Abstract
Background
Recent studies have indicated the presence of multipotent mesenchymal stromal cells (MSCs) in human lung diseases. Excess airway smooth muscle, myofibroblasts and activated fibroblasts have each been noted in asthma, suggesting that mesenchymal progenitor cells play a role in asthma pathogenesis. We therefore sought to determine whether MSCs are present in the lungs of ovalbumin (OVA)-sensitized and challenged mice, a model of allergic airways disease.
Methods
Balb/c mice were sensitized and challenged with PBS or OVA over a 25 day period. Flow cytometry as well as colony forming and differentiation potential were used to analyze the emergence of MSCs along with gene expression studies using immunochemical analyses, quantitative polymerase chain reaction (qPCR), and gene expression beadchips.
Results
A CD45-negative subset of cells expressed Stro-1, Sca-1, CD73 and CD105. Selection for these markers and negative selection against CD45 yielded a population of cells capable of adipogenic, osteogenic and chondrogenic differentiation. Lungs from OVA-treated mice demonstrated a greater average colony forming unit-fibroblast (CFU-F) than control mice. Sorted cells differed from unsorted lung adherent cells, exhibiting a pattern of gene expression nearly identical to bone marrow-derived sorted cells. Finally, cells isolated from the bronchoalveolar lavage of a human asthma patient showed identical patterns of cell surface markers and differentiation potential.
Conclusions
In summary, allergen sensitization and challenge is accompanied by an increase of MSCs resident in the lungs that may regulate inflammatory and fibrotic responses.
Introduction
Adult bone marrow contains a minority population of mesenchymal stem cells that are thought to contribute to the regeneration of connective tissues such as bone, cartilage, muscle, ligaments, tendons, fat and stroma [1]. These cells demonstrate cell surface markers consistent with a mesenchymal origin (e.g., Stro-1, Sca-1, CD73, CD90, CD105) but fail to express markers associated with a hematopoietic (CD34, CD45) or endothelial cell (CD31) origin. Subsequently, multipotent mesenchymal stem cells were isolated from peripheral sites, including peripheral blood [2], adipose tissue [3], articular synovium [4] and trabecular bone [5]. More recently, it has been suggested that most organs apparently carry their own population of pluripotent mesenchymal stem cells in a perivascular compartment that participate in tissue repair [6]. The perivascular niche has been demonstrated to be the source of mesenchymal stem cells in adipose tissue [7], skeletal muscle [8] and other organs [9].
With regard to the lung, mesenchymal stem cells have been isolated from the bronchial tissue of patients undergoing lobectomy for primary lung tumors [10]. Our laboratory has isolated mesenchymal stem cells from the tracheal aspirates of premature infants undergoing mechanical ventilation for respiratory distress [11]. Mesenchymal stem cells of donor sex identity have been found in lung allografts years after transplantation, suggesting that these stromal cells may originate from the lung tissue itself, perhaps from the perivascular compartment [12]. Finally, we [13] and others [14,15] have shown that, in addition to the above cell types, mesenchymal stem cells may undergo differentiation to myofibroblasts.
Recent work suggests that there is a hierarchy of multipotent mesenchymal stromal cells ranging from true self-renewing stem cells with multilineage differentiation capacity to those with more restricted differentiation potential, until a state of complete restriction to the fibroblast is reached [16]. Since the clonogenicity or self-renewal of most mesenchymal stem cell isolates has not been thoroughly tested, we now refer to cells with the surface markers and differentiation potential of mesenchymal stem cells as mesenchymal stromal cells (MSCs), which have a more restricted differentiation potential.
There is abundant evidence in the literature suggesting that mesenchymal progenitor cells are involved in asthma pathogenesis. Excess airway smooth muscle, caused in part by an increase in the number of smooth muscle cells, has been well-described in asthma [17,18]. Patients with severe asthma also demonstrate an accumulation of myofibroblasts in the airway subepithelium [19-22]. These alterations may extend beyond the central airways to the distal airways and lung parenchyma [23]. Finally, in contrast to control subjects, "activated" fibroblasts have been identified in the bronchoalveolar lavage (BAL) fluid of patients with asthma [24]. We therefore hypothesized that multipotent MSCs, which may serve as progenitor cells of smooth muscle cells and myofibroblasts, are increased in the lungs of ovalbumin-sensitized and -challenged mice, an animal model of allergic airways disease. Previous studies have demonstrated hyperplasia of myofibroblasts in both the large and small airways of these mice, including the terminal bronchioles, alveolar ducts and alveolar walls [25].
Materials and methods
OVA-sensitization and challenge
Mice (BALB/cByJ, Jackson Labs, Bar Harbor, ME) were sensitized to sterile LPS-free OVA (Pierce, Rockford, IL) or PBS control by intraperitoneal injection and serially challenged over a month with intranasal instillations of OVA or PBS (Figure 1), as previously described [26,27]. This study was approved by the Institutional Animal Care and Use Committee.
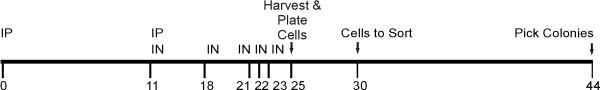
Figure 1.
Schema for OVA sensitization and challenge of mice, isolation of a total lung fibroblast fraction, and sorting of a mesenchymal stromal cell fraction. Mice were sensitized twice by intraperitoneal (IP) injections at day 0 and day 11 of OVA followed by intranasal (IN) challenges at days 11, 18, 21, 22, and 23. Lungs were isolated, digested with collagenase, and adherent cells plated with daily media changes to remove nonadherent cells. At the end of 5 days, adherent cells were lifted, sorted and, in two additional weeks, processed for colony formation.
Flow cytometry of whole lung digests and adherent cells
Lungs from PBS- and OVA-treated mice were perfused with 5 mM EDTA, minced into 1-2 mm blocks and digested for 1 hr in 5 mg/ml collagenase. Cells were obtained by filtration through a 70 μm mesh. Red blood cells were lysed with an ammonium chloride buffer. Cells were washed in 10% serum-containing DMEM with 4 mM glucose, 1 mM sodium pyruvate, and 2 mM L-glutamine and plated at 106 cells/100 mm dish. After 5 days, adherent cells were removed from the plates by trypsin, washed with Ca2+, Mg2+-containing PBS and blocked with 1% BSA in PBS for 20 min at 4°C. Within three hours after immunostaining (below), cells were sorted using a BD Biosciences FACSDiVa High-Speed Cell Sorter (San Diego, CA) with 350 nm, 488 nm, and 633 nm lasers. Cells were stained with 2.5 μg/mL of FITC- or AlexaFluor (AF)-conjugated antibodies. Typically, anti-Stro-1 was conjugated to AF 350, anti-CD34 was conjugated to FITC, anti-CD105 was coupled to AF555, anti-CD73 was coupled to AF633 and anti-CD45 was conjugated to AF750. Cells were negatively selected for CD34 and CD45 and positively selected for Stro-1, CD73 and CD105 binding (see text). Cells were collected in 1 mL DMEM supplemented with 10% FBS and cultured for 2 weeks to allow colony formation before expansion for study.
