Abstract
Mycobacterium avium causes systemic infections through primary intestinal lesions in pigs. However, its pathogenesis is not well understood. The aim of this study was to confirm the effects on swine after enteral infection. One hundred and twelve pigs with hepatic lesions infected with M. avium were used in this study. We investigated the involvement of other organs and the distribution of hepatic lesions in the lobular structure. Most lesions involved the mesenteric lymph nodes. Hepatic lymph nodes were the secondary nodes involved. In 74 cases (66.1%), the hepatic lesions were predominantly distributed in the portal tract of the affected livers. The other 38 cases (33.9%) showed granulomatous lesions in the hepatic lobule. Many cases showed interface hepatitis. There was a significant relationship between focal lesions within hepatic lobule and splenic lesions. These findings suggest that granulomatous lesions formed in hepatic lobules upon establishment of bacteremia in pigs systemically infected with M. avium.
Résumé
Chez les porcs Mycobacterium avium cause des infections systémiques suite à une lésion intestinale primaire. Toutefois, la pathogénie de cette condition n’est pas clairement comprise. L’objectif de la présente étude était de confirmer les effets chez le porc suite à une infection entérale. Cent douze porcs infectés avec M. avium présentant des lésions hépatiques ont été utilisés dans cette étude. Nous avons examiné l’implication des autres organes et la distribution des lésions hépatiques dans la structure lobulaire. La plupart des lésions impliquaient les nœuds lymphatiques mésentériques. Les nœuds lymphatiques hépatiques étaient ceux impliqués en second lieu. Dans 74 cas (66,1 %), les lésions hépatiques étaient distribuées de manière prédominante dans l’espace porte des foies affectés. Les 38 autres cas (33,9 %) présentaient des lésions granulomateuses dans le lobule hépatique. Plusieurs cas montraient une hépatite d’interface. Il y avait une relation significative entre les lésions focales à l’intérieur des lobules hépatiques et des lésions spléniques. Ces trouvailles suggèrent que les lésions granulomateuses se sont formées dans les lobules hépatiques suite à une bactériémie chez les porcs infectés par M. avium de manière systémique.
(Traduit par Docteur Serge Messier)
Introduction
Mycobacterium avium complex (MAC), which is a complex of M. avium and M. intracellulare, is an ubiquitous organism and certain animals act as reservoirs in nature (1). Among the members of the MAC family, M. avium is the most predominant pathogen identified in humans and some animal species (1–3). This organism is classified into 4 subspecies. Mycobacterium avium subsp paratuberculosis is the etiological agent for severe enteritis in ruminants known as Johne’s disease (4). Mycobacterium avium subsp avium originates from avian species and is most virulent in poultry (3). Mycobacterium avium subsp silvaticum has been isolated from the parenchymatous organs of wood pigeons with a tuberculous pathology (5). Mycobacterium avium subsp hominissuis is the most prevalent opportunistic pathogen for humans and pigs (6,7).
Mycobacteriosis by M. avium subsp hominissuis is the one of major diseases in swine populations (6–8). Sporadic subclinical infections in swine at slaughterhouses have been reported in several industrial countries (6,7,9,10). However, elimination of the disease is very difficult if the breeding environment at the hog farm is contaminated by the pathogen. Therefore, the occurrence of mycobacteriosis causes continuous financial losses for hog farms (11). Mycobacterial lesions, localized primarily in the lymph nodes of the digestive tract, are frequently observed in nature and a number of systemically infected pigs have been identified in affected swine populations (3,9,10). Hepatic lesions are frequently observed in systemically infected individuals (12). Therefore, the tonsil and intestinal mucosa are thought to be the initial infective focus and sources of the bacterial excretion (13,14). The excretion of M. avium in the feces of pigs has also been documented (15,16). From such evidence, it was generally considered that swine are primarily infected by the ingestion of mycobacteria from the contaminated surrounding environment and lymphatic metastasis to parenchymal organs occurs through the intestinal epithelium (2,17). However, this has not been fully confirmed in pigs.
