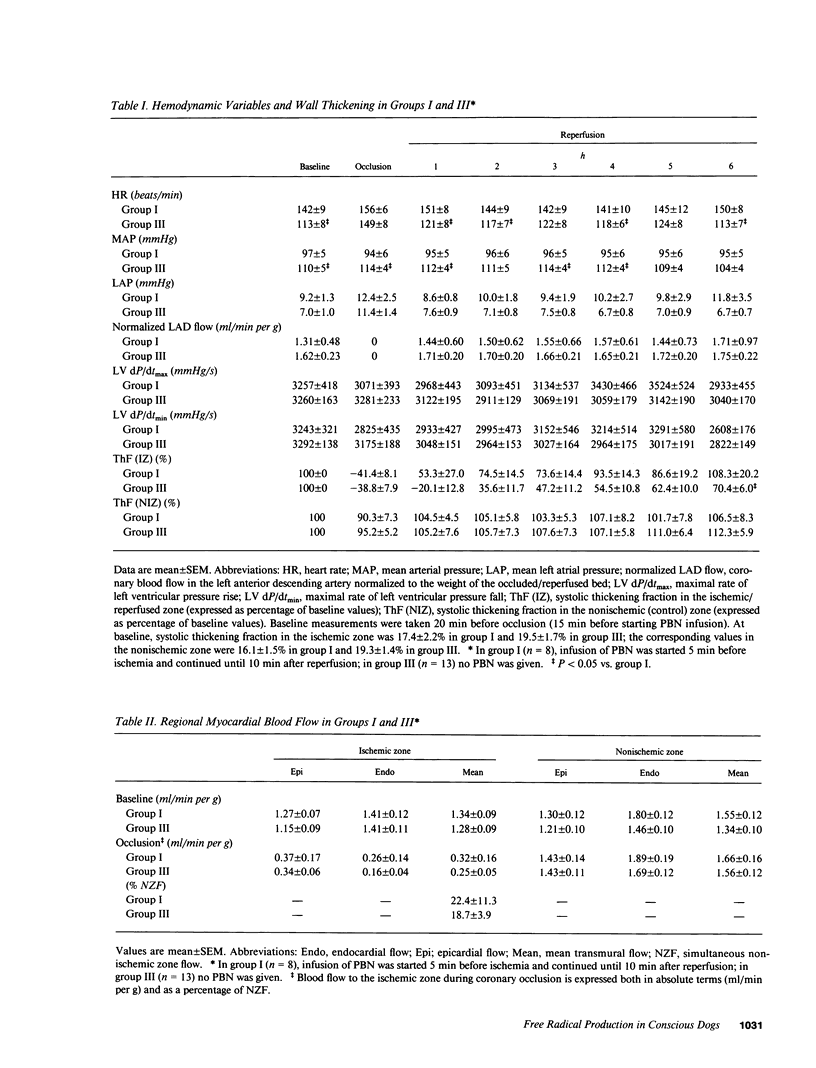
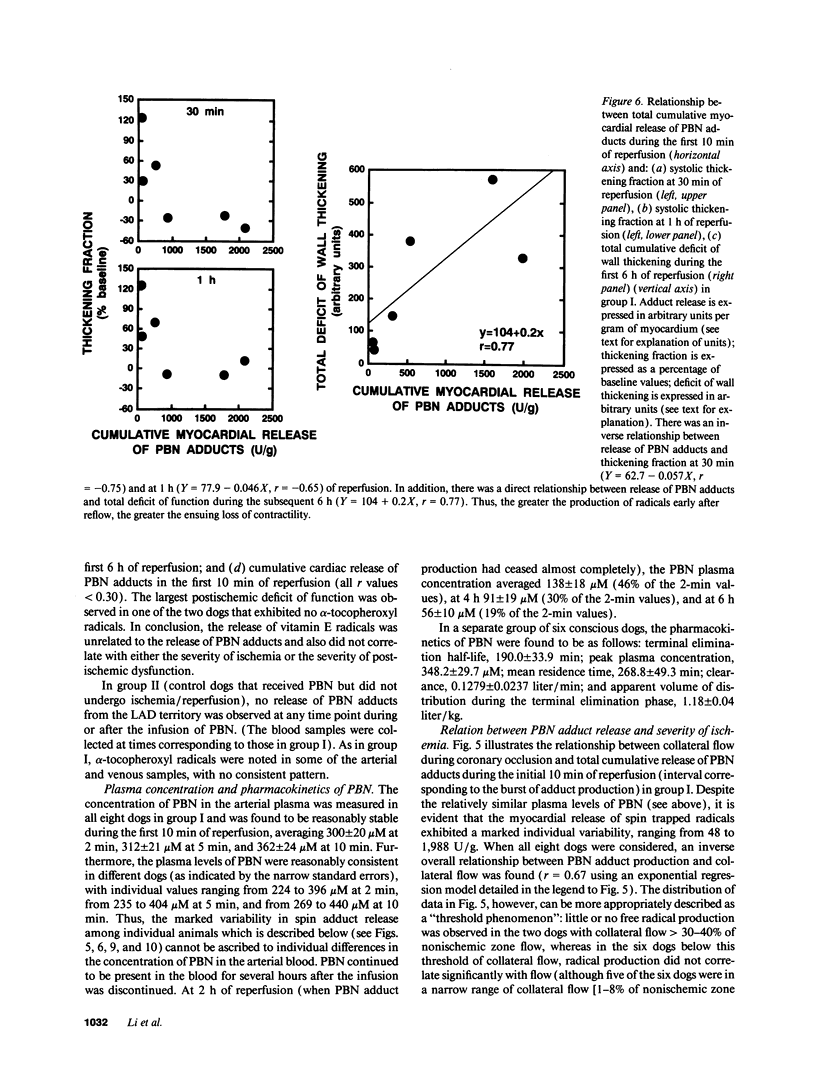
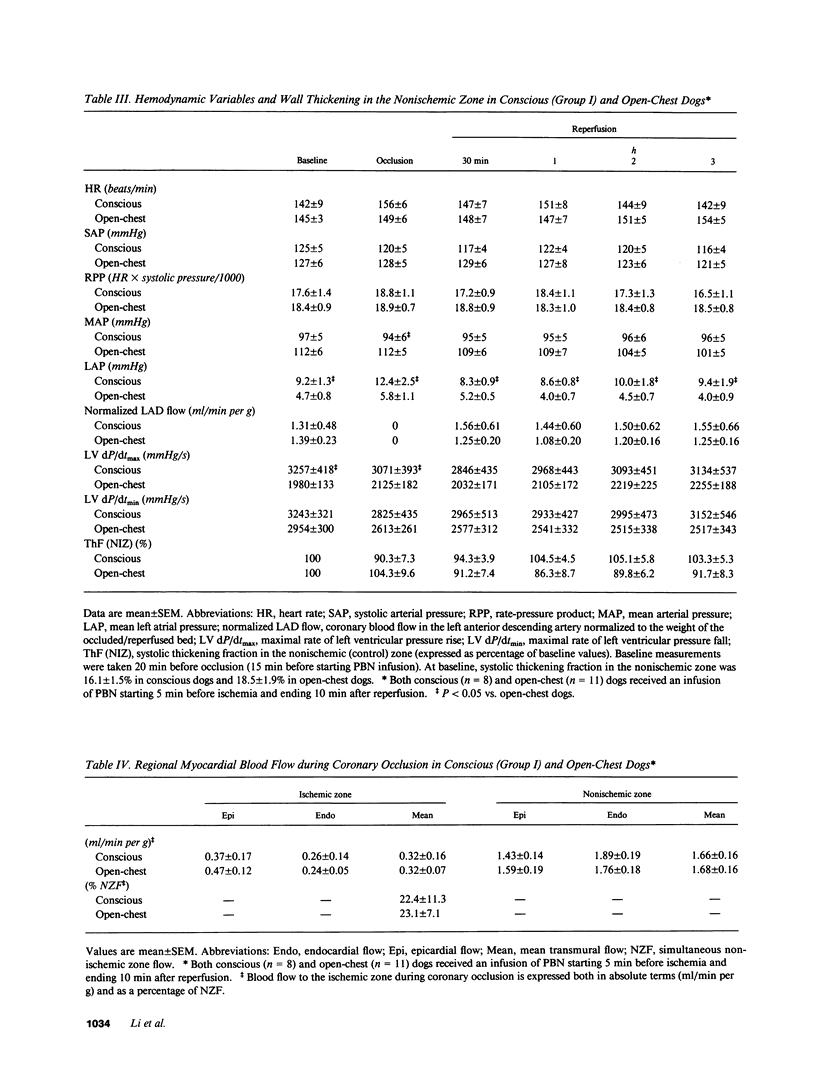
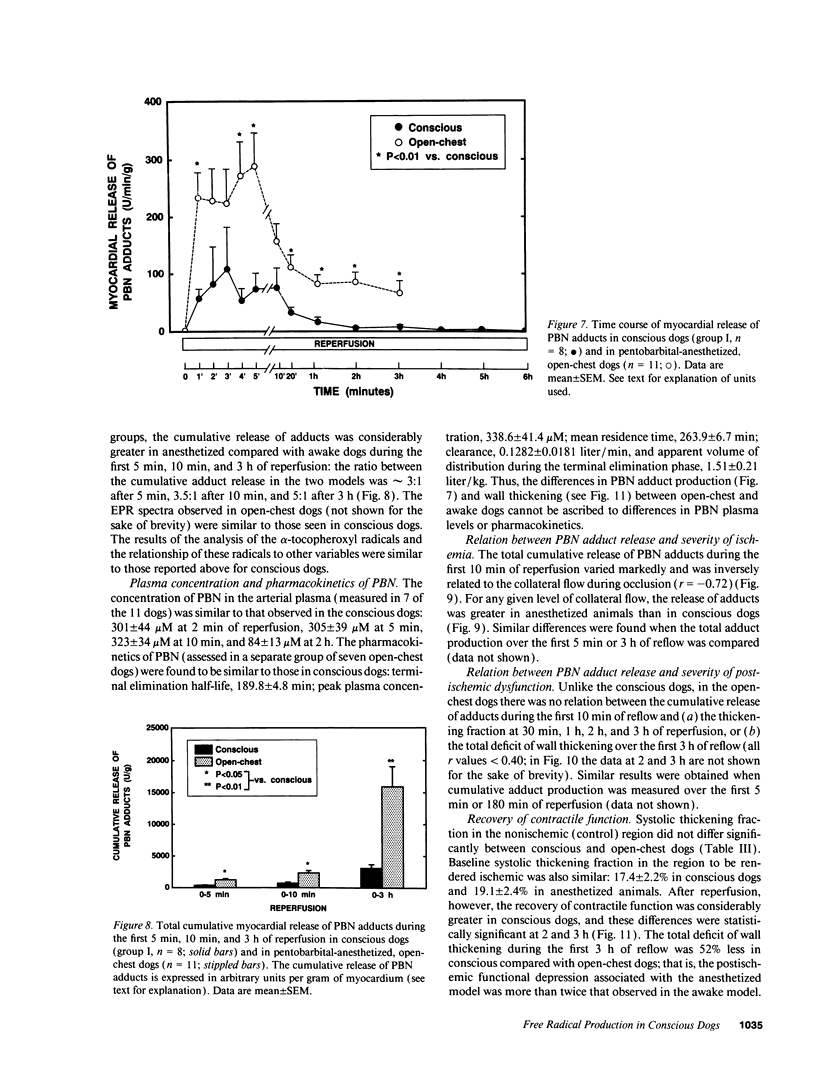
Abstract
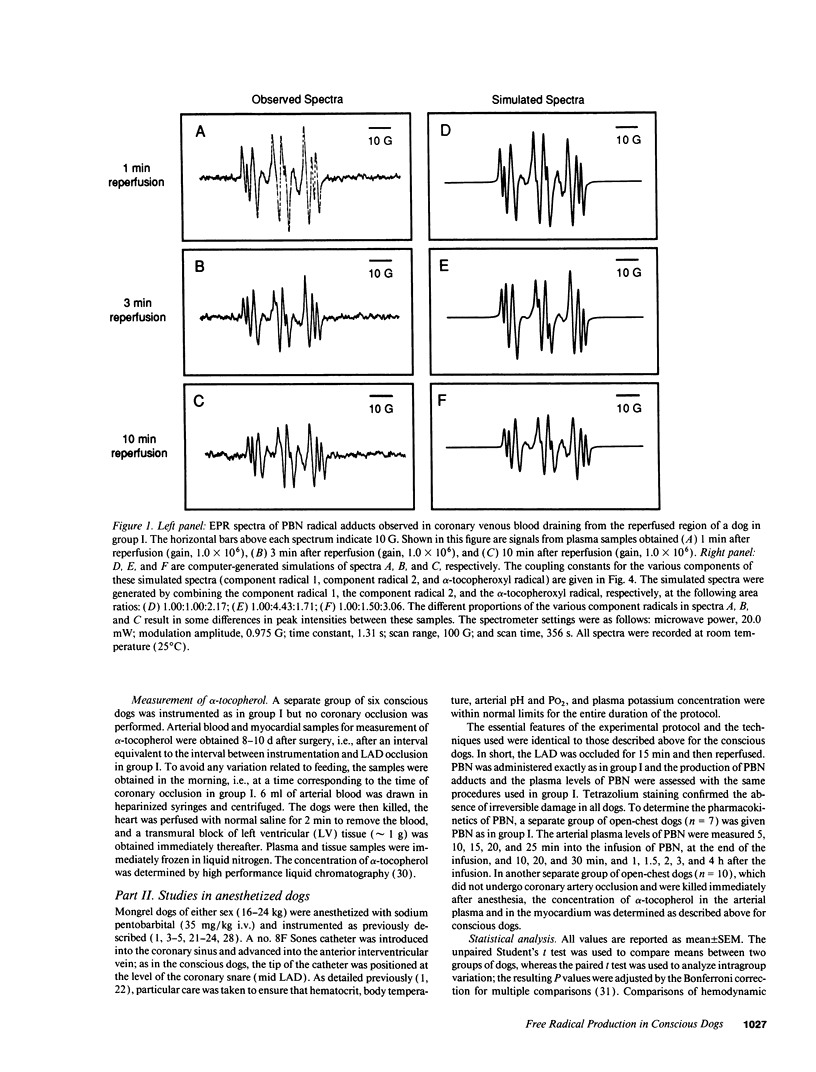
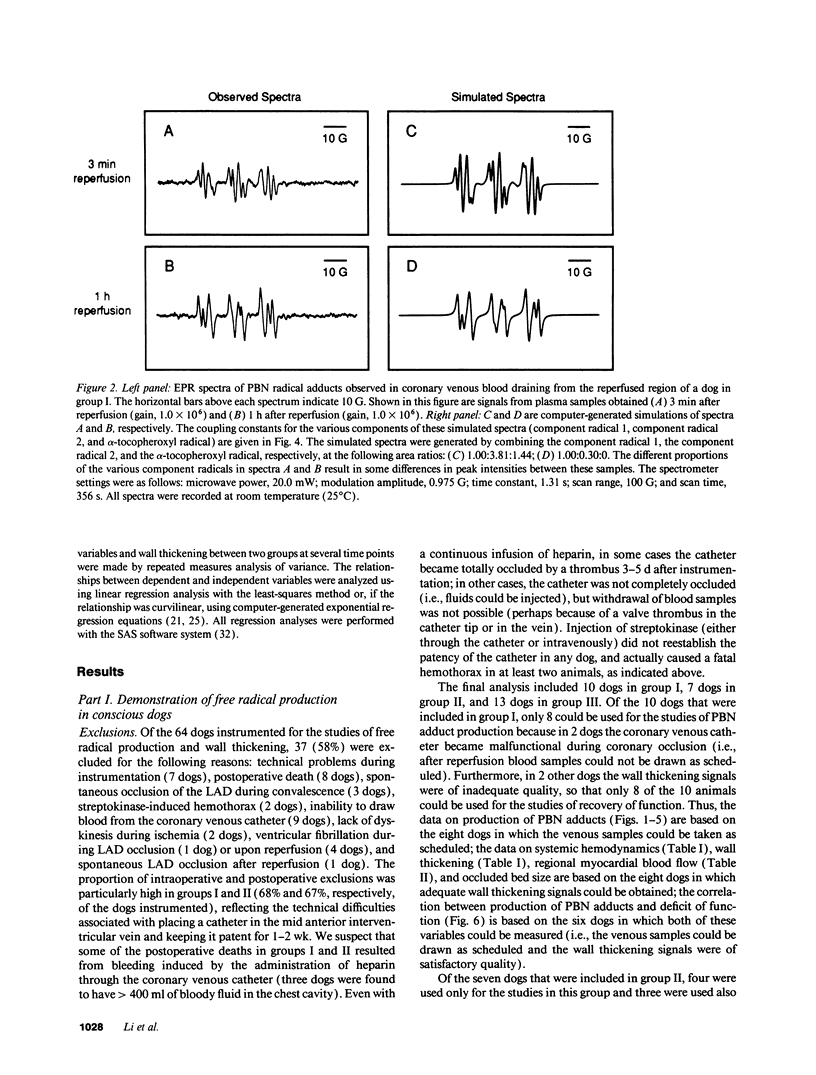
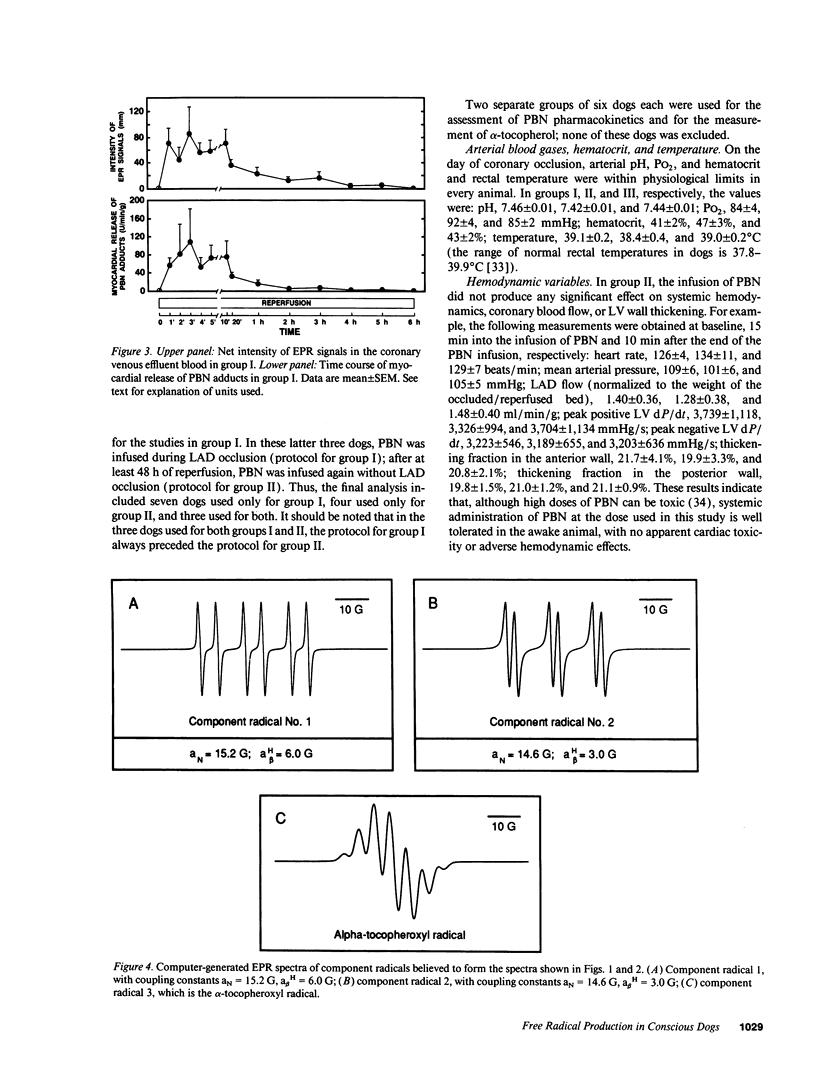
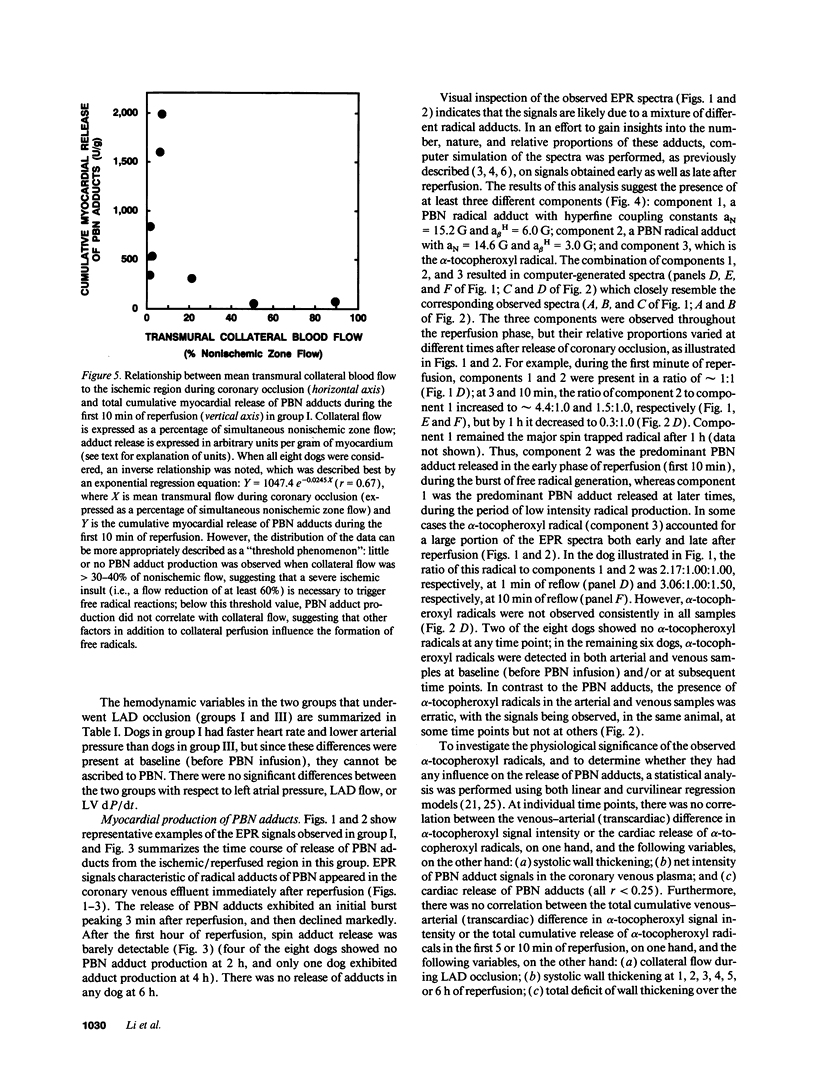
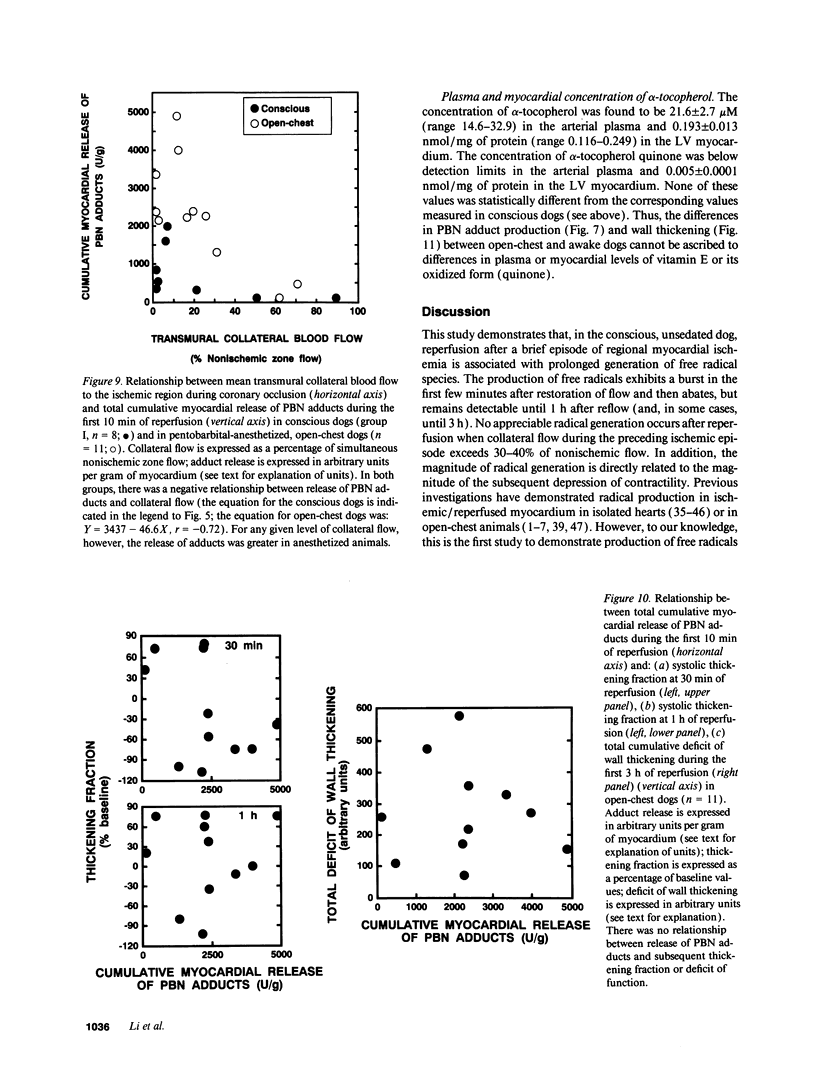
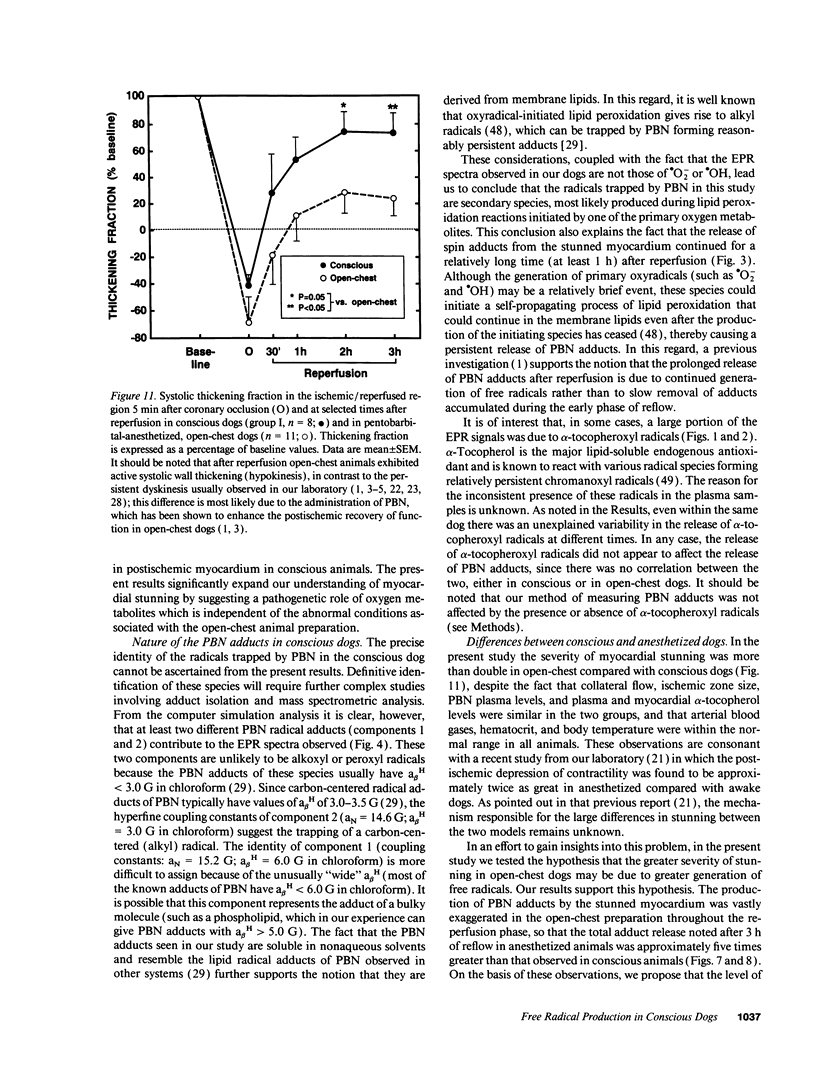
Conscious dogs undergoing a 15-min coronary occlusion were given alpha-phenyl N-tert-butyl nitrone (PBN) and the local coronary venous plasma was analyzed by electron paramagnetic resonance spectroscopy. A prolonged myocardial release of PBN radical adducts was observed, which exhibited a burst in the initial minutes of reflow (peaking at 3 min) and then abated but continued for 1-3 h after reperfusion. Computer simulation revealed the presence of at least two PBN adducts (aN = 15.2 G and a beta H = 6.0 G; aN = 14.6 G and a beta H = 3.0 G), both consistent with the trapping of secondary carbon-centered radicals. No appreciable PBN adduct production was observed when collateral flow exceeded 30-40% of nonischemic flow, indicating that a flow reduction of at least 60% is necessary to trigger free radical reactions. There was a direct relationship between the magnitude of PBN adduct production and the severity of contractile dysfunction (r = 0.77), suggesting that the radicals generated upon reperfusion play a causal role in the subsequent stunning. The total release of PBN adducts after 3 h of reperfusion