Abstract
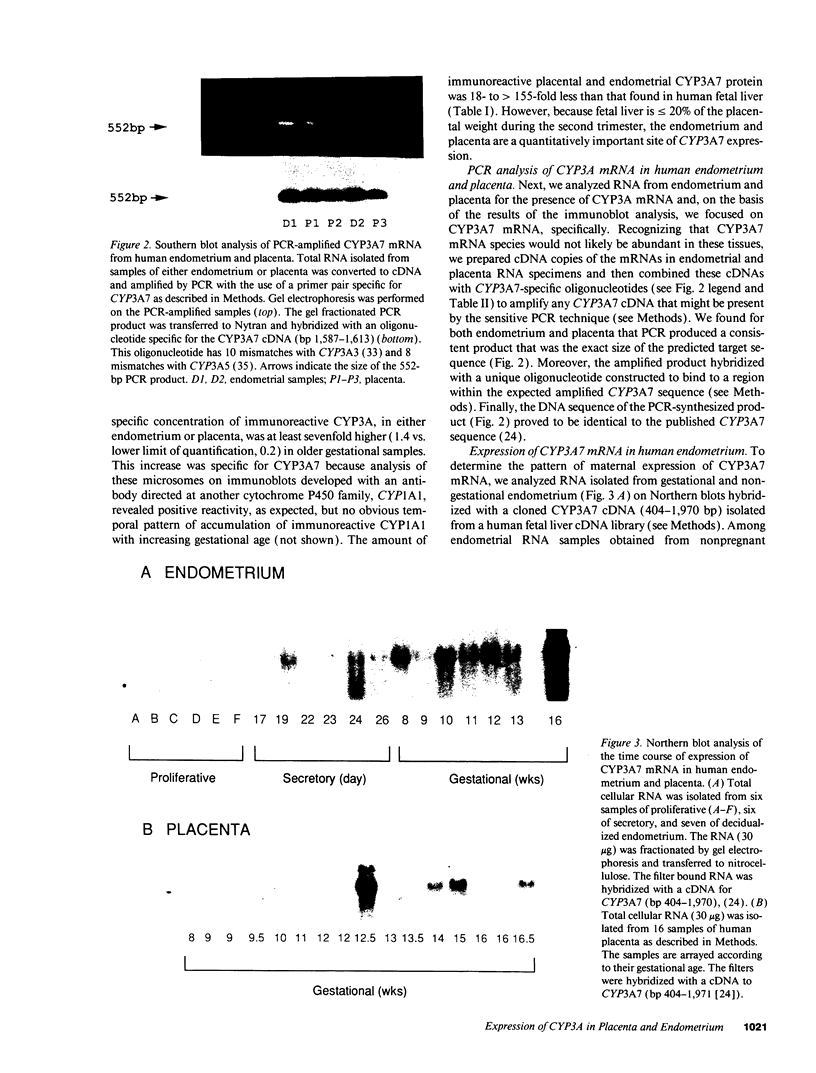
Placenta and endometrium carry out steroidogenic biotransformation reactions such as 6-beta-hydroxylation of cortisol, a reaction characteristic of the dominant family of cytochromes P450 in human liver, CYP3A. To investigate the possible role in these extrahepatic tissues of the CYP3A microsomal hemoproteins, we analyzed placental and endometrial microsomes on Western blots developed with an anti-human CYP3A antibody. We found an immunoreactive 51,500 D protein that migrated between CYP3A3 (HLp) and CYP3A5 (HLp2) identical with CYP3A7 (HFLa). CYP3A7, a form found prominently in human fetal liver microsomes, was first isolated as a liver 16-alpha-dehydroepiandrosterone-sulfate hydroxylase. Northern blot analysis of total RNA isolated from placenta or from endometrium demonstrated a single band that cross-hybridized with a CYP3A7 cDNA. Amplification of the same RNA samples with the use of primers specific for CYP3A7, produced a 552-bp segment that had the predicted size and the same DNA sequence as does liver CYP3A7 cDNA. Hybridizable endometrial CYP3A7 mRNA was detected more frequently (six of seven samples) and in higher amounts (approximately 12-fold higher) in pregnant compared with nonpregnant women (4 of 12 samples). In addition, during the secretory phase of the menstrual cycle CYP3A7 expression was sixfold higher than in the one sample from the proliferative phase that had detectable CYP3A7 mRNA. Moreover, the amounts of placental and endometrial CYP3A7 mRNA and protein increased substantially from the first to the second trimester of pregnancy. We conclude that placenta and endometrium express the same P450 as is found in fetal liver. These tissues represent a previously unrecognized and quantitatively important site for 6-beta-hydroxylation and 16-alpha-hydroxylation of specific steroid precursors, possibly for protection of the fetus from the toxic effects of endogenous steroids and foreign substrates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLINER D. L., SALHANICK H. A. The presence of 6-beta-hydroxylase in human placenta. J Clin Endocrinol Metab. 1956 Jul;16(7):903–905. doi: 10.1210/jcem-16-7-903. [DOI] [PubMed] [Google Scholar]
- Beaune P. H., Umbenhauer D. R., Bork R. W., Lloyd R. S., Guengerich F. P. Isolation and sequence determination of a cDNA clone related to human cytochrome P-450 nifedipine oxidase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8064–8068. doi: 10.1073/pnas.83.21.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork R. W., Muto T., Beaune P. H., Srivastava P. K., Lloyd R. S., Guengerich F. P. Characterization of mRNA species related to human liver cytochrome P-450 nifedipine oxidase and the regulation of catalytic activity. J Biol Chem. 1989 Jan 15;264(2):910–919. [PubMed] [Google Scholar]
- Branchaud C. L., Goodyer C. G., Lipowski L. S. Progesterone and estrogen production by placental monolayer cultures: effect of dehydroepiandrosterone and luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1983 Apr;56(4):761–766. doi: 10.1210/jcem-56-4-761. [DOI] [PubMed] [Google Scholar]