This is the first study to examine the relationship between the dissemination to other organs and the distribution of hepatic granulomatous lesions with M. avium infection. The findings of this investigation will help strengthen our understanding of the pathogenesis of disseminated M. avium infections in pigs.
Materials and methods
Materials for histological examination
Systemically infected pigs (n = 276) that were used in a previous study were examined in this study (12). All of the candidate pigs were reared on the Okinawa islands, approximately 500 km from the main islands of Japan. Most of the porcine breed was based on triple crossbred pigs (F1 hybrid Landrace × Duroc and their female parents Large-Yorkshire) or F1 (Berkshire × Duroc). A systemically infected pig was defined as a carcass with mycobacterial granulomatous lesions, as determined using histology, in the sub-maxillary or mesenteric lymph nodes, and any of the visceral organs. We excluded similar granulomatous lesions caused by Rhodococcus equi, Salmonella, toxoplasma, or ascarid through routine laboratory examination. The isolation of acid-fast bacilli was conducted using a previously described method (12). Mycobacterium avium infection was identified using DNA-DNA hybridization with the Amplicor Mycobacterium (Roche Diagnostic, Tokyo, Japan) and colonies isolated from affected organs. In addition, the isolates were examined by restriction fragment length polymorphism (RFLP) analysis of the insertion sequence (IS) 1245 and IS901. The tissues for histopathological examination were processed according to a generalized method; samples were fixed in 20% phosphate-buffered formalin, processed in paraffin wax, and sectioned into 4 μm thick slices. Sections were stained using hematoxylin and eosin (HE). The existence of acid-fast bacilli on these tissues was confirmed using Fite-Faraco staining. Cases with hepatic lesions confirmed to be caused by M. avium infection were included in this analysis. These porcine livers were used for analyzing the distribution of granulomatous lesions according to the zonal classification of hepatic parenchyma described by Rappaport (18) and the classification of hepatic lobules. The tissues were classified into 3 groups: group A — individuals with lesions localized in the portal area; group B — individuals with lesions localized in the both the portal area and the hepatic lobule; and group C — individuals with lesions localized in the hepatic lobule. The classifications were done by examining at least 2 different tissue sections from each pig. Granulomatous lesions were classified into 2 categories with reference to a previously described classification (12). Briefly, “poorly demarcated granuloma” was characterized by clusters of macrophages and lymphocytes without capsulization. “Well-formed granuloma” was characterized by proliferative reactions with capsulization. In addition, the basic histopathological changes in hepatic lesions were examined based on results of HE staining. Histological assessment was done by 2 pathologists and 2 practitioners specialized in hepatology.
Statistical analysis
Statistical analysis of the data was done using computer software (Statview-J5.0; Abacus Concept, Berkeley, California, USA). Comparisons between groups, such as the distribution of granulomatous lesions in porcine liver, were done using χ2 tests. Values of P < 0.01 were considered statistically significant.
Results
Identification of species
One hundred and twenty mycobacteria were isolated from the affected tissues of 276 systemically infected pigs. Of these, 119 strains of the positive culture were identified as M. avium hominisuiss, which had a high IS1245 copy number (between 6 and 28) but did not have IS901, based on the RFLP pattern (data not shown). Overall, the hepatic lesions were confirmed in 112 of 119 systemically infected swine.
Organ involvement
In 112 cases with hepatic lesions infected with M. avium, the appearance of lesions in other organs and regional lymph nodes were evaluated and shown in Table I. The involvement of the spleen (8.0%) and lungs (6.2%) was relatively low. The hepatic lymph nodes (80.4%) were the second most commonly involved lymph nodes after the mesenteric lymph nodes (98.2%). The involvement of pulmonary lymph nodes (43.8%) was comparatively high.
Table I.
