2+]cyt to the second peak between the wild type and G protein mutants (data not shown). As the PLC inhibitor U-73122 preferentially inhibited the second peak (Fig. 2, C and D), this failure to alter the relative magnitudes of either peak in the [Ca2+]cyt response indicates that plant G proteins are unlikely to be involved in the PLC-mediated Ca2+ signaling pathway. The nature of the mechanism underlying the regulation of nucleotide-induced [Ca2+]cyt responses by AGB1 is still unknown. However, it is possible that the observed hyperresponsiveness to nucleotides in the agb1 mutant is attributable to a general lack of cellular control in desensitizing extracellular nucleotide signaling, as is the case for touch-induced desensitization of ATP release (Weerasinghe et al., 2009).
Interestingly, similar to agb1-2 mutant plants, the double mutant gpa1-4;agb1-2 showed significantly enhanced [Ca2+]cyt responses to ATP addition. If this process were controlled by canonical heterotrimeric G protein signaling, Gβ(γ)-mediated signaling should also be affected upon disruption of Gα and vice versa, because Gβγ and Gα are mutually dependent for appropriate membrane targeting and for interaction with receptors (Marrari et al., 2007). Hence, we might have concluded that AGB1 is epistatic to GPA1. However, the gpa1 mutant displayed no detectable differences compared with the wild type in the nucleotide-induced [Ca2+]cyt elevation. Moreover, the behavior of the double mutant was indistinguishable from that of the single mutant in these assays. Therefore, we conclude that AGB1 is involved in nucleotide-induced Ca2+ signaling independently of the Gα-subunit, rather than that AGB1 acts downstream of GPA1 in this signaling pathway. This conclusion is similar to that made in published reports describing the phenotypes altered only in agb1 mutants on silique shape (Lease et al., 2001), cell death associated with the unfolded protein response (Wang et al., 2007), and root waving and skewing responses (Pandey et al., 2008). In agreement with the conclusion of Pandey et al. (2008), we propose that the AGB1 subunit functions in an NTP-induced [Ca2+]cyt response without forming a Gαβγ heterotrimeric complex.
In experiments using Arabidopsis seedlings expressing aequorin, we found that the addition of single doses of ATP, ADP, or poorly hydrolyzable analogs in the presence of a plant apyrase inhibitor, NGXT191, induced multiple temporal [Ca2+]cyt oscillations, in contrast to the transient [Ca2+]cyt response in the absence of ectoapyrase inhibition (Fig. 7; Supplemental Table S1). Our initial conclusion from these experiments was that nucleotide hydrolysis by ectoapyrases resulted in a rapid loss of extracellular nucleotides and that a sustained [Ca2+]cyt response was seen only when these enzymes were inhibited. However, this conclusion is inconsistent with the data in which a single application of poorly hydrolyzable analogs, ATPγS or ADPβS, did not cause oscillations (data not shown) but that oscillations occurred in the presence of the apyrase inhibitor (Supplemental Table S1). ATPγS and ADPβS are known to be relatively resistant to hydrolysis by ectonucleotidases (Picher et al., 1996; Gendaszewska-Darmach et al., 2003). Therefore, the long-term hydrolysis rate is likely not the critical component causing the [Ca2+]cyt oscillations observed (Fig. 7). Therefore, one possibility is that initial ATP addition triggers the serial release of small amounts of endogenous ATP, which is essential for the subsequent calcium oscillations that are only apparent with ectoapyrase inhibition. This model would explain why poorly hydrolyzable nucleotides were also able to cause the [Ca2+]cyt oscillations.
In mammals, ATP release can be induced by ATP or also other agonists, such as ADP and UTP (Yang et al., 1994; Matsuo et al., 1997; Cotrina et al., 1998; Anderson et al., 2004). Moreover, several reports described an interaction between ATP release and [Ca2+]cyt oscillations. For example, spontaneous [Ca2+]cyt oscillations required nucleotide release in canine renal epithelia (Geyti et al., 2008). ATP is also rhythmically released to control the intercellular synchronization of [Ca2+]cyt oscillations in rat cardiac myocytes in a phenomenon dependent on purinoceptor signaling (Kawahara and Nakayama, 2007; Nakayama et al., 2007). In general, the release of ATP to the extracellular matrix can be mediated by exocytosis, anion channels, and ABC transporters in mammals (Lazarowski et al., 2003). In plants, ATP release was reported to occur mediated by AtPGP1 and vesicle trafficking (Thomas et al., 2000; Kim et al., 2006). We found that BFA, not NPA, specifically impaired the nucleotide-induced [Ca2+]cyt oscillations (Fig. 7E; Table I). This result strengthens our hypothesis that the nucleotide-induced [Ca2+]cyt oscillations require ATP release mediated by vesicle trafficking, such as exocytosis. However, we cannot rule out the possibility that MDR/PGPs, other than AtPGP1, are required, since verapamil, which inhibited nucleotide-induced [Ca2+]cyt responses (Table I), also acts as an inhibitor of mammalian MDR/PGPs (Horio et al., 1989) as well as Ca2+ channels.