- Burger H. J., Schuetz J. D., Schuetz E. G., Guzelian P. S. Paradoxical transcriptional activation of rat liver cytochrome P-450 3A1 by dexamethasone and the antiglucocorticoid pregnenolone 16 alpha-carbonitrile: analysis by transient transfection into primary monolayer cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2145–2149. doi: 10.1073/pnas.89.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Cresteil T., Beaune P., Kremers P., Flinois J. P., Leroux J. P. Drug-metabolizing enzymes in human foetal liver: partial resolution of multiple cytochromes P 450. Pediatr Pharmacol (New York) 1982;2(3):199–207. [PubMed] [Google Scholar]
- Csaba G., Inczefi-Gonda A. Specificity of the dexamethasone-induced steroid receptor in Tetrahymena. Experientia. 1989 Feb 15;45(2):174–175. doi: 10.1007/BF01954865. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre G., Crevat-Pisano P., Dragna S., Covo J., Barra Y., Cano J. P. Involvement of the macrolide antibiotic inducible cytochrome P-450 LM3c in the metabolism of midazolam by microsomal fractions prepared from rabbit liver. Biochem Pharmacol. 1988 May 15;37(10):1947–1953. doi: 10.1016/0006-2952(88)90541-2. [DOI] [PubMed] [Google Scholar]
- Ged C., Rouillon J. M., Pichard L., Combalbert J., Bressot N., Bories P., Michel H., Beaune P., Maurel P. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989 Oct;28(4):373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Martin M. V., Beaune P. H., Kremers P., Wolff T., Waxman D. J. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1986 Apr 15;261(11):5051–5060. [PubMed] [Google Scholar]
- Guengerich F. P., Müller-Enoch D., Blair I. A. Oxidation of quinidine by human liver cytochrome P-450. Mol Pharmacol. 1986 Sep;30(3):287–295. [PubMed] [Google Scholar]
- Herrington D. M., Gordon G. B., Achuff S. C., Trejo J. F., Weisman H. F., Kwiterovich P. O., Jr, Pearson T. A. Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. J Am Coll Cardiol. 1990 Nov;16(6):862–870. doi: 10.1016/s0735-1097(10)80334-1. [DOI] [PubMed] [Google Scholar]
- Hostetler K. A., Wrighton S. A., Molowa D. T., Thomas P. E., Levin W., Guzelian P. S. Coinduction of multiple hepatic cytochrome P-450 proteins and their mRNAs in rats treated with imidazole antimycotic agents. Mol Pharmacol. 1989 Mar;35(3):279–285. [PubMed] [Google Scholar]
- Kauma S., Matt D., Strom S., Eierman D., Turner T. Interleukin-1 beta, human leukocyte antigen HLA-DR alpha, and transforming growth factor-beta expression in endometrium, placenta, and placental membranes. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1430–1437. doi: 10.1016/0002-9378(90)90601-3. [DOI] [PubMed] [Google Scholar]
- Kauppila A., Tuimala R., Ylikorkala O., Reinilä M., Ylöstalo P. Placental steroid synthesis from DHEAS during dexamethasone therapy. Obstet Gynecol. 1979 Jul;54(1):39–42. doi: 10.1097/00006250-197907000-00010. [DOI] [PubMed] [Google Scholar]
- Kitada M., Igoshi N., Kamataki T., Itahashi K., Imaoka S., Komori M., Funae Y., Rikihisa T., Kanakubo Y. Immunochemical similarity of P-450 HFLa, a form of cytochrome P-450 in human fetal livers, to a form of rat liver cytochrome P-450 inducible by macrolide antibiotics. Arch Biochem Biophys. 1988 Jul;264(1):61–66. doi: 10.1016/0003-9861(88)90570-x. [DOI] [PubMed] [Google Scholar]
- Kitada M., Kamataki T., Itahashi K., Rikihisa T., Kanakubo Y. P-450 HFLa, a form of cytochrome P-450 purified from human fetal livers, is the 16 alpha-hydroxylase of dehydroepiandrosterone 3-sulfate. J Biol Chem. 1987 Oct 5;262(28):13534–13537. [PubMed] [Google Scholar]
- Kitada M., Kamataki T., Itahashi K., Rikihisa T., Kanakubo Y. Significance of cytochrome P-450 (P-450 HFLa) of human fetal livers in the steroid and drug oxidations. Biochem Pharmacol. 1987 Feb 15;36(4):453–456. doi: 10.1016/0006-2952(87)90350-9. [DOI] [PubMed] [Google Scholar]