Determination of colony forming unit-fibroblast (CFU-F)
Lungs from PBS- and OVA-treated mice were perfused with 5 mM EDTA, minced into 1-2 mm blocks and digested for 1 hr in 5 mg/ml collagenase. Cells were obtained by filtration through a 70 μm mesh. Trypan blue-excluding cells from each animal were serially diluted and plated in DMEM with 10% FBS at 100 cells/well in 2 mL of medium into a 30 mm well of a 6 well dish (in triplicate). Cells were allowed to grow in 10% FBS/DMEM for 14 days. After 14 days, plates were stained with 0.5% toluidine blue for 5 min, washed three times with PBS, and independent colonies of primary mouse lung fibroblasts were counted, as described previously [28]. A similar procedure was followed to determine the CFU-F of sorted Stro-, CD73, CD105-positive mouse lung MSCs.
Analysis of cultured sorted cells from the lungs of OVA-sensitized and challenged mice
Cells (passage 3-5 post-sort) were dissociated from plastic using cell dissociation buffer (PBS supplemented with 5 mM EDTA) and analyzed for the surface markers described above, as well as CD90.2 (rat anti-mouse Thy 1.2, clone 30-H12, BD Pharmingen) and Sca-1 (rat anti-mouse Ly 6-A/E, clone e13-161.7, BD Pharmingen). Cells were typically stained with 2 μg/mL FITC- or AF488-conjugated antibodies or isotypic control immunoglobulins and 50 μg/mL propidium iodide.
Fluorescence microscopy
The pulmonary artery was perfused with EDTA. Mouse lung vasculature was perfused with 5 mM EDTA in PBS injected through the pulmonary artery until the lung blanched. Lungs were inflated to 30 cm H2O pressure with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) and placed in formalin overnight. Paraffin-embedded lungs were sectioned with 5 μm-thick sections. AlexaFluor (AF) dye antibody conjugates were prepared using N-hydroxy succinimide esterified dye conjugation reactions with 20 μg antibody and 100 μg activated dye in 10 mM sodium bicarbonate buffer (pH 8.5) for 1 hour. The reaction was quenched by making it 500 mM Tris HCl pH 7.2 for 1 hour, and the conjugated antibody was purified by separating it from quenched dye over a G-50 spin column (GE Healthcare, Piscataway, NJ). Slides were probed with 2 μg/mL AF or fluorescein isothiocyanate (FITC)-conjugated anti-Stro-1 (clone STRO-1, R&D Systems, Minneapolis, MN), anti-CD34 clone MEC14.7, anti-CD45 clone 30-F11, anti-CD73 clone TY/11.8, anti CD105 clone MJ7/18 (each from BioLegend, San Diego, CA) and Cy3- or FITC-conjugated mouse anti-α-smooth muscle actin (clone 1A4, Sigma-Aldrich). Nuclei were visualized with 10 μg/mL Hoechst 33258 (Sigma-Aldrich). Cells were imaged using either an Olympus XI74 microscope (Center Valley, PA) for epifluorescence, a Zeiss Axioplan microscope equipped with an ApoTome and digital AxioCamMR CCD camera, or a Zeiss LSM 150 confocal microscope (Thornwood, NY).
Differentiation of clonal MSCs
Passage two sorted cells were serially diluted in 96 well plates to 0.3 cells per well. After 2 weeks in this media, individual colonies were trypsinized, dispersed into 100 mm dishes, allowed to grow to confluence and plated on fibronectin-coated slides. Myogenic differentiation was induced by addition of 10 ng/mL transforming growth factor (TGF)-β, 1 μg/mL insulin, 0.55 μg/mL transferrin and 670 ng/mL selenium (Invitrogen, Carlsbad, CA) for 6 days. These cells were immunostained for α-actin (clone 1A4, Sigma-Aldrich) and myosin heavy chain (clone hSM-V, Sigma-Aldrich). Adipogenic differentiation was induced by culture in DMEM supplemented with 5% fetal bovine serum (FBS), 10 μM dexamethasone, 50 μM indomethacin, 100 μM isobutylmethylxanthine (IBMX) and 10 μg/mL insulin for 21 days, as described [11] Formalin-fixed cells were stained for neutral lipids with Oil Red O. Osteogenic differentiation was accomplished by culture in DMEM supplemented with 5% FBS, 100 nM dexamethasone, 10 mM β-glycerophosphate and 50 μg/mL ascorbic acid for 21 days, as described [11]. Formalin-fixed cells were stained for calcium phosphate with alizarin red.
To further assess differentiation state, cells were solubilized and RNA extracted using a guanidine isothiocyanate-phenol protocol (Trizol, InVitrogen). Quantitative two-step real time PCR for differentiation marker transcripts was performed using an Eppendorf realplex2 (Westbury, NY). Transcripts were examined for myocardin (MYOCD, forward sequence, CCCATGGACTCTGCCTATGC; reverse sequence, CTGAGGATCTGTAGCTGCAGGAAT), smooth muscle myosin heavy chain (MHC11, forward sequence, GCCGACACAGCCTACAGAAG; reverse sequence, TTTCCGGCTCCAGACTCA), transgelin (SM22, forward sequence, GCCAACAAGGGTCCATCCTA; reverse sequence, CTCCAGCTCCTCGTCATACTTC), peroxisome proliferator-activated receptor (PPAR)-γ (forward sequence, GGCCCACCAACTTCGGAATC; reverse sequence, GTAAAGGGCTTGATGTCAAAGGAA), fatty acid binding protein 4/adipocyte protein-2 (FABP4, forward sequence, GGCTTTGCCACAAGGAAAGTG; reverse sequence, CGCCCAGTTTGAAGGAAATCTC), osteopontin/secreted phosphoprotein 1 (SSP1, forward sequence, CTCACCATTCGGATGAGTCTGAT; reverse sequence, AGGGACGATTGGAGTGAAAGTG) and osteocalcin/bone γ-carboxyglutamate (gla) protein (BGLAP, forward sequence, CAGCTTGGCCCAGACCTA; reverse sequence, GCCAGGGTCAGCAGAGTG), Expression levels were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward sequence, TCCACTCACGGCAAATTCAAC); reverse sequence, CGCTCCTGGAAGATGGTGATG).