following a 15-min coronary occlusion was found to be approximately five times greater in open-chest compared with conscious dogs; at the same time, the recovery of wall thickening was markedly less in open-chest dogs. This study represents the first application of spin trapping to a conscious animal model of myocardial ischemia. The results demonstrate (a) that free radicals are generated in the stunned myocardium in the absence of the artificial or abnormal conditions associated with previously used models (isolated hearts, open-chest preparations), and (b) that both the severity of postischemic dysfunction and the magnitude of the attendant free radical production are greatly exaggerated in the open-chest dog, implying that previous conclusions derived from this model may not be applicable to conscious animals or to humans. This investigation also provides a method to measure free radicals in awake animals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo C. M., Kramer J. H., Dickens B. F., Weglicki W. B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987 Aug 31;221(1):101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Leiboff R. H., Mergner G. W., Dickens B. F., Weglicki W. B. Spin trapping of oxygen and carbon-centered free radicals in ischemic canine myocardium. Free Radic Biol Med. 1987;3(5):313–316. doi: 10.1016/s0891-5849(87)80037-0. [DOI] [PubMed] [Google Scholar]
- Baker J. E., Felix C. C., Olinger G. N., Kalyanaraman B. Myocardial ischemia and reperfusion: direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2786–2789. doi: 10.1073/pnas.85.8.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., Jeroudi M. O., Patel B. S., Aruoma O. I., Halliwell B., Lai E. K., McCay P. B. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial "stunning" is a manifestation of reperfusion injury. Circ Res. 1989 Sep;65(3):607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- Bolli R., Jeroudi M. O., Patel B. S., DuBose C. M., Lai E. K., Roberts R., McCay P. B. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4695–4699. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., McCay P. B. Use of spin traps in intact animals undergoing myocardial ischemia/reperfusion: a new approach to assessing the role of oxygen radicals in myocardial "stunning". Free Radic Res Commun. 1990;9(3-6):169–180. doi: 10.3109/10715769009145674. [DOI] [PubMed] [Google Scholar]
- Bolli R., Myers M. L., Zhu W. X., Roberts R. Disparity of reperfusion arrhythmias after reversible myocardial ischemia in open chest and conscious dogs. J Am Coll Cardiol. 1986 May;7(5):1047–1056. doi: 10.1016/s0735-1097(86)80222-4. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Hartley C. J., Thornby J. I., Jeroudi M. O., Roberts R. Nonuniform transmural recovery of contractile function in stunned myocardium. Am J Physiol. 1989 Aug;257(2 Pt 2):H375–H385. doi: 10.1152/ajpheart.1989.257.2.H375. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Lai E. K., McCay P. B. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988 Aug;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Li X. Y., Triana J. F., Lai E. K., McCay P. B. Iron-mediated radical reactions upon reperfusion contribute to myocardial "stunning". Am J Physiol. 1990 Dec;259(6 Pt 2):H1901–H1911. doi: 10.1152/ajpheart.1990.259.6.H1901. [DOI] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Hartley C. J., Michael L. H., Repine J. E., Hess M. L., Kukreja R. C., Roberts R. Attenuation of dysfunction in the postischemic 'stunned' myocardium by dimethylthiourea. Circulation. 1987 Aug;76(2):458–468. doi: 10.1161/01.cir.76.2.458. [DOI] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Thornby J. I., O'Neill P. G., Roberts R. Time course and determinants of recovery of function after reversible ischemia in conscious dogs. Am J Physiol. 1988 Jan;254(1 Pt 2):H102–H114. doi: 10.1152/ajpheart.1988.254.1.H102. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Webb A., Ingold K. U. A mild, rapid, and efficient method of lipid extraction for use in determining vitamin E/lipid ratios. Lipids. 1985 Jan;20(1):29–39. doi: 10.1007/BF02534359. [DOI] [PubMed] [Google Scholar]
- Charlat M. I., O'Neill P. G., Egan J. M., Abernethy D. R., Michael L. H., Myers M. L., Roberts R., Bolli R. Evidence for a pathogenetic role of xanthine oxidase in the "stunned" myocardium. Am J Physiol. 1987 Mar;252(3 Pt 2):H566–H577. doi: 10.1152/ajpheart.1987.252.3.H566. [DOI] [PubMed] [Google Scholar]