- Kitada M., Kamataki T. Partial purification and properties of cytochrome P450 from homogenates of human fetal livers. Biochem Pharmacol. 1979 Mar 15;28(6):793–797. doi: 10.1016/0006-2952(79)90360-5. [DOI] [PubMed] [Google Scholar]
- Kitada M., Taneda M., Ohi H., Komori M., Itahashi K., Nagao M., Kamataki T. Mutagenic activation of aflatoxin B1 by P-450 HFLa in human fetal livers. Mutat Res. 1989 Sep;227(1):53–58. doi: 10.1016/0165-7992(89)90068-7. [DOI] [PubMed] [Google Scholar]
- Kitada M., Taneda M., Ohta K., Nagashima K., Itahashi K., Kamataki T. Metabolic activation of aflatoxin B1 and 2-amino-3-methylimidazo[4,5-f]-quinoline by human adult and fetal livers. Cancer Res. 1990 May 1;50(9):2641–2645. [PubMed] [Google Scholar]
- Ko M. S., Nakauchi H., Takahashi N. The dose dependence of glucocorticoid-inducible gene expression results from changes in the number of transcriptionally active templates. EMBO J. 1990 Sep;9(9):2835–2842. doi: 10.1002/j.1460-2075.1990.tb07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori M., Nishio K., Fujitani T., Ohi H., Kitada M., Mima S., Itahashi K., Kamataki T. Isolation of a new human fetal liver cytochrome P450 cDNA clone: evidence for expression of a limited number of forms of cytochrome P450 in human fetal livers. Arch Biochem Biophys. 1989 Jul;272(1):219–225. doi: 10.1016/0003-9861(89)90213-0. [DOI] [PubMed] [Google Scholar]
- Komori M., Nishio K., Kitada M., Shiramatsu K., Muroya K., Soma M., Nagashima K., Kamataki T. Fetus-specific expression of a form of cytochrome P-450 in human livers. Biochemistry. 1990 May 8;29(18):4430–4433. doi: 10.1021/bi00470a024. [DOI] [PubMed] [Google Scholar]
- Komori M., Nishio K., Ohi H., Kitada M., Kamataki T. Molecular cloning and sequence analysis of cDNA containing the entire coding region for human fetal liver cytochrome P-450. J Biochem. 1989 Feb;105(2):161–163. doi: 10.1093/oxfordjournals.jbchem.a122632. [DOI] [PubMed] [Google Scholar]
- LAMBERT M., PENNINGTON G. W. THE ESTIMATION OF POLAR STEROIDS IN LIQUOR AMNII. J Endocrinol. 1965 Jul;32:287–293. doi: 10.1677/joe.0.0320287. [DOI] [PubMed] [Google Scholar]
- LIPMAN M. M., KATZ F. H., JAILER J. W. An alternate pathway for cortisol metabolism. 6 beta-hydroxycortisol production by human tissue slices. J Clin Endocrinol Metab. 1962 Mar;22:268–272. doi: 10.1210/jcem-22-3-268. [DOI] [PubMed] [Google Scholar]
- Ladona M. G., Spalding D. J., Ekman L., Linström B., Rane A. Human fetal and adult liver metabolism of ethylmorphine. Relation to immunodetected cytochrome P-450 PCN and interactions with important fetal corticosteroids. Biochem Pharmacol. 1989 Oct 1;38(19):3147–3155. doi: 10.1016/0006-2952(89)90607-2. [DOI] [PubMed] [Google Scholar]
- McManus M. E., Burgess W. M., Veronese M. E., Huggett A., Quattrochi L. C., Tukey R. H. Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res. 1990 Jun 1;50(11):3367–3376. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mohan P. F., Cleary M. P. Dehydroepiandrosterone and related steroids inhibit mitochondrial respiration in vitro. Int J Biochem. 1989;21(10):1103–1107. doi: 10.1016/0020-711x(89)90050-5. [DOI] [PubMed] [Google Scholar]