Western analyses
Mouse lung MSCs were washed in PBS, homogenized in 50 mM Tris (pH 7.5), 100 mM NaCl, 50 mM NaF, 40 mM β-glycerophosphate, 2 mM EDTA, 200 μM Na3VO4, and 1% Triton X-100 containing complete protease inhibitors (Roche Diagnostics, Indianapolis, IN). After centrifugation at 10,000 g for 30 min to remove nuclei and cell particles, supernatants were resolved by SDS-PAGE and transferred to nitrocellulose. Membranes were probed with mouse monoclonal anti-α-smooth muscle actin (MAb 1A4; Calbiochem, San Diego, CA), mouse monoclonal anti-myosin heavy chain (MHC) (MAb HSM-V; Sigma-Aldrich, St. Louis, MO) or mouse monoclonal anti-β-actin (Sigma). Antibody binding was detected in Westerns with a peroxidase-conjugated anti-rabbit or anti-mouse IgG and chemiluminescence (Thermo-Pierce, Rockford, IL).
Gene arrays
We examined the gene expression profile of MSCs using the Illumina MouseWG-6 expression BeadChip platform (San Diego, CA). This system detects 26,766 coding transcripts with well-established annotations, 6,277 coding transcripts with provisional annotations and 56 non-coding transcripts with well-established annotations. Total RNA was extracted using the RNeasy Plus Mini kit (Qiagen, Valencia, CA). Further preparation and analysis was carried out by the University of Michigan Sequencing Core, according to the chip manufacturer's recommended protocol. Hybridized biotinylated cRNA was detected with streptavidin-Cy3 and quantitated using an Illumina BeadArray Reader. The gene expression profile of lung MSCs was compared to primary mouse lung fibroblasts as well as primary mouse bone marrow MSCs, which were isolated from the femurs of OVA-treated mice by flow cytometry, using gates identical to those used for lung MSCs.
For microarray statistical analysis, background-corrected values for each probe on the BeadChip array were extracted using GenomeStudio Data Analysis Software (Illumina). Detection p-values were computed using a non-parametric method. Probe signals are ranked relative to signals of negative controls. Detection p-value = 1-R/N, where R is the rank of the gene signal relative to negative controls and N is number of negative controls. Statistical differences in gene expression between lung and bone marrow-derived cells were calculated using the Illumina Custom differential expression algorithm. Multiple tests were corrected by the Benjamini and Hochberg false discovery rate [29]. For qPCR, data were normalized for GAPDH, and differences in gene expression analyzed by paired t test.
Isolation and characterization of MSCs from the BAL of a patient with asthma
This study was approved by the University of Michigan Institutional Review Board. After informed consent, BAL fluid was obtained from three pediatric asthma patients who were undergoing diagnostic flexible bronchoscopy. Cells were sedimented for 5 min at 500 × g, washed in media, counted, and serial dilutions of 100,000 to 100 cells were plated and allowed to grow for 21 days CellCe, Single colonies were isolated, expanded, and assessed for cell surface markers and pluripotency. Cell surface markers and differentiation potential were compared to human neonatal lung MSCs [11] and primary human lung fibroblasts (Lonza, Walkersville, MD). Cell surface markers were examined by flow cytometry using human-specific antibodies (BioLegend).. Cells were differentiated along adipogenic and osteogenic lines as described above. For chondrogenic differentiation, cells were differentiated along cartilage lines by first sedimenting (10 min at 500 × g) 200,000 trypsinized cells in a sterile 15 mL polypropylene culture tube and maintaining them as a pellet for 21 days in serum-free DMEM composed with 10 ng TGF-β1 and 10 ng bone morphogenetic protein-4/mL [30]. At the end of this time cell pellets were fixed overnight in formalin, processed for paraffin embedding and sectioning, and stained for chondroitin sulfate with Alcian blue[31]. A nuclear fast red counterstain was used to help distinguish nuclei acids from acid mucopolysaccharides.
Results
OVA sensitization and challenge is associated with the appearance of Stro-1, CD73, and CD105-positive cells with high colony forming potential
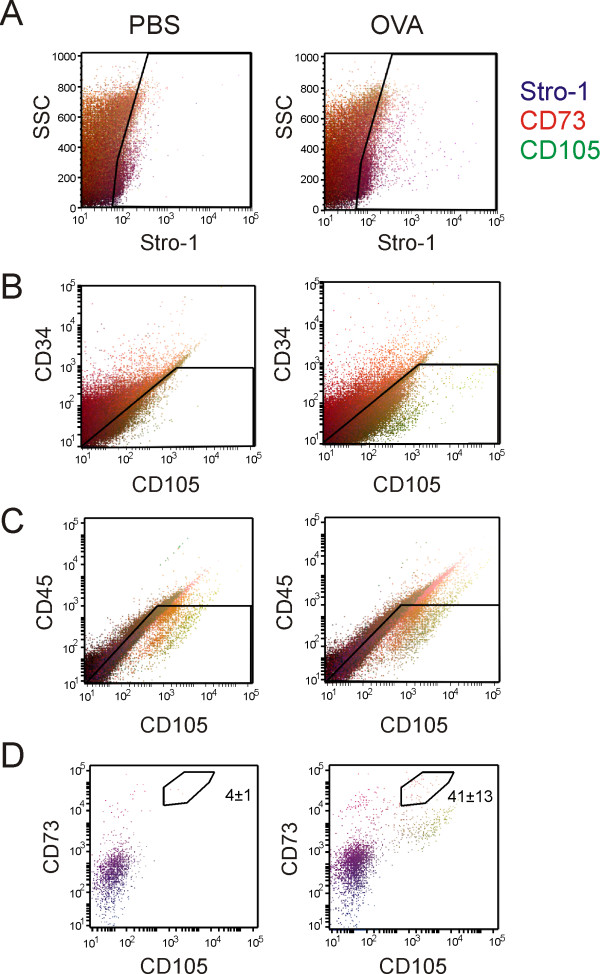
We used flow cytometry to examine cells for cell surface markers typically associated with MSCs (Figure 2). Cells from whole lung digests were plated on plastic for five days to enrich for adherent lung fibroblast cells. We stained live cells from PBS- and OVA-treated mice for Stro-1, CD73, CD105, CD34 and CD45. We found a subpopulation of fibroblast-like cells from OVA-treated mice which showed greater Stro-1 expression (Figure 2A). We found distinct side populations of cells from OVA-treated mice which showed greater CD105 expression (Figures 2B and 2C), Both populations gated in the same forward scattering area. To eliminate hematopoietic cells, CD34 and CD45-positive cells were delineated by the windows shown (Figures 2B and 2C) and further gated for CD73 and CD105 expression, leaving a population of Stro-1-, CD73- and CD105-positive cells (Figure 2D). This population represents about 0.04% of the original lung cell digest (Table 1) and is here designated a lung MSC population. These cells were rarely detectable in PBS-treated lungs (0.004%).
Figure 2.