When monitored at the single cell level, applied ATP induced [Ca2+]cyt oscillations (Fig. 8B; Supplemental Fig. S3A; Supplemental Movie S2) without addition of the ectoapyrase inhibitor NGXT191. However, such oscillations were limited to specific cell types and were variable in occurrence. Considering that the aequorin-based [Ca2+]cyt measurement results from integrated data of [Ca2+]cyt elevations/oscillations in single cells (Dodd et al., 2006), our observations suggest that ectoapyrase activity may vary between cell types, possibly in an environmentally responsive manner, and is likely low around those root cells exhibiting [Ca2+]cyt oscillations visualized with YC3.6. The addition of NGXT191 may act to lower ectoapyrase activity around all cell types, allowing aequorin to monitor oscillations that develop across the entire plant. Inclusion of the inhibitor with the ATP did not elicit clear oscillations in the root tip cells analyzed, suggesting that the oscillatory changes revealed by aequorin arise from outside this region.
It is impossible to exclude the possibility that some fraction of NGXT191 might cross the plasma membrane and inhibit intracellular apyrase activity. Although the cellular functions of plant intracellular apyrases have not been studied, there is a well-developed model for apyrase function in the Golgi and ER in yeast and Caenorhabditis elegans related to the control of protein glycosylation (Hirschberg et al., 1998; Uccelletti et al., 2008). It was reported that glycosylation of Ca2+ sensors in the ER regulates store-operated Ca2+ influx (Taylor, 2006; Czyz et al., 2009). It is interesting to speculate that disruption of the regulation system of intracellular Ca2+ flux by NGXT191 might contribute to ATP-induced [Ca2+]cyt oscillations. Evidence of intracellular apyrase activity correlated to protein glycosylation rate in ER would be needed to support this model. It is also important to note that, although NGXT191 has been used to inhibit apyrases in plants, it is known to have low, but measurable, inhibitor activity against alkaline phosphatase (Windsor et al., 2003). Results obtained with any pharmacological reagent should be interpreted with caution, and it is for precisely this reason that we sought independent confirmation of the ATP-induced calcium oscillations utilizing transgenic plants expressing the YC3.6 calcium reporter (see below).
What is the function of nucleotide-induced [Ca2+]cyt oscillations? In plants, specific [Ca2+]cyt oscillations are known to be crucial to the control of stomatal function, pollen tube and root hair growth, and the response of legume root hairs to rhizobia-produced Nod factor signals (Evans et al., 2001; Monshausen et al., 2008; Oldroyd and Downie, 2008; McAinsh and Pittman, 2009). It is tempting to propose that extracellular ATP functions as a component of these processes to elicit the [Ca2+]cyt oscillations. For example, previous reports demonstrated a critical role for ectoapyrases during the early steps of legume root hair infection by rhizobia (Etzler et al., 1999; McAlvin and Stacey, 2005; Govindarajulu et al., 2009). Presumably, these ectoapyrases are controlling the level of extracellular ATP at the root hair surface. Indeed, the addition of exogenous ADP to soybean (Glycine max) root hairs was shown to significantly increase nodulation (Govindarajulu et al., 2009). Utilizing a reporter system that directly imaged extracellular ATP levels, Kim et al. (2006) demonstrated the release of extracellular ATP from the tips of growing legume root hairs. Kim et al. (2006) and Wu et al. (2008) also implicated extracellular ATP in the plant cell response to specific pathogen-associated elicitors (e.g. chitin). Consistent with these findings, chitin addition to aequorin-expressing Arabidopsis seedlings in the presence of NGXT191 induced [Ca2+]cyt oscillations similar to those shown in Figure 7C (K. Tanaka and G. Stacey, unpublished data). Hence, the data are suggestive of a critical and largely unexplored role for extracellular ATP release in mediating [Ca2+]cyt signaling essential for a variety of plant cellular responses.