- Molowa D. T., Schuetz E. G., Wrighton S. A., Watkins P. B., Kremers P., Mendez-Picon G., Parker G. A., Guzelian P. S. Complete cDNA sequence of a cytochrome P-450 inducible by glucocorticoids in human liver. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5311–5315. doi: 10.1073/pnas.83.14.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Rane A. Epoxide hydrolase in human placenta at different stages of pregnancy. Dev Pharmacol Ther. 1983;6(2):83–93. doi: 10.1159/000457282. [DOI] [PubMed] [Google Scholar]
- Pasanen M., Pelkonen O. Human placental xenobiotic and steroid biotransformations catalyzed by cytochrome P450, epoxide hydrolase, and glutathione S-transferase activities and their relationships to maternal cigarette smoking. Drug Metab Rev. 1989;21(3):427–461. doi: 10.3109/03602538909030305. [DOI] [PubMed] [Google Scholar]
- Pichard L., Fabre I., Fabre G., Domergue J., Saint Aubert B., Mourad G., Maurel P. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990 Sep-Oct;18(5):595–606. [PubMed] [Google Scholar]
- Powell W. A., Mitchell B. F., Challis J. R. Effects of steroids on progesterone output by explants of human chorion. Gynecol Obstet Invest. 1986;22(2):64–72. doi: 10.1159/000298893. [DOI] [PubMed] [Google Scholar]
- Rabe T., Hösch R., Runnebaum B. Diagnosis of intrauterine fetal growth retardation (IUGR) and placental insufficiency by a dehydroepiandrosterone sulfate (DHAS) loading test. Biol Res Pregnancy Perinatol. 1983;4(3):130–136. [PubMed] [Google Scholar]
- Romano W. M., Lukash L. A., Challis J. R., Mitchell B. F. Substrate utilization for estrogen synthesis by human fetal membranes and decidua. Am J Obstet Gynecol. 1986 Dec;155(6):1170–1175. doi: 10.1016/0002-9378(86)90139-0. [DOI] [PubMed] [Google Scholar]
- Sakyo K., Ito A., Mori Y. Dehydroepiandrosterone sulfate stimulates collagenase synthesis without affecting the rates of collagen and noncollagen protein syntheses by rabbit uterine cervical fibroblasts. Biol Reprod. 1987 Mar;36(2):277–281. doi: 10.1095/biolreprod36.2.277. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz E. G., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. II. Evidence that glucocorticoids regulate induction of cytochrome P-450 by a nonclassical receptor mechanism. J Biol Chem. 1984 Feb 10;259(3):2007–2012. [PubMed] [Google Scholar]
- Schuetz E. G., Wrighton S. A., Safe S. H., Guzelian P. S. Regulation of cytochrome P-450p by phenobarbital and phenobarbital-like inducers in adult rat hepatocytes in primary monolayer culture and in vivo. Biochemistry. 1986 Mar 11;25(5):1124–1133. doi: 10.1021/bi00353a027. [DOI] [PubMed] [Google Scholar]
- Schuetz J. D., Molowa D. T., Guzelian P. S. Characterization of a cDNA encoding a new member of the glucocorticoid-responsive cytochromes P450 in human liver. Arch Biochem Biophys. 1989 Nov 1;274(2):355–365. doi: 10.1016/0003-9861(89)90449-9. [DOI] [PubMed] [Google Scholar]
- Schulz S., Klann R. C., Schönfeld S., Nyce J. W. Mechanisms of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells by dehydroepiandrosterone: role of isoprenoid biosynthesis. Cancer Res. 1992 Mar 1;52(5):1372–1376. [PubMed] [Google Scholar]
- Shimada T., Guengerich F. P. Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A. 1989 Jan;86(2):462–465. doi: 10.1073/pnas.86.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Iwasaki M., Martin M. V., Guengerich F. P. Human liver microsomal cytochrome P-450 enzymes involved in the bioactivation of procarcinogens detected by umu gene response in Salmonella typhimurium TA 1535/pSK1002. Cancer Res. 1989 Jun 15;49(12):3218–3228. [PubMed] [Google Scholar]