OVA treatment increases the fraction of mesencymal stromal cell antigen-positive lung cells. Collagenase dispersible cells were prepared from the lungs of PBS- (left column) or OVA-treated mice (right column). Single cell suspensions from PBS or OVA-treated mouse lungs were allowed to adhere to polystyrene plates for 5 days, trypsinized, blocked with 1% BSA and incubated with AF350-conjugated anti-Stro-1, AF488 anti-CD34, AF750 anti-CD45, AF555 anti-CD105, and AF633 anti-CD73. Data from 100,000 events are shown, with Stro-1 fluorescence intensity in blue, CD73 in red and CD105 in green. Gates were drawn to select a Stro-1-positive (A), low CD34 (B) and low CD45 population (C). In panel D, the gates indicated in panels A, B and C were used to produce the CD73 vs. CD105 plots shown (dot size is adjusted to make the lower cell numbers visible). The average number of cells in the various CD73-, CD105-positive subpopulations shown is provided Table 1. There was a significant increase in the number of CD34-, CD45-negative, CD73-, CD105-positive cells in lungs from OVA-treated mice.
Table 1.
Populations of Stro-1-, CD105-positive, CD34-CD45-negative cells from PBS- and OVA-treated lungs.
| Population of Cells Analysed | PBS | OVA |
|---|---|---|
| Total population | 100,000 | 100,000 |
| High Stro-1, low CD34, low CD45, low CD73, high CD105 | 14 ± 7 | 36 ± 19 |
| High Stro-1, low CD34, low CD45, medium CD73, high CD105 | 12 ± 6 | 138 ± 61 |
| High Stro-1, low CD34, low CD45, high CD73, high CD105 | 4 ± 1 | 41 ± 13* |
Cells obtained and sorted as described above were counted from 3 sets of 5 PBS- or OVA-treated mice (*P < 0.05, unpaired t-test).
The potential of adherent cells to form colonies was assessed by colony-forming unit-fibroblast (CFU-F) assay [28]. Sorted cells and primary mouse lung fibroblasts were serially diluted and 100 cells were plated in DMEM with 10% FBS. After 14 days, plates were fixed and stained. Sorted cells demonstrated a CFU-F of 29.0 ± 1.4 (mean ± SEM, n = 4) out of 100 cells plated, significantly greater than unsorted lung cells from either PBS (1.5 ± 0.1)- or OVA-treated (8.9 ± 2.2) mice (n = 6 for both groups, P < 0.05, ANOVA).
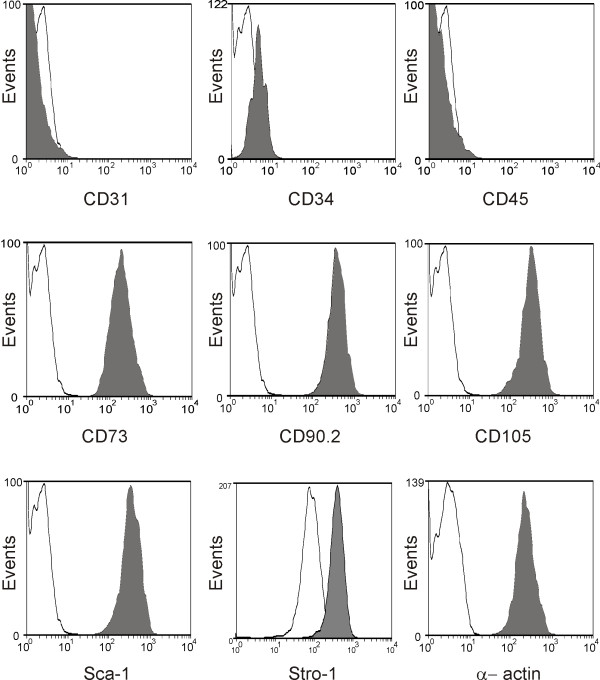
Sorted and clonal cells were trypsinized after two further passages and processed for flow cytometry using the same antibodies with which they were initially sorted. The sorted cells retained MSC cell surface antigens after propagation, and also stained positively for CD90.2 and Sca-1 (Figure 3). In the mouse lung, the CD45-, CD31-negative, Sca-1-positive phenotype has been associated with the mesenchymal progenitor cell lineage [32]
Figure 3.
Sorted cells retain cell surface markers typically associated with multipotent stromal cells. The CD34-, CD45-negative, Stro-1-, CD73-, CD105-positive cell fraction was plated, grown to confluence, passaged three times, removed with cell dissociation buffer, and bound to either AF488-conjugated control Ig (open curve) or antibody to the indicated antigen (grey curve). Cells in G0/G1 phase were gated by staining with propidium iodide. Event number is indicated on the y-axis.
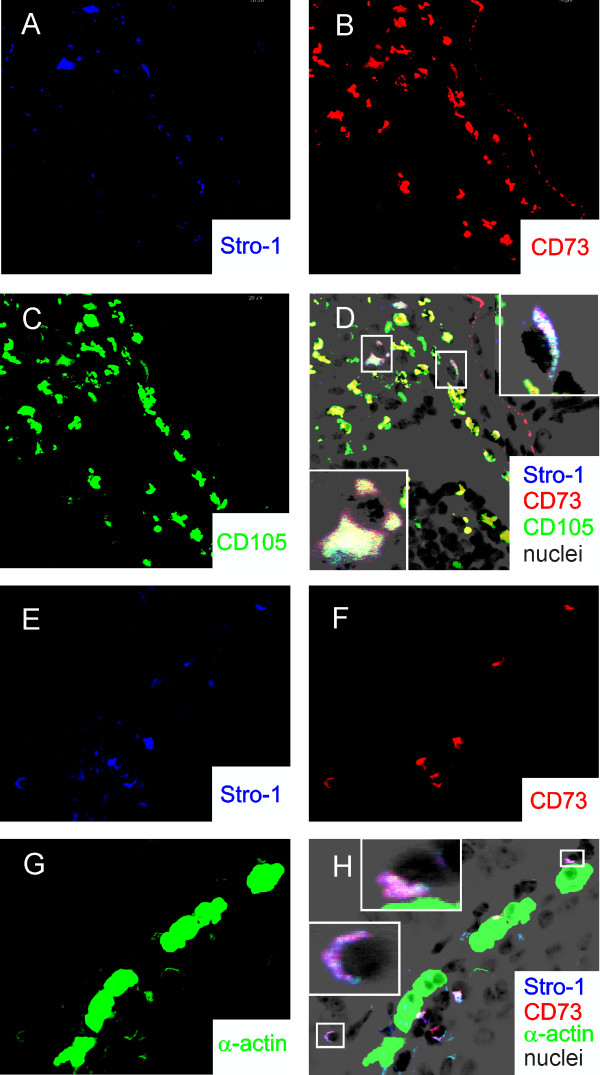
We examined sections from PBS- and OVA-treated mouse lungs for surface markers typically associated with MSCs including Stro-1, CD73 and CD105. In humans, Stro-1 is almost exclusively found on bone marrow-derived stromal cells [33]. Stro-1 localization has also been noted in mouse mesenchymal cells of the bone marrow [34] and developing periodontium [35]. CD73 (SH3/SH4) and CD105 (SH2/endoglin) are localized at the plasma membrane of bone marrow mesenchymal stem cells [36]. A small number of peribronchial cells stained positively for Stro-1, CD73, CD105 and α-smooth muscle actin (Figure 4).