Involvement of organs and lymph nodes of pigs with hepatic lesions caused by Mycobacterium avium infection
| Organ involved | Number of individuals | Percentagea |
|---|---|---|
| Spleen | 9 | 8.0 |
| Lungs | 7 | 6.2 |
| Hepatic LNs | 90 | 80.4 |
| Pulmonary LNs | 49 | 43.8 |
| Mesenteric LNs | 110 | 98.2 |
| Submandibular LNs | 60 | 53.6 |
| Inguinal LNs | 29 | 25.9 |
Percentage of the total number of included pigs (n = 112).
LNs — lymph nodes.
Distribution of granulomatous lesions in porcine liver
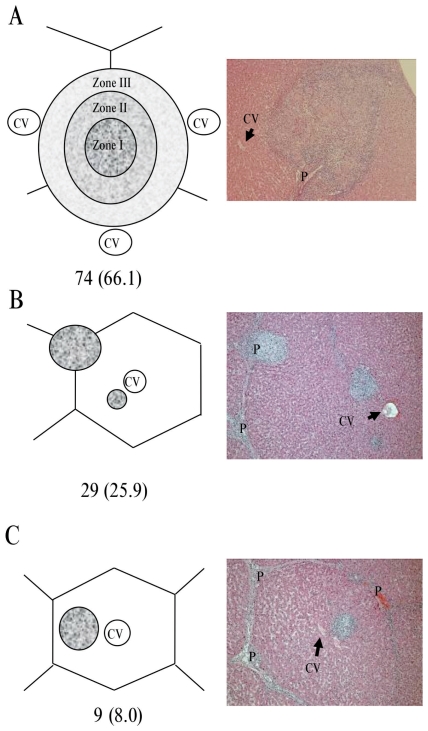
The localization of granulomatous lesions was described based on the classification of liver lobules. Most of the lesions were localized in the portal tract, but the remaining porcine tissues showed localization only in the hepatic lobules. Overall, 74 of 112 cases (66.1%) were classified in group A, 29 of 112 cases (25.9%) were classified in group B, and the remaining 9 of 112 cases (8.0%) were classified in group C (Figure 1).
Figure 1.
Classification scheme based on the distribution of the granulomatous lesions in relation to the hepatic acinar structure. A — Granuloma originating from the portal tract. Three zones are shown according to the zonal classification of hepatic parenchyma described by Rappaport (18). Granuloma localized in zone I exist in the portal tract without expansion. Granuloma localized in zones II and III are expanding from zone I. B — Co-localized granuloma originating from the portal tract and the hepatic lobule. C — Granuloma localized in the hepatic lobule. Each photo shows an example of each classification (×100). The numbers in parentheses show the percentage of the total number (n = 112) of samples evaluated. CV — central vein. P — portal tract.
Histological changes in the portal tract and hepatic lobule
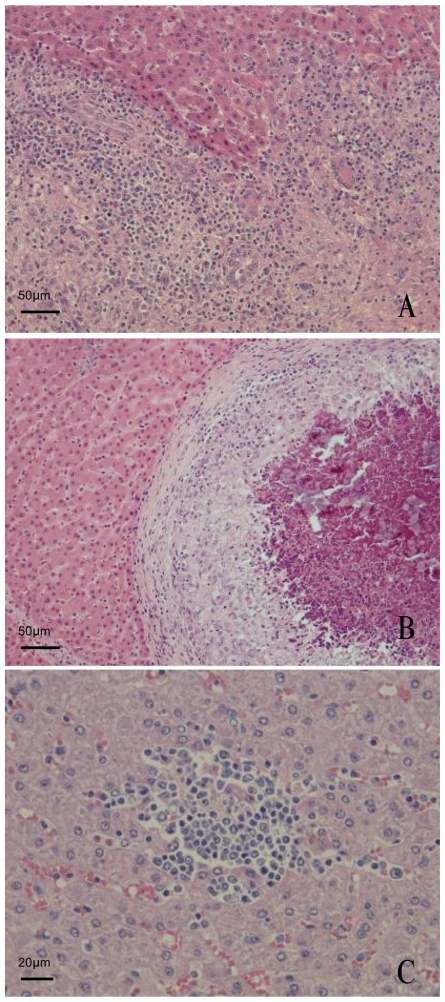
Two histological characteristics of granulomatous lesions were found in the liver: Poorly demarcated granuloma (Figure 2A) and well-formed granuloma (Figure 2B). In the lesions that showed poorly demarcated granuloma, interface hepatitis with infiltration of numerous eosinophils and lymphocytes was usually observed (Figure 2A). In the hepatic lobules, focal necrosis with infiltration of lymphocytes was frequently observed (Figure 2C). In the lesions that showed well-formed granulomas, the proliferative granulomas displaced the normal structure of the hepatic lobule with limiting plates (Figure 2B).