When monitored at the cellular level using the YC3.6 Ca2+ sensor, we observed that NTP addition elicited changes in [Ca2+]cyt that did not occur synchronously across the root. Thus, ATP and GTP caused elevations in [Ca2+]cyt that were broadly similar in magnitude and progression through the root tip but that were markedly different in magnitude and spatial and temporal characteristics from CTP (Fig. 8; Supplemental Fig. S2; Supplemental Movies S2, S4, and S5). These NTP-specific [Ca2+]cyt signatures may well provide information to the root regarding the NTP driving the Ca2+ signaling and have the potential to elicit stimulus-specific downstream responses (McAinsh and Pittman, 2009). Further investigation of this new and emerging role for extracellular nucleotides as a critical extracellular signal in plants is likely to provide novel insight into the basic mechanisms of plant cell growth and environmental response.
MATERIALS AND METHODS
Reagents
All chemicals and enzymes, except those mentioned elsewhere, were purchased from Sigma-Aldrich. Stock solution of nucleotides, antagonists of mammalian P2 receptors (Tocris Bioscience), and calcium channel blockers were prepared in water at 100 mm and stored at −20°C until use. NGXT191 (a kind gift of Dr. Stanley J. Roux), NPA (Frinton Laboratories), U-73122, and BFA were dissolved in dimethyl sulfoxide. The final concentration of the solvent under assay conditions did not exceed 0.1% (v/v).
[Ca2+]cyt Measurement by Aequorin Luminometry and Confocal Microscopy
Aequorin transgenic seedlings in the Columbia background (kindly provided by Dr. Marc R. Knight, University of Oxford) were sterilized for 5 min in 5% sodium hypochlorite solution and then rinsed twice and resuspended in sterile water. The seeds were then sown along a straight line on half-strength Murashige and Skoog medium containing 1% (w/v) Suc, 1% (w/v) agar, 0.05% (w/v) MES (pH 5.7), and Gamborg’s B5 vitamins. After incubation at 4°C for 3 d to synchronize seed germination, petri dishes with the seeds were placed vertically in a plant growth chamber set at 22°C under continuous light conditions. Five-day-grown seedlings were transferred individually to individual wells of a 96-well microplate and incubated overnight at room temperature in 50 μL of reconstitution buffer containing 2 mm MES (pH 5.7), 10 mm CaCl2, and 10 μm coelenterazine (Nanolight Technology). After overnight incubation, chemicals were applied at the indicated final concentrations. Photon emissions were detected using an intensified CCD camera (Photek 216; Photek, Ltd.). At the end of each experiment, remaining aequorin was discharged by the addition of an equal volume of solution containing 2 m CaCI2 and 20% (v/v) ethanol. Luminescence values were calibrated as calcium concentrations, according to Knight et al. (1996).
For cytoplasmic calcium imaging using confocal microscopy, Arabidopsis (Arabidopsis thaliana) seedlings expressing the FRET-based Ca2+ sensor YC3.6 (Nagai et al., 2004) were mounted in a thin layer of Phytagel medium on 22- × 40-mm coverglass as described by Blancaflor et al. (1998). The root tips of 5-d-old seedlings were exposed by cutting a window in the gel to which a 1× solution of 10 μm ATP, CTP, GTP, or medium without NTPs was added. Roots were ratio imaged with the Zeiss LSM 510 microscope (Carl Zeiss) at intervals of 5 or 3 s using the 40× objective with the 458-nm line of the argon laser exciting the YC3.6. The cyan fluorescent protein (473–505 nm) and FRET-dependent (536–546 nm) emission were collected by using a 458-nm primary dichroic mirror and the Meta detector of the microscope. Images were pseudocolored (overlays of the cyan fluorescent protein channel in green and the FRET channel in red) using Zeiss LSM software. Ratios were calculated using iVision software (BioVision Technologies), statistical analysis was done using Excel (Microsoft), and graphs were generated using KaleidaGraph (Synergy Software).