- Charlat M. L., O'Neill P. G., Hartley C. J., Roberts R., Bolli R. Prolonged abnormalities of left ventricular diastolic wall thinning in the "stunned" myocardium in conscious dogs: time course and relation to systolic function. J Am Coll Cardiol. 1989 Jan;13(1):185–194. doi: 10.1016/0735-1097(89)90569-x. [DOI] [PubMed] [Google Scholar]
- Cobb F. R., Bache R. J., Greenfield J. C., Jr Regional myocardial blood flow in awake dogs. J Clin Invest. 1974 Jun;53(6):1618–1625. doi: 10.1172/JCI107712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. B., Davies M. J., Hearse D. J., Slater T. F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987 Nov;61(5):757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- Jeroudi M. O., Triana F. J., Patel B. S., Bolli R. Effect of superoxide dismutase and catalase, given separately, on myocardial "stunning". Am J Physiol. 1990 Sep;259(3 Pt 2):H889–H901. doi: 10.1152/ajpheart.1990.259.3.H889. [DOI] [PubMed] [Google Scholar]
- Jewett S. L., Eddy L. J., Hochstein P. Is the autoxidation of catecholamines involved in ischemia-reperfusion injury? Free Radic Biol Med. 1989;6(2):185–188. doi: 10.1016/0891-5849(89)90116-0. [DOI] [PubMed] [Google Scholar]
- Jugdutt B. I. Different relations between infarct size and occluded bed size in barbiturate-anesthetized versus conscious dogs. J Am Coll Cardiol. 1985 Nov;6(5):1035–1046. doi: 10.1016/s0735-1097(85)80306-5. [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Arroyo C. M., Dickens B. F., Weglicki W. B. Spin-trapping evidence that graded myocardial ischemia alters post-ischemic superoxide production. Free Radic Biol Med. 1987;3(2):153–159. doi: 10.1016/s0891-5849(87)80011-4. [DOI] [PubMed] [Google Scholar]