Figure 4.
Presence of Stro-1-, CD73-, CD105-positive cells in the lungs of OVA-treated mice. A-D. Sections from OVA-sensitized and -challenged mouse lungs (at 630× original magnification) were stained for nuclei with Hoescht 33258. These are shown as a negative grayscale image in black. AF633-conjugated anti Stro-1 is shown in blue (A), AF555-conjugated anti-CD73 is shown in red (B) and AF488-conjugated anti-CD105 is shown in green (C). In D, the merged image shows co-localization of CD73 and CD105 (yellow/orange) and Stro-1, CD73 and CD105 (white). The insets show isolated cells at higher magnification. (There is some non-specific crossreactivity with the epithelial basement membrane in the red channel.) E-H. Lung sections from OVA-treated mice were stained for nuclei with Hoechst 33258 (black), AF633-conjugated anti-Stro-1 (blue, E), AF555-conjugated anti-CD73 (red, F) and AF488-conjugated anti-α-smooth muscle actin (green, G). In H, the merged image shows co-localization of Stro-1 and CD73 (magenta), Stro-1 and α-actin (turquoise) and Stro-1, CD73 and α-actin (white). The insets show isolated cells at higher magnification. Some positive cells lie adjacent to the airway smooth muscle layer.
Sorted Stro-1(+), CD73(+), CD105(+) cells may be differentiated along a variety of mesenchymal lineages
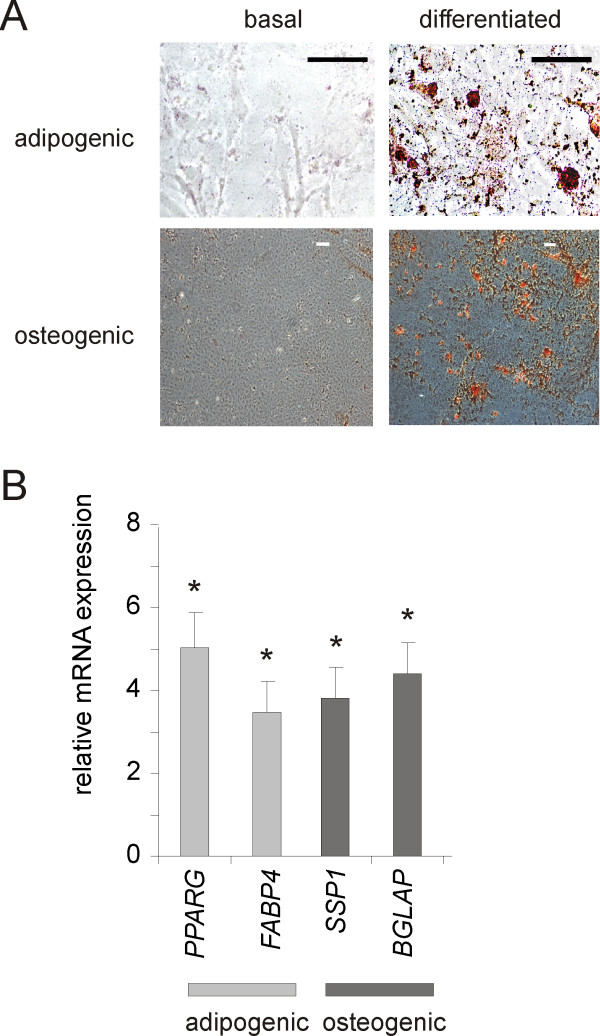
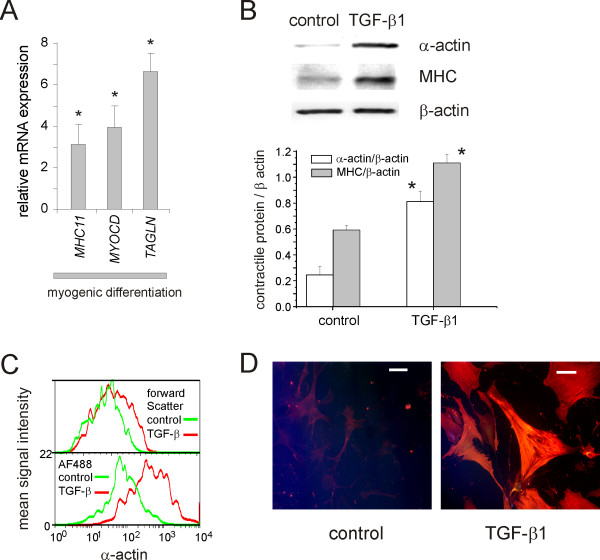
Cells from Stro-1-, CD73-, CD105-positive, CD34-, CD45-negative subpopulations were seeded onto plastic dishes. Only the high CD73, high CD105 cells grew well and were studied further. To isolate individual clones, passage two sorted cells were serially diluted in 96 well plates to 0.3 cells per well. After two weeks of growth in this media, wells with cells were trypsinized. Cells were dispersed into 100 mm dishes, allowed to grow to confluence, plated on fibronectin-coated slides and differentiated along adipogenic, osteogenic and myofibroblast lines (Figures 5, 6). When cells are treated with adipogenic medium (10 μM dexamethasone, 100 μM IBMX, 50 μM indomethacin and 100 μg/mL insulin), cells accumulated neutral lipid vesicles (Figure 5A) and expressed more mRNA encoding PPARG and FABP4 (Figure 5B). Osteogenic differentiation with 0.1 μM dexamethasone, 10 mM β-glycerophosphate and 50 μg/ml ascorbic acid caused cells to increase calcium phosphate deposition as well as mRNA expression of SSP1 and BGLAP. TGF-β treatment induced expression of MYOCD, MHC11, and TAGLN (Figure 6A). Western analyses of these cells demonstrated significantly more α-actin and MHC (Figure 6B). The size of α-actin-positive cells was increased, as assessed by increased forward scatter (Figure 6C). When cells were treated with TGF-β, many differentiated into filamentous α-actin- and MHC-expressing myofibroblasts (Figure 6D). On the basis of their cell surface markers, ability form colonies, and differentiation potential, these cells meet established criteria for MSCs [37].
Figure 5.
Sorted Stro-1(+), CD73(+), CD105(+) cells may be differentiated along adipogenic and osteogenic lines. In panel A, cells were differentiated for 21 days as described in the text. Adipogenic differentiation was monitored by staining for neutral lipids with Oil Red-O (stains red). Osteogenic differentiation was assessed by staining of calcium phosphate with alizarin red (red). B. Four different single cell clones isolated from different sorting experiments were exposed to adipogenic and osteogenic media (see text). Bars depict the fold increase in gene expression by quantitative PCR (mean ± SEM, *p < 0.05, paired t test).
Figure 6.