- Stehr-Green P. A., Welty E., Steele G., Steinberg K. Evaluation of potential health effects associated with serum polychlorinated biphenyl levels. Environ Health Perspect. 1986 Dec;70:255–259. doi: 10.1289/ehp.8670255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey R. H., Okino S., Barnes H., Griffin K. J., Johnson E. F. Multiple gene-like sequences related to the rabbit hepatic progesterone 21-hydroxylase cytochrome P-450 1. J Biol Chem. 1985 Oct 25;260(24):13347–13354. [PubMed] [Google Scholar]
- Watkins P. B., Wrighton S. A., Maurel P., Schuetz E. G., Mendez-Picon G., Parker G. A., Guzelian P. S. Identification of an inducible form of cytochrome P-450 in human liver. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6310–6314. doi: 10.1073/pnas.82.18.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins P. B., Wrighton S. A., Schuetz E. G., Maurel P., Guzelian P. S. Macrolide antibiotics inhibit the degradation of the glucocorticoid-responsive cytochrome P-450p in rat hepatocytes in vivo and in primary monolayer culture. J Biol Chem. 1986 May 15;261(14):6264–6271. [PubMed] [Google Scholar]
- Waxman D. J., Attisano C., Guengerich F. P., Lapenson D. P. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 beta-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988 Jun;263(2):424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Ram P. A., Notani G., LeBlanc G. A., Alberta J. A., Morrissey J. J., Sundseth S. S. Pituitary regulation of the male-specific steroid 6 beta-hydroxylase P-450 2a (gene product IIIA2) in adult rat liver. Suppressive influence of growth hormone and thyroxine acting at a pretranslational leve;. Mol Endocrinol. 1990 Mar;4(3):447–454. doi: 10.1210/mend-4-3-447. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Maurel P., Schuetz E. G., Watkins P. B., Young B., Guzelian P. S. Identification of the cytochrome P-450 induced by macrolide antibiotics in rat liver as the glucocorticoid responsive cytochrome P-450p. Biochemistry. 1985 Apr 23;24(9):2171–2178. doi: 10.1021/bi00330a010. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Molowa D. T., Guzelian P. S. Identification of a cytochrome P-450 in human fetal liver related to glucocorticoid-inducible cytochrome P-450HLp in the adult. Biochem Pharmacol. 1988 Aug 1;37(15):3053–3055. doi: 10.1016/0006-2952(88)90299-7. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Ring B. J., Watkins P. B., VandenBranden M. Identification of a polymorphically expressed member of the human cytochrome P-450III family. Mol Pharmacol. 1989 Jul;36(1):97–105. [PubMed] [Google Scholar]
- Wrighton S. A., Thomas P. E., Willis P., Maines S. L., Watkins P. B., Levin W., Guzelian P. S. Purification of a human liver cytochrome P-450 immunochemically related to several cytochromes P-450 purified from untreated rats. J Clin Invest. 1987 Oct;80(4):1017–1022. doi: 10.1172/JCI113154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton S. A., Vandenbranden M. Isolation and characterization of human fetal liver cytochrome P450HLp2: a third member of the P450III gene family. Arch Biochem Biophys. 1989 Jan;268(1):144–151. doi: 10.1016/0003-9861(89)90575-4. [DOI] [PubMed] [Google Scholar]
- Yamazoe Y., Murayama N., Shimada M., Kato R. Thyroid hormone suppression of hepatic levels of phenobarbital-inducible P-450b and P-450e and other neonatal P-450s in hypophysectomized rats. Biochem Biophys Res Commun. 1989 Apr 28;160(2):609–614. doi: 10.1016/0006-291x(89)92476-5. [DOI] [PubMed] [Google Scholar]