- Laxson D. D., Homans D. C., Dai X. Z., Sublett E., Bache R. J. Oxygen consumption and coronary reactivity in postischemic myocardium. Circ Res. 1989 Jan;64(1):9–20. doi: 10.1161/01.res.64.1.9. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Sun J. Z., Bradamante S., Piccinini F., Bolli R. Effects of the spin trap alpha-phenyl N-tert-butyl nitrone on myocardial function and flow: a dose-response study in the open-chest dog and in the isolated rat heart. Free Radic Biol Med. 1993 Mar;14(3):277–285. doi: 10.1016/0891-5849(93)90024-o. [DOI] [PubMed] [Google Scholar]
- Manders W. T., Vatner S. F. Effects of sodium pentobarbital anesthesia on left ventricular function and distribution of cardiac output in dogs, with particular reference to the mechanism for tachycardia. Circ Res. 1976 Oct;39(4):512–517. doi: 10.1161/01.res.39.4.512. [DOI] [PubMed] [Google Scholar]
- McCay P. B., Lai E. K., Poyer J. L., DuBose C. M., Janzen E. G. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984 Feb 25;259(4):2135–2143. [PubMed] [Google Scholar]
- McCay P. B. Vitamin E: interactions with free radicals and ascorbate. Annu Rev Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- Mergner G. W., Weglicki W. B., Kramer J. H. Postischemic free radical production in the venous blood of the regionally ischemic swine heart. Effect of deferoxamine. Circulation. 1991 Nov;84(5):2079–2090. doi: 10.1161/01.cir.84.5.2079. [DOI] [PubMed] [Google Scholar]