TGF-β induces myofibroblastic differentiation of lung MSCs. In panel A, three different clonal cell lines were incubated for 6 days under serum-free conditions without or with 10 ng TGF-β/mL. A. qPCR reactions were run on the cDNA produced from RNA from the cells assessing the relative expression of myosin heavy chain 11 (MHC11), transgelin (TAGLN), or myocardin (MYOCD). Expression of all three was significantly elevated with TGF-β treatment (N = 3, p < 0.05). B. Western analyses demonstrated increases in α-actin and myosin heavy chain (MHC) synthesis. Signals were quantified by densitometry and normalized to β-actin (N = 3, p < 0.05). C. TGF-β increases cell size and α-smooth muscle actin expression. Forward scatter (top panel) and α-actin immunostaining (bottom panel) for cells serum deprived for 6 days (green) or treated with TGF-β (10 ng/ml) for 6 days (orange). D. TGF-β produces an increase in cell size, α-actin, and MHC immunostaining. Myofibroblastic differentiation was assessed by staining with AF488-conjugated anti-MHC (green) and anti α-actin-Cy3 conjugate (red). Co localization of α-actin and MHC Is shown In yellow to orange. White bars indicate 100 μm.
Sorted lung MSCs have a significantly different gene expression pattern from either unsorted lung fibroblasts
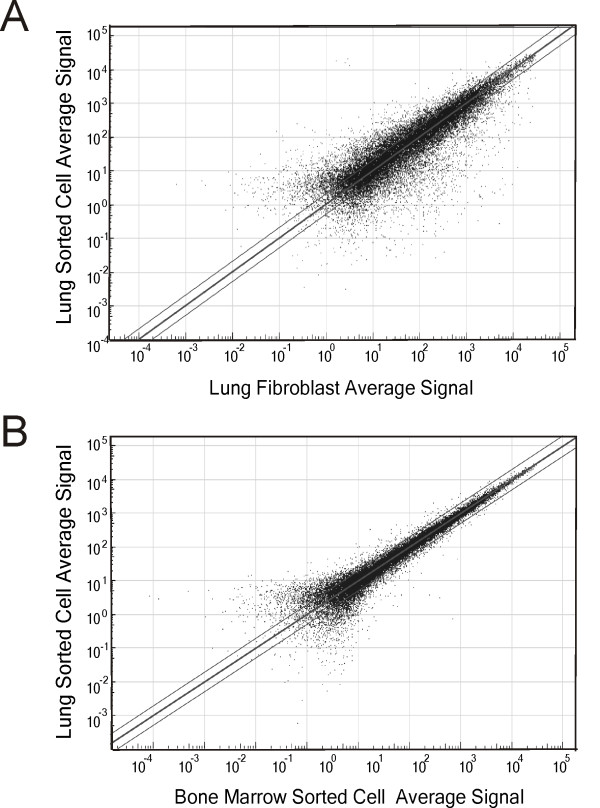
To better characterize the differences between sorted lung MSCs and other resident fibroblast-like cells, mRNA samples from three different sorted clonal lines were used to analyse patterns of gene expression. Out of 33043 mouse mRNA transcripts, a detection p ≤ 0.05 for 12,331 genes of mouse lung MSCs was obtained. Global transcript expression concordance (r2) between the sorted lung MSCs and adherent unsorted lung fibroblasts was 0.7539 (Figure 7A). We found a differential p value of ≤0.05 for 5559 genes between sorted mouse lung MSCs and unsorted adherent lung fibroblasts. On the other hand, there was high concordance between lung and bone marrow MSCs (r2 = 0.9872) (Figure 7B). Thus, the sorted multipotent stromal cells represent a distinct subpopulation of cells within the lung.
Figure 7.
Concordance of gene probe detection p-values between sorted lung multipotent stromal cells, lung fibroblasts (A) and bone marrow sorted cells (B). Parallel lines signify 1.5 fold change based on average probe signal. There was not a high degree of concordance between lung MSCs and lung fibroblasts, suggesting that lung multipotent stromal cells represent a unique population of cells in the lung.
Of the 5559 genes differing between lung MSCs and lung fibroblasts, 2480 were upregulated. These genes included selected cytokines and paracrine factors; receptors, signaling intermediates and transcriptional regulators of the TGF-β1 and TNF-α pathways; and receptor tyrosine kinases and their downstream signaling intermediates (Table 2 and Additional File 1, Table S1, supplement).
Table 2.
Selected genes upregulated in sorted lung MSCs relative to lung fibroblasts.
| Symbol average | Lung MSCs average | Lung fibroblast | Fold difference | Differential p-value |
|---|---|---|---|---|
| Secreted cytokines and paracrine factors | ||||
| Prl2c3 | 20800 | 2.91 | 7147.77 | 1.00E-13 |
| Prl2c4 | 16700 | 2.49 | 6706.83 | 1.01E-13 |
| Prl2c2 | 16400 | 2.37 | 6919.83 | 1.01E-13 |
| Efnb2 | 250 | 42.4 | 5.90 | 2.98E-08 |
| Prl2c5 | 224 | 71.5 | 3.13 | 7.15E-08 |
| Grem1 | 17700 | 2520 | 7.02 | 1.42E-06 |
| Ptges3 | 2080 | 763 | 2.73 | 1.20E-05 |
| Fgf10 | 3710 | 853 | 4.35 | 0.000233 |
| Ctf1 | 265 | 109 | 2.43 | 0.000226 |
| Ccl25 | 171 | 58.9 | 2.90 | 0.000239 |
| Fgf7 | 7600 | 3580 | 2.12 | 0.00038 |
| Efna4 | 176 | 85.3 | 2.06 | 0.00268 |
| Bmper | 2060 | 1020 | 2.02 | 0.0041 |
| Lefty1 | 411 | 244 | 1.68 | 0.0163 |
| Gdf11 | 52.3 | 7.81 | 6.70 | 0.0279 |
| Thbs1 | 961 | 300 | 3.20 | 0.0293 |
| Cx3cl1 | 384 | 81.6 | 4.71 | 0.0311 |
| Sema3a | 638 | 14.8 | 43.11 | 0.0367 |
| Spp1 | 12500 | 8920 | 1.40 | 0.0469 |
| TGF-β1 signaling | ||||
| Smad2 | 334 | 86.4 | 3.87 | 5.67E-07 |
| Smad3 | 889 | 410 | 2.17 | 5.67E-07 |
| Mapk13 | 387 | 104 | 3.72 | 1.28E-06 |
| Hdac2 | 2440 | 1300 | 1.88 | 1.58E-05 |
| Trrap | 207 | 54.2 | 3.82 | 4.02E-05 |
| Hdac4 | 179 | 69.6 | 2.57 | 0.000433 |
| Mapk11 | 115 | 30.3 | 3.80 | 0.000661 |
| Tgfbrap1 | 603 | 195 | 3.09 | 0.00172 |
| Nfatc3 | 287 | 121 | 2.37 | 0.00226 |
| Hdac3 | 1080 | 377 | 2.86 | 0.00241 |
| Hdac10 | 60.3 | 19.4 | 3.11 | 0.00378 |
| Acvr2b | 177 | 107 | 1.65 | 0.0308 |
| Endogl1 | 77.5 | 39.1 | 1.98 | 0.0462 |
Abbreviations are: Prl2c3, encoding prolactin family 2, subfamily c, member 3; Prl2c4, prolactin family 2, subfamily c, member 4 ; Prl2c2. prolactin family 2, subfamily c, member 2; Efnb2, ephrin B2; Prl2c5, prolactin family 2, subfamily c, member 5; Grem1, gremlin 1; Ptges3, prostaglandin E synthase 3; Fgf10, fibroblast growth factor 10; Ctf1, cardiotrophin 1; Ccl25, chemokine (C-C motif) ligand 25; Fgf7, fibroblast growth factor 7 or keratinocyte growth factor; Efna4, ephrin A4; Bmper, BMP-binding endothelial regulator; Lefty1, left right determination factor 1; Gdf11, growth differentiation factor 11 (BMP 11); Thbs1, thrombospondin 1; Cx3cl1, chemokine (C-X3-C motif) ligand 1; Sema3a, sema domain, secreted, (semaphorin) 3A; Spp1, secreted phosphoprotein 1, osteopontin; Smad2, MAD homolog 2 (Drosophila); Smad3, MAD homolog 3 (Drosophila); Mapk13, mitogen activated protein kinase 13 (p38); Hdac2, histone deacetylase 2; Trrap, transformation/transcription domain-associated protein; Hdac4, histone deacetylase 4; Mapk11, mitogen-activated protein kinase 11 (p38 δ); Tgfbrap1, transforming growth factor, beta receptor associated protein 1; Nfatc3, nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 3; Hdac3, histone deacetylase 3; Hdac10, histone deacetylase 10; Acvr2b, activin receptor IIB; Endogl1, endoglin (CD105);