Figure 2.
Typical granulomatous lesions and focal necrosis in the porcine liver. A — Poorly demarcated granuloma in the portal tract. It should be noted that this represents interface hepatitis with infiltration of numerous eosinophils and lymphocytes. B — Well-formed granuloma in the hepatic lobule. Note, the surrounding sinusoids are dilated by compression of the around hepatic lobule structure with the limiting plate. C — Focal necrosis in the hepatic lobule. Some lymphocytes have aggregated without the appearance of epithelioid cells in the lobule.
Relationship between the presence of lesions in other tissues and the distribution of granulomatous lesions in the liver
We investigated the relationship between dissemination and distribution of the lesions in the liver. Groups B and C (shown in Figure 1) were considered to represent the distribution of lesions in the hepatic lobules in this analysis. Seventy-four samples were classified as group A and 38 samples as B and C. As a consequence, there was a significant difference between the distribution of granulomatous lesions in hepatic lobules and the involvement of the spleen, pulmonary lymph nodes, submandibular lymph nodes, and inguinal lymph nodes (Table II). However, there was no significant difference between the distribution of granulomatous lesions in hepatic lobules and involvement of the lungs, hepatic lymph nodes, and mesenteric lymph nodes (Table II).
Table II.
Relationships between the presence of lesions in other tissues and the distribution of granulomatous lesions in the porcine liver
| Organ involved | Distribution |
||
|---|---|---|---|
| A (n = 74) | B + C (n = 38) | P-value | |
| Spleen (n = 9) | 1 (1.4%) | 8 (21.1%) | < 0.01 |
| Lungs (n = 7) | 3 (4.1%) | 4 (10.5%) | 0.04 |
| Hepatic LNs (n = 90) | 59 (79.7%) | 31 (81.6%) | 0.62 |
| Pulmonary LNs (n = 49) | 23 (31.1%) | 26 (68.4%) | < 0.01 |
| Mesenteric LNs (n = 110) | 72 (97.3%) | 38 (100.0%) | — |
| Submandibular LNs (n = 60) | 35 (47.3%) | 25 (65.8%) | < 0.01 |
| Inguinal LNs (n = 29) | 14 (18.9%) | 15 (39.5%) | < 0.01 |
Numbers in parentheses are percentages of the total number of samples classified into each category based on the distribution of groups A and B + C, representing “A”, “B,” and “C” in Figure 1.
LNs — lymph nodes.
In the following analysis, the relationships between dissemination and infiltration of eosinophils (n = 75/112) or focal necrosis in lobules (n = 78/112) were examined (Table III). As a result, there were relationships between the involvement of each of the hepatic lymph nodes, pulmonary lymph nodes, mesenteric lymph nodes, and sub-mandibular lymph nodes and the infiltration of eosinophils within the hepatic lesion. In addition, there were relationships between the involvement of each of the spleen, hepatic lymph nodes, pulmonary lymph nodes, mesenteric lymph nodes, submandibular lymph nodes, and inguinal lymph nodes and focal necrosis in hepatic lobules.
Table III.