- Ning X. H., Zweng T. N., Gallagher K. P. Ejection- and isovolumic contraction-phase wall thickening in nonischemic myocardium during coronary occlusion. Am J Physiol. 1990 Feb;258(2 Pt 2):H490–H499. doi: 10.1152/ajpheart.1990.258.2.H490. [DOI] [PubMed] [Google Scholar]
- Pietri S., Culcasi M., Cozzone P. J. Real-time continuous-flow spin trapping of hydroxyl free radical in the ischemic and post-ischemic myocardium. Eur J Biochem. 1989 Dec 8;186(1-2):163–173. doi: 10.1111/j.1432-1033.1989.tb15191.x. [DOI] [PubMed] [Google Scholar]
- Rankin J. S., McHale P. A., Arentzen C. E., Ling D., Greenfield J. C., Jr, Anderson R. W. The three-dimensional dynamic geometry of the left ventricle in the conscious dog. Circ Res. 1976 Sep;39(3):304–313. doi: 10.1161/01.res.39.3.304. [DOI] [PubMed] [Google Scholar]
- Shuter S. L., Davies M. J., Garlick P. B., Hearse D. J., Slater T. F. Studies on the effects of antioxidants and inhibitors of radical generation on free radical production in the reperfused rat heart using electron spin resonance spectroscopy. Free Radic Res Commun. 1990;9(3-6):223–232. doi: 10.3109/10715769009145680. [DOI] [PubMed] [Google Scholar]
- Templeton G. H., Wildenthal K., Willerson J. T., Mitchell J. H. Influence of acute myocardial depression on left ventricular stiffness and its elastic and viscous components. J Clin Invest. 1975 Aug;56(2):278–285. doi: 10.1172/JCI108091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A., Hess M. L. The oxygen free radical system: a fundamental mechanism in the production of myocardial necrosis. Prog Cardiovasc Dis. 1986 May-Jun;28(6):449–462. doi: 10.1016/0033-0620(86)90027-7. [DOI] [PubMed] [Google Scholar]