Identification of Heterotrimeric G Protein Mutants in the Aequorin Transgenic Background
Heterotrimeric G protein mutants in the aequorin transgenic background were screened from F2 generation pools after cross-pollination between the gpa1-4;agb1-2 double mutant (kindly provided by Alan M. Jones) and aequorin transgenic Arabidopsis. Homozygous T-DNA insertions were selected by PCR using gene-specific primers (Jones et al., 2003; Ullah et al., 2003) and a T-DNA left border-specific primer, LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′). The presence of the aequorin transgene was confirmed in the F2 generation by detection of bioluminescence in its dissected cotyledon using reconstitution buffer and discharging solution (see above); homozygosity was then determined in the F3 generation by the same method.
Statistical Analysis
All experiments were repeated at least three times, and the data obtained were analyzed using ANOVA followed by Student’s t test or the Tukey-Kramer multiple comparison test. A difference with P < 0.05 was considered significant.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of a PLC inhibitor and a Ca2+ channel inhibitor on CTP-induced [Ca2+]cyt response in Arabidopsis seedlings.
Supplemental Figure S2. Effects of ATP, GTP, or CTP on [Ca2+]cyt response in the root tip of Arabidopsis seedlings monitored by YC3.6.
Supplemental Figure S3. Effects of ATP and cold shock on [Ca2+]cyt response in Arabidopsis root tips.
Supplemental Table S1. Nucleotide-induced [Ca2+]cyt oscillations in Arabidopsis.
Supplemental Table S2. Nucleotide-induced [Ca2+]cyt oscillations in heterotrimeric G protein mutants.
Supplemental Movie S1. NGXT191 elicited ADP-induced [Ca2+]cyt oscillations.
Supplemental Movie S2. Effect of ATP on [Ca2+]cyt response in the root tip of Arabidopsis seedlings.
Supplemental Movie S3. Effect of cold shock on [Ca2+]cyt response in the root tip of Arabidopsis seedlings.
Supplemental Movie S4. Effect of GTP on [Ca2+]cyt response in the root tip of Arabidopsis seedlings.
Supplemental Movie S5. Effect of CTP on [Ca2+]cyt response in the root tip of Arabidopsis seedlings.
Supplementary Material
Acknowledgments
We are grateful to Alan M. Jones (University of North Carolina) for heterotrimeric G protein mutant Arabidopsis, Dr. Marc R. Knight (University of Oxford) for aequorin transgenic Arabidopsis, and Dr. Stanley J. Roux (University of Texas) for providing NGXT191. Special thanks to Drs. Gary A. Weisman and Seth D. Findley (University of Missouri) for critical comments on the manuscript.
References
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 [DOI] [PubMed] [Google Scholar]
- Anderson CM, Bergher JP, Swanson RA. (2004) ATP-induced ATP release from astrocytes. J Neurochem 88: 246–256 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP, Slabas AR. (2009) Extracellular ATP is a regulator of pathogen defence in plants. Plant J 60: 436–448 [DOI] [PubMed] [Google Scholar]
- Chivasa S, Ndimba BK, Simon WJ, Lindsey K, Slabas AR. (2005) Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 17: 3019–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. (2007) Calcium signaling. Cell 131: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. (1998) Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyz A, Brutkowski W, Fronk J, Duszynski J, Zablocki K. (2009) Tunicamycin desensitizes store-operated Ca2+ entry to ATP and mitochondrial potential. Biochem Biophys Res Commun 381: 176–180 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM. (2003) Is ATP a signaling agent in plants? Plant Physiol 133: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, et al. (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58: 903–913 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Jakobsen MK, Baker AJ, Telzerow A, Hou SW, Laplaze L, Barrot L, Poethig RS, Haseloff J, Webb AA. (2006) Time of day modulates low-temperature Ca2+ signals in Arabidopsis. Plant J 48: 962–973 [DOI] [PubMed] [Google Scholar]
- Etzler ME, Kalsi G, Ewing NN, Roberts NJ, Day RB, Murphy JB. (1999) A nod factor binding lectin with apyrase activity from legume roots. Proc Natl Acad Sci USA 96: 5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NH, McAinsh MR, Hetherington AM. (2001) Calcium oscillations in higher plants. Curr Opin Plant Biol 4: 415–420 [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. (2006) Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7: 423–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresi NP, Laxalt AM, Tonon CV, Casalongue CA, Lamattina L. (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Kolukisaoglu HU, Bouchard R, Billion K, Berger J, Saal B, Frangne N, Koncz-Kalman Z, Koncz C, Dudler R, et al. (2003) TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol Biol Cell 14: 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendaszewska-Darmach E, Maszewska M, Zaklos M, Koziolkiewicz M. (2003) Degradation of extracellular nucleotides and their analogs in HeLa and HUVEC cell cultures. Acta Biochim Pol 50: 973–984 [PubMed] [Google Scholar]