Relationships between the presence of lesions in other tissues and histological findings in the porcine liver
| Organ involved | Insertion of eosinophils (n = 75) |
Focal necrosis in lobules (n = 78) |
||||
|---|---|---|---|---|---|---|
| + | − | P-value | + | − | P-value | |
| Spleen (n = 9) | 6 (8.0%) | 3 (4.0%) | 0.04 | 8 (10.3%) | 1 (1.3%) | < 0.01 |
| Lungs (n = 7) | 5 (6.7%) | 2 (2.7%) | 0.01 | 4 (5.1%) | 3 (3.8%) | 0.50 |
| Hepatic LNs (n = 90) | 65 (86.7%) | 25 (33.3%) | < 0.01 | 66 (84.6%) | 24 (30.8%) | < 0.01 |
| Pulmonary LNs (n = 9) | 31 (41.3%) | 18 (24.0%) | < 0.001 | 36 (46.2%) | 13 (16.7%) | < 0.01 |
| Mesenteric LNs (n = 110) | 73 (97.3%) | 37 (49.3%) | < 0.001 | 74 (94.9%) | 36 (46.2%) | < 0.01 |
| Submandibular LNs (n = 60) | 39 (52.0%) | 21 (28.0%) | < 0.01 | 44 (56.4%) | 16 (20.5%) | < 0.01 |
| Inguinal LNs (n = 29) | 17 (22.7%) | 12 (16.0%) | 0.07 | 22 (28.2%) | 7 (9.0%) | < 0.01 |
Numbers in parentheses are percentages of the total number of histology findings.
LNs — lymph nodes.
Discussion
Previous studies have shown that the liver is the predominant target organ for MAC infection in pigs and human patients with immunodeficiencies (12,19–23). However, it is not clear why hepatic lesions are observed in most systemically infected individuals and how the organism spreads to the other internal organs from the liver or regional lymph nodes in the alimentary tract. We hypothesized that it would be possible to answer these questions by examining the distribution of hepatic lesions.
Some histopathological findings of hepatic granuloma in MAC infection have already been reported (24,25), but the distribution of hepatic lesions has not been described with great certainty. In this analysis using pigs, lesions were predominantly found in the portal tract, but the remaining cases had lesions within the hepatic lobule. Interestingly, a significant relationship was observed between focal lesions within the hepatic lobule and splenic lesions. Hersche et al (26) evaluated 200 cases of hepatic tuberculosis and reported that miliary or diffuse granulomas tend to be located inside hepatic lobules, whereas local forms are predominately located in the portal regions. Similarly, Oliva et al (27) reported that hepatic lesions are localized to the portal tract in patients with hepatic tuberculosis without obvious clinical evidence of pulmonary tuberculosis. These findings suggest that the lesions form in hepatic lobules upon establishment of bacteremia (28). Moreover, the lesions localized to the portal tract are formed once the organism reaches the liver via the portal vein (27). This perspective is supported by findings that the prevalence of hepatic lesions is similar to that in mesenteric lymph nodes (12). In addition, the findings that the lesions predominantly formed in portal tracts can be explained because the portal tract of the liver is a region with abundant capillary lymph vessels (29) and is a pathological niche that induces the initial antigen-specific immune response (30,31). Consequently, lesions in the portal tract might cause further dissemination to hepatic lymph nodes, a finding that may be supported by the evidence of frequent involvement of hepatic lymph nodes.
This study revealed significant associations between the appearance of eosinophils in the hepatic lesions and the dissemination to other organs. Histologically, the appearance of eosinophils and lymphocytes is a characteristic finding indicative of the exudative reaction phase in pigs with disseminated infection (12). Continuous inflammation around the portal tract causes interface hepatitis (previously known as piecemeal necrosis) with subsequent expansion of the portal area in human chronic hepatitis (32). Similar findings were observed in this study; namely, the destruction of the limiting plate by interface hepatitis and expanded granulomatous lesions with invasion into the lobule. Thus, enlargement of granulomatous lesions with interface hepatitis may be responsible for the hematogenous dissemination in pigs systemically infected with M. avium.