Isolation of mesenchymal progenitor cells with surface markers of MSCs from a patient with asthma
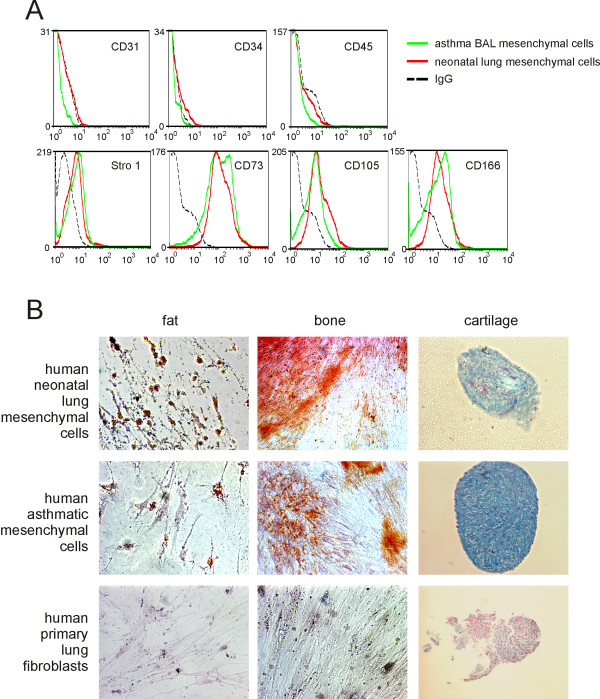
We have isolated pluripotent mesenchymal cells from the tracheal aspirates of premature infants undergoing mechanical ventilation for respiratory distress [11]. Activated fibroblasts have previously been isolated from human asthma patients [24]. We examined the BAL fluid of three children with asthma who were undergoing diagnostic flexible bronchoscopy. After two weeks of culture, one sample yielded colonies of adherent cells. These cells stained low for CD31, CD34 or CD45 and high for Stro-1, CD73, CD105, and CD166 (Figure 8A). Unlike normal human lung fibroblasts, but similar to pluripotent mesenchymal cells from human neonates [11], these cells could be differentiated along adipogenic, osteogenic and chondrogenic lines (Figure 8B). Together, these data suggest that pluripotent mesenchymal cells may be identified in the BAL of human asthma patients.
Figure 8.
Isolation of a mesenchymal progenitor cells from the BAL of a patient with asthma. A. Cells are negative for CD31, CD34 and CD45, and positive for Stro-1, CD73, CD105 and CD166. Mesenchymal cells from a patient with asthma (green) are compared with neonatal lung mesenchymal cells (red). IgG control is shown in black. B. Colonies of cells derived from the tracheal aspirates of human premature infants and a patient with asthma were isolated, grown and treated for differentiation along fat, bone or cartilage lines. Responses were compared with primary human lung fibroblasts. Shown are oil red o staining for fat droplets (left column of panels), alizarin red staining for calcium phosphate (middle column of panels) or alcian blue staining for acid mucopolysaccharide sulfates (nuclear fast red counterstain).
Discussion
Mesenchymal stem cells are multipotent cells capable of differentiation into a select range of mesenchymal cell types influenced by their microenvironment. They are identified by their cell surface markers, high proliferation rate, ability to form colonies and differentiation potential [37]. More recent work shows that mesenchymal stem cells are not after all uniform, and that pluripotent mesenchymal cells may be separated into a hierarchy with differential self-renewal potential and multipotency [16].
Using cell sorting, we isolated a population of CD34- and CD45-negative, Stro-1-positive, high CD73- and CD105-positive cells. In humans, Stro-1 is almost exclusively found on bone marrow-derived MSCs [33]. Stro-1 has been described in bone marrow-derived stem cells in the mouse [34]. CD73 (SH3/SH4) and CD105 (SH2/endoglin) are localized at the plasma membrane of bone marrow mesenchymal stromal cells [36]. The CD34- and CD45-negative, Stro-1-positive, high CD73- and CD105-positive cells grew on plastic, demonstrated ample colony-forming potential, and were capable of differentiation to osteogenic, adipogenic, and myogenic phenotypes. Taken together, these data suggest that OVA-sensitization and -challenge increases the population of MSCs in the lung.
While we did not determine the precise function of MSCs in allergic airways disease, we consider two possibilities to be most likely. First, MSCs could constitute a source of airway myofibroblasts, leading to increased airway smooth muscle mass. Patients with severe asthma also demonstrate an accumulation of myofibroblasts in the airway subepithelium [19-21]. These alterations may extend beyond the central airways to the distal airways and lung parenchyma [23]. After allergen challenge, myofibroblasts in the subepithelium of the asthmatic airway may migrate to the smooth muscle layer [22]. Previous studies have noted increased airway smooth muscle cell number in fatal and non-fatal asthma [17,18], as well as OVA-sensitized and -challenged mice [38]. Mesenchymal stem cells generally express low levels of α-smooth muscle actin, but may express high levels of this contractile protein upon TGF-β treatment [11]. In multipotent stem cells from tracheal aspirates of premature infants, autocrine production of TGF-β further drives myofibroblastic differentiation,[13]. Finally, sorted lung MSCs were enriched for many transcripts which could play a role in myofibroblast differentiation and fibrosis including those encoding CD105/endoglin, part of the TGF-β receptor complex; the TGF-β family protein Gdf11 [39,40]; the TGF-β antagonists Gremlin, Lefty1 and BMP-binding endothelial regulator [41,42]; SMADs 2 and 3; NFATc3, a transcription factor involved in TGF-β1 signaling in airway smooth muscle [43]; and osteopontin which has been shown to be required for TGF-β expression and lung fibrosis in bleomycin- [44] and OVA-treated mice [45].