- Geyti CS, Odgaard E, Overgaard MT, Jensen ME, Leipziger J, Praetorius HA. (2008) Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch 455: 1105–1117 [DOI] [PubMed] [Google Scholar]
- González FA, Weisman GA, Erb L, Seye CI, Sun GY, Velázquez B, Hernández-Pérez M, Chorna NE. (2005) Mechanisms for inhibition of P2 receptors signaling in neural cells. Mol Neurobiol 31: 65–79 [DOI] [PubMed] [Google Scholar]
- Govindarajulu M, Kim SY, Libault M, Berg RH, Tanaka K, Stacey G, Taylor CG. (2009) GS52 ecto-apyrase plays a critical role during soybean nodulation. Plant Physiol 149: 994–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Musset B, Renigunta V, Limberg SH, Dalpke AH, Sus R, Heeg KM, Preisig-Muller R, Daut J. (2004) Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci USA 101: 9479–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg CB, Robbins PW, Abeijon C. (1998) Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem 67: 49–69 [DOI] [PubMed] [Google Scholar]
- Horio M, Chin KV, Currier SJ, Goldenberg S, Williams C, Pastan I, Gottesman MM, Handler J. (1989) Transepithelial transport of drugs by the multidrug transporter in cultured Madin-Darby canine kidney cell epithelia. J Biol Chem 264: 14880–14884 [PubMed] [Google Scholar]
- Ito K, Dulon D. (2002) Nonselective cation conductance activated by muscarinic and purinergic receptors in rat spiral ganglion neurons. Am J Physiol Cell Physiol 282: C1121–C1135 [DOI] [PubMed] [Google Scholar]
- Jaffe MJ. (1973) The role of ATP in mechanically stimulated rapid closure of the Venus’s flytrap. Plant Physiol 51: 17–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ. (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16: 2652–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Ecker JR, Chen JG. (2003) A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol 131: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Nakayama Y. (2007) Fluctuations in the concentration of extracellular ATP synchronized with intracellular Ca2+ oscillatory rhythm in cultured cardiac myocytes. Chronobiol Int 24: 1035–1048 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G. (2009) The double life of ATP. Sci Am 301: 84–90, 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Sivaguru M, Stacey G. (2006) Extracellular ATP in plants: visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol 142: 984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795 [DOI] [PubMed] [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC. (2001) A mutant Arabidopsis heterotrimeric G-protein beta subunit affects leaf, flower, and fruit development. Plant Cell 13: 2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Boehm S. (2004) Regulation of neuronal ion channels via P2Y receptors. Purinergic Signal 1: 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RR, Dearnaley JDW. (2000) Extracellular nucleotide effects on electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci 153: 1–6 [Google Scholar]
- Lüttge U, Schöch EV, Ball E. (1974) Can externally applied ATP supply energy to active ion uptake mechanisms of intact plant cells? Aust J Plant Physiol 1: 211–220 [Google Scholar]
- Mahoney MG, Randall CJ, Linderman JJ, Gross DJ, Slakey LL. (1992) Independent pathways regulate the cytosolic [Ca2+] initial transient and subsequent oscillations in individual cultured arterial smooth muscle cells responding to extracellular ATP. Mol Biol Cell 3: 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. (2007) Assembly and trafficking of heterotrimeric G proteins. Biochemistry 46: 7665–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Katsuragi T, Fujiki S, Sato C, Furukawa T. (1997) ATP release and contraction mediated by different P2-receptor subtypes in guinea-pig ileal smooth muscle. Br J Pharmacol 121: 1744–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. (2009) Shaping the calcium signature. New Phytol 181: 275–294 [DOI] [PubMed] [Google Scholar]