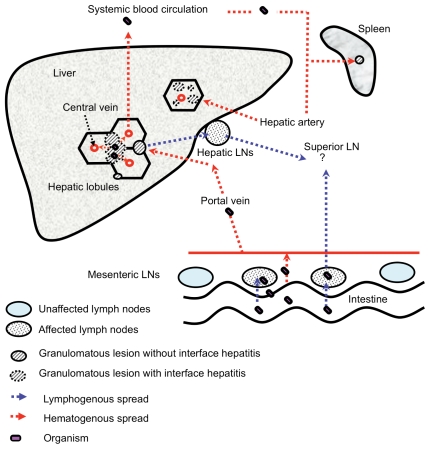
In immunocompromised patients, such as those with AIDS, retroperitoneal and mesenteric lymph nodes were the most commonly involved tissues (19–23). However, the existence of retroperitoneal lymphadenitis has not been reported in pigs (12). Recently, it was shown that, in orally infected mice, the bacteria remain viable in mesenteric lymph nodes after exposure to a small oral inoculum of mycobacteria, and can disseminate later (33). According to evidence obtained from such mouse models and histological analysis of autopsy cases of AIDS patients, bacilli from the mesenteric lymph nodes of early lesions spread via the lymphatic system to the superior lymph nodes and then to the blood circulation as immune function declines (2,34). In pigs experimentally infected orally with M. avium, such pathogenesis was not observed (13,14). Thus, further dissemination from the mesenteric lymph nodes may not be a common occurrence in pigs, although lymphatic dissemination has been considered to be the primary pathway for distribution throughout the abdominal cavity in humans. In conclusion, we believe that bacterial spread from hepatic lesions is a more critical event than dissemination from the regional lymph nodes of the alimentary tract in pigs. This pathogenesis is summarized in Figure 3.
Figure 3.
Proposed mechanism for the dissemination of M. avium infection in pigs. This figure illustrates the proposed pathogenesis of disseminated M. avium infection in pigs based on our results and the results of prior studies. The organism reaches the intestinal mucosa after ingestion of the organism. The organism may then infect epithelial cells and may be transported to mesenteric lymph nodes by phagocytic cells (2). Further spread through the lymphatic system to the superior deep lymph nodes may occur in rare instances. Meanwhile, some organisms reach the liver through the portal vein. In the liver, granulomas are predominantly formed in portal tracts. Persistent inflammatory reactions to the organisms in the portal tract cause destruction of the limiting plate and the organism can enter the blood stream in the hepatic lobule. Then, the organism spreads through the blood circulation to reticuloendothelial organs such as the spleen or bone marrow. Many granulomatous lesions are formed secondarily in the hepatic lobules by hematogenous seeding. LNs — lymph nodes.
The limitation of this study is that the hypothesis is based on histopathological observation. Further studies with tracer systems, such as quantitative assays with fluorescently labeled organisms (35), are warranted to test this hypothesis.
Acknowledgments
The authors thank all staff involved in the inspections of the porcine carcasses.