- Tosaki A., Braquet P. DMPO and reperfusion injury: arrhythmia, heart function, electron spin resonance, and nuclear magnetic resonance studies in isolated working guinea pig hearts. Am Heart J. 1990 Oct;120(4):819–830. doi: 10.1016/0002-8703(90)90197-6. [DOI] [PubMed] [Google Scholar]
- Triana J. F., Li X. Y., Jamaluddin U., Thornby J. I., Bolli R. Postischemic myocardial "stunning". Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ Res. 1991 Sep;69(3):731–747. doi: 10.1161/01.res.69.3.731. [DOI] [PubMed] [Google Scholar]
- Vatner S. F., Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med. 1975 Nov 6;293(19):970–976. doi: 10.1056/NEJM197511062931906. [DOI] [PubMed] [Google Scholar]
- Vatner S. F., Franklin D., Braunwald E. Effects of anesthesia and sleep on circulatory response to carotid sinus nerve stimulation. Am J Physiol. 1971 May;220(5):1249–1255. doi: 10.1152/ajplegacy.1971.220.5.1249. [DOI] [PubMed] [Google Scholar]
- Vatner S. F., Higgins C. B., Patrick T., Franklin D., Braunwald E. Effects of cardiac depression and of anesthesia on the myocardial action of a cardiac glycoside. J Clin Invest. 1971 Dec;50(12):2585–2595. doi: 10.1172/JCI106759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Williams R., Rayburn B. K., Smith D., Weisfeldt M. L., Flaherty J. T. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem. 1989 Nov 15;264(32):18890–18895. [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]