- McAlvin CB, Stacey G. (2005) Transgenic expression of the soybean apyrase in Lotus japonicus enhances nodulation. Plant Physiol 137: 1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. (2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AS, Hoogner KR, Peer WA, Taiz L. (2002) Identification, purification, and molecular cloning of N-1-naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol 128: 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. (2004) Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Kawahara K, Hachiro T, Yamauchi Y, Yoneyama M. (2007) Possible involvement of ATP-purinoceptor signalling in the intercellular synchronization of intracellular Ca2+ oscillation in cultured cardiac myocytes. Biosystems 90: 179–187 [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Downie JA. (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59: 519–546 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Insel PA. (2000) Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem 275: 11735–11739 [DOI] [PubMed] [Google Scholar]
- Pandey S, Monshausen GB, Ding L, Assmann SM. (2008) Regulation of root-wave response by extra large and conventional G proteins in Arabidopsis thaliana. Plant J 55: 311–322 [DOI] [PubMed] [Google Scholar]
- Picher M, Sevigny J, D’Orleans-Juste P, Beaudoin AR. (1996) Hydrolysis of P2-purinoceptor agonists by a purified ectonucleotidase from the bovine aorta, the ATP-diphosphohydrolase. Biochem Pharmacol 51: 1453–1460 [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492 [PubMed] [Google Scholar]
- Rudd JJ, Franklin-Tong VE. (2001) Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol 151: 7–33 [DOI] [PubMed] [Google Scholar]
- Shen J, Harada N, Nakazawa H, Yamashita T. (2005) Involvement of the nitric oxide-cyclic GMP pathway and neuronal nitric oxide synthase in ATP-induced Ca2+ signalling in cochlear inner hair cells. Eur J Neurosci 21: 2912–2922 [DOI] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol 140: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ. (2003) Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 131: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart DM. (1973) Reduction of water permeability in potato tuber slices by cyanide, ammonia, 2,4-dinitrophenol, and oligomycin and its reverse by adenosine 5′-triphosphate and cytidine 5′-triphosphate. Plant Physiol 51: 485–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Brady SR, Sun Y, Muday GK, Roux SJ. (2003) Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol 131: 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW. (2006) Store-operated Ca2+ entry: a STIMulating stOrai. Trends Biochem Sci 31: 597–601 [DOI] [PubMed] [Google Scholar]
- Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ. (2000) A role for ectophosphatase in xenobiotic resistance. Plant Cell 12: 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelletti D, Pascoli A, Farina F, Alberti A, Mancini P, Hirschberg CB, Palleschi C. (2008) APY-1, a novel Caenorhabditis elegans apyrase involved in unfolded protein response signalling and stress responses. Mol Biol Cell 19: 1337–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy J, Farkas GL. (1973) ATP stimulates the formation of nucleases in excised Avena leaves. Z Pflanzenphysiol 69: 394–401 [Google Scholar]
- Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM. (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visegrady A, Grama L, Somogyi B, Lustyik G. (2000) Characterization of intracellular calcium oscillations induced by extracellular nucleotides in HEp-2 cells. J Photochem Photobiol B 58: 80–86 [DOI] [PubMed] [Google Scholar]
- Wang S, Narendra S, Fedoroff N. (2007) Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA 104: 3817–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasinghe RR, Swanson SJ, Okada SF, Garrett MB, Kim SY, Stacey G, Boucher RC, Gilroy S, Jones AM. (2009) Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett 583: 2521–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor B, Roux SJ, Lloyd A. (2003) Multiherbicide tolerance conferred by AtPgp1 and apyrase overexpression in Arabidopsis thaliana. Nat Biotechnol 21: 428–433 [DOI] [PubMed] [Google Scholar]
- Wolf C, Hennig M, Romanovicz D, Steinebrunner I. (2007) Developmental defects and seedling lethality in apyrase AtAPY1 and AtAPY2 double knockout mutants. Plant Mol Biol 64: 657–672 [DOI] [PubMed] [Google Scholar]
- Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ. (2007) Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol 144: 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Liu YS, Wu JY. (2008) The signaling role of extracellular ATP and its dependence on Ca2+ flux in elicitation of Salvia miltiorrhiza hairy root cultures. Plant Cell Physiol 49: 617–624 [DOI] [PubMed] [Google Scholar]
- Wu SJ, Wu JY. (2008) Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J Exp Bot 59: 4007–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cheek DJ, Westfall DP, Buxton IL. (1994) Purinergic axis in cardiac blood vessels: agonist-mediated release of ATP from cardiac endothelial cells. Circ Res 74: 401–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.