References
- 1.Biet F, Boschiroli ML, Thorel MF, Guilloteau LA. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC) Vet Res. 2005;36:411–436. doi: 10.1051/vetres:2005001. [DOI] [PubMed] [Google Scholar]
- 2.Horsburgh CR., Jr The pathophysiology of disseminated Mycobacterium avium complex disease in AIDS. J Infect Dis. 1999;179:461–465. doi: 10.1086/314804. [DOI] [PubMed] [Google Scholar]
- 3.Thoen CO. Mycobacterium avium infections in animals. Res Microbiol. 1994;145:173–177. doi: 10.1016/0923-2508(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 4.Johne HA, Frothinghan L. Ein eigenthuemlicher tall van tuberckulose beim rind. Dtsch Z Tiermed Pathol. 1895;21:438–454. [Google Scholar]
- 5.McDiarmid A. The occurrence of tuberculosis in the wild wood-pigeon. J Comp Pathol. 1948;58:128–133. doi: 10.1016/s0368-1742(48)80010-x. [DOI] [PubMed] [Google Scholar]
- 6.Matlova L, Dvorska L, Palecek K, Maurenc L, Bartos M, Pavlik I. Impact of sawdust and wood shavings in bedding on pig tuberculous lesions in lymph nodes, and IS1245 RFLP analysis of Mycobacterium avium subsp. hominissuis of serotypes 6 and 8 isolated from pigs and environment. Vet Microbiol. 2004;102:227–236. doi: 10.1016/j.vetmic.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Johansen TB, Olsen I, Jensen MR, Dahle UR, Holstad G, Djønne B. New probes used for IS1245 and IS1311 restriction fragment length polymorphism of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis isolates of human and animal origin in Norway. BMC Microbiol. 2007;7:14. doi: 10.1186/1471-2180-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingos M, Amado A, Botelho A. IS1245 RFLP analysis of strains of Mycobacterium avium subspecies hominissuis isolated from pigs with tuberculosis lymphadenitis in Portugal. Vet Rec. 2009;164:116–120. doi: 10.1136/vr.164.4.116. [DOI] [PubMed] [Google Scholar]
- 9.Komijn RE, de Haas PE, Schneider MM, et al. Prevalence of Mycobacterium avium in slaughter pigs in The Netherlands and comparison of IS1245 restriction fragment length polymorphism patterns of porcine and human isolates. J Clin Microbiol. 1999;37:1254–1259. doi: 10.1128/jcm.37.5.1254-1259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibiya K, Kasumi Y, Nishiuchi Y, et al. Descriptive analysis of the prevalence and the molecular epidemiology of pigs infected with Mycobacterium avium complex that were slaughtered on the Okinawa main islands. Comp Immunol Microbiol Infect Dis. 2010 doi: 10.1016/j.cimid.2009.03.002. (In press) [DOI] [PubMed] [Google Scholar]
- 11.Pavlik I, Matlova L, Dvorska L, et al. Tuberculous lesions in pigs in the Czech Republic during 1990–1999: Occurrence, causal factors and economic losses. Veterinarni Medicina. 2003;48:113–125. [Google Scholar]
- 12.Hibiya K, Kasumi Y, Sugawara I, Fujita J. Histopathological classification of systemic Mycobacterium avium complex infections in slaughtered domestic pigs. Comp Immunol Microbiol Infect Dis. 2008;31:347–366. doi: 10.1016/j.cimid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Acland HM, Whitlock RH. Mycobacterium avium serotype 4 infection of swine: The attempted transmission by contact and the sequence of morphological changes in inoculated pigs. J Comp Pathol. 1986;96:247–266. doi: 10.1016/0021-9975(86)90045-9. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Yokomizo Y, Okutomo M, Nishimori K, Yugi H, Shoya S. Light and electron microscopic observations on granulomatous lesions in pigs dosed with Mycobacterium intracellulare. J Comp Pathol. 1984;94:509–519. doi: 10.1016/0021-9975(84)90055-0. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen JB. Experimental infection with Mycobacterium avium, serotype 2, in pigs. 4. Contact infection from orally inoculated pigs. Acta Vet Scand. 1978;19:58–72. doi: 10.1186/BF03547642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellsworth SR, Kirkbride CA, Johnson DD. Excretion of Mycobacterium avium from lesions in the intestine and tonsils of infected swine. Am J Vet Res. 1980;41:1526–1530. [PubMed] [Google Scholar]