Another possible function of resident multipotent stromal cells is immunomodulation. Lung-resident mesenchymal stem cells from human lung allografts inhibit T cell proliferation in vitro [46]. Intravenously-administered murine mesenchymal stem cells derived from plastic-adherent bone marrow cells protect against bleomycin lung injury through the expression of interleukin-1 receptor antagonist [47-49]. In addition, intratracheal pluripotent mesenchymal stem cells have been shown to decrease endotoxin-induced lung injury [50]. In the present study, sorted lung MSCs were enriched in transcripts encoding the pro-inflammatory IL-6 family member cardiotrophin. This cytokine also is involved in ASM hypertrophy [43]. In addition, cultured lung multipotent stromal cells also produced MCP-1/CCL2 and KC/CXCL1, cytokines that would be expected to increase airway inflammation. Several members of the prolactin 2c gene family were greatly enriched in lung multipotent stromal cells. While humans possess only one prolactin gene used for both endocrine and paracrine functions, the prolactins are a multigene family in mice [51,52]. The prolactin-like peptides, also referred to as proliferins, have been implicated in wound-healing [53], angiogenesis [54] and B cell tolerance [55].
We successfully isolated MSCs from a patient with asthma, proof of concept that MSCs may be isolated from the BAL of these patients. As noted above, multipotent mesenchymal stem cells have been isolated from the tracheal aspirates of premature infants undergoing mechanical ventilation for respiratory distress [11] and the bronchoalveolar lavage of patients who have undergone lung transplantation [12]. In the latter studies, cells were grown from colonies identified 7-21 days after initial plating on plastic [11,12]. In the present study, MSCs were identified by flow cytometry after only 5 days of adherence to plastic, and by fluorescence microscopy at the time of allergen challenge. Together, these data provide stronger evidence that multipotent mesenchymal cells are present in the lung in vivo. However, at this time it is premature to say whether MSCs play a pathogenic role in human asthma.
In summary, we have found that allergen-sensitization and -challenge leads to the emergence of a lung cell population with the surface markers, differentiation potential and clonogenic potential of multipotent stromal cells. Further animal studies will be required to determine the physiologic role of these cells. Finally, additional work is needed to determine whether lung MSCs play a role in human asthma.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JKB contributed aspects of all experimental designs, analyses and manuscript preparation; APP contributed to conceptual analyses of experiments; PDB contributed to conceptual analyses of gene chip experiments; MJL performed tissue processing, sectioning, and immunostaining; AEB assisted in developing differentiation assays; JL performed Western analyses; AMG performed ELISA and multiplex analyses; MBH contributed to conceptual design and analyses of experiments, manuscript preparation, and funding. All authors read and approved the final manuscript.
Supplementary Material
Additional selected genes upregulated in sorted lung MSCs relative to lung fibroblasts. This file contains a Table, Table S1, listing additional genes upregulated in sorted lung MSCs relative to total lung fibroblasts
Contributor Information
J Kelley Bentley, Email: kbentley@umich.edu.
Antonia P Popova, Email: antoniap@umich.edu.
Paul D Bozyk, Email: paulbozy@umich.edu.
Marisa J Linn, Email: mlinn@umich.edu.
Amy E Baek, Email: abaek@umich.edu.
Jing Lei, Email: leijing@umich.edu.
Adam M Goldsmith, Email: amgold@umich.edu.
Marc B Hershenson, Email: mhershen@umich.edu.
Acknowledgements
This work was supported by National Institutes of Health grants HL079339 and HL090134 (M.B.H.)
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak D. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG, Tuan RS. Characterization of Multipotential Mesenchymal Progenitor Cells Derived from Human Trabecular Bone. Stem Cells. 2003;21:681–693. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- Tare RS, Babister JC, Kanczler J, Oreffo RO. Skeletal stem cells: Phenotype, biology and environmental niches informing tissue regeneration. Mol Cell Endocrinol. 2008;288:11–21. doi: 10.1016/j.mce.2008.02.017. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85:962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE. et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med. 2007;175:1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- Lama VN, Smith L, Badri L, Flint AJ, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH. et al. Evidence for Tissue-Resident Mesenchymal Stem Cells in Human Adult Lung from Studies of Transplanted Allografts. J Clin Invest. 2007;117(4):989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB. Autocrine production of TGF-{beta}1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–743. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The Bone Marrow Functionally Contributes to Liver Fibrosis. Gastroenterology. 2006;130(6):1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Cho M, Suh DS, Yoon MS, Chang CL, Jung JS. et al. Cancer-Derived Lysophosphatidic Acid Stimulates Differentiation of Human Mesenchymal Stem Cells to Myofibroblast-Like Cells. Stem Cells. 2008;26(3):789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human Mesenchymal Stem Cells Self-Renew and Differentiate According to a Deterministic Hierarchy. PLoS One. 2009;4(8):e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis. 1993;148(3):720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of Smooth Muscle in Mild to Moderate Asthma without Changes in Cell Size or Gene Expression. Am J Respir Crit Care Med. 2004;169(9):1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- Roche WR, Williams JH, Beasley R, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. doi: 10.1016/S0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. American Journal of Respiratory Cell & Molecular Biology. 1990;3(5):507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, laRocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-B expression in mucosa biopsies in asthma and chronic bronchitis. Am J Resp Crit Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–673. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- Dolhnikoff M, da Silva LFF, de Araujo BB, Gomes HAP, Fernezlian S, Mulder A, Lindeman JH, Mauad T. The outer wall of small airways is a major site of remodeling in fatal asthma. Journal of Allergy and Clinical Immunology. 2009;123(5):1090–1097. doi: 10.1016/j.jaci.2009.02.032. e1091. [DOI] [PubMed] [Google Scholar]
- Larsen K, Tufvesson E, Malmstrom J, Morgelin M, Wildt M, Andersson A, Lindstrom A, Malmstrom A, Lofdahl CG, Marko-Varga G. et al. Presence of activated mobile fibroblasts in bronchoalveolar lavage from patients with mild asthma. Am J Respir Crit Care Med. 2004;170:1049–1056. doi: 10.1164/rccm.200404-507OC. [DOI] [PubMed] [Google Scholar]
- Xisto