- 17.Hibiya K, Higa F, Tateyama M, Fujita J. The pathogenesis and the development mechanism of Mycobacterium avium complex infection. Kekkaku. 2007;82:903–918. [PubMed] [Google Scholar]
- 18.Rappaport AM, Borowy ZJ, Lougheed WM, Lotto WN. Subdivision of hexagonal liver lobules into a structural and functional unit. Role in hepatic physiology and pathology. Anat Rec. 1954;119:11–33. doi: 10.1002/ar.1091190103. [DOI] [PubMed] [Google Scholar]
- 19.Torriani FJ, Behling CA, McCutchan JA, Haubrich RH, Havlir DV. Disseminated Mycobacterium avium complex: Correlation between blood and tissue burden. J Infect Dis. 1996;173:942–949. doi: 10.1093/infdis/173.4.942. [DOI] [PubMed] [Google Scholar]
- 20.Torriani FJ, McCutchan JA, Bozzette SA, Grafe MR, Havlir DV. Autopsy findings in AIDS patients with Mycobacterium avium complex bacteremia. J Infect Dis. 1994;170:1601–1605. doi: 10.1093/infdis/170.6.1601. [DOI] [PubMed] [Google Scholar]
- 21.Klatt EC, Jensen DF, Meyer PR. Pathology of Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome. Hum Pathol. 1987;18:709–714. doi: 10.1016/s0046-8177(87)80242-3. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Dayem HM, Omar WS, Aziz M, et al. Disseminated Mycobacterium avium complex. Review of Ga-67 and TI-201 scans and autopsy findings. Clin Nucl Med. 1996;21:547–556. doi: 10.1097/00003072-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Modilevsky T, Sattler FR, Barnes PF. Mycobacterial disease in patients with human immunodeficiency virus infection. Arch Intern Med. 1989;149:2201–2205. [PubMed] [Google Scholar]
- 24.Orenstein MS, Tavitian A, Yonk B, et al. Granulomatous involvement of the liver in patients with AIDS. Gut. 1985;26:1220–1225. doi: 10.1136/gut.26.11.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klatt EC, Jensen DF, Meyer PR. Pathology of Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome. Hum Pathol. 1987;18:709–714. doi: 10.1016/s0046-8177(87)80242-3. [DOI] [PubMed] [Google Scholar]
- 26.Hersch C. Tuberculosis of the liver. A study of 200 cases. S Afr Med J. 1964;38:857–863. [PubMed] [Google Scholar]
- 27.Oliva A, Duarte B, Jonasson O, Nadimpalli V. The nodular form of local hepatic tuberculosis. A review. J Clin Gastroenterol. 1990;12:166–173. doi: 10.1097/00004836-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Huang WT, Wang CC, Chen WJ, Cheng YF, Eng HL. The nodular form of hepatic tuberculosis: A review with five additional new cases. J Clin Pathol. 2003;56:835–839. doi: 10.1136/jcp.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec. 2008;291:643–652. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 30.Yoneyama H, Matsuno K, Zhang Y, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract–associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193:35–50. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuno K, Ezaki T. Dendritic cell dynamics in the liver and hepatic lymph. Int Rev Cytol. 2000;197:83–136. doi: 10.1016/s0074-7696(00)97003-7. [DOI] [PubMed] [Google Scholar]
- 32.Fontaine H, Nalpas B, Poulet B, et al. Hepatitis activity index is a key factor in determining the natural history of chronic hepatitis C. Human Pathology. 2001;32:904–909. doi: 10.1053/hupa.2001.28228. [DOI] [PubMed] [Google Scholar]
- 33.Petrofsky M, Bermudez LE. CD4+ T cells but Not CD8+ or gammadelta+ lymphocytes are required for host protection against Mycobacterium avium infection and dissemination through the intestinal route. Infect Immun. 2005;73:2621–2627. doi: 10.1128/IAI.73.5.2621-2627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bermudez LE, Wagner D, Sosnowska D. Mechanisms of Mycobacterium avium pathogenesis. Arch Immunol Ther Exp (Warsz) 2000;48:521–527. [PubMed] [Google Scholar]
- 35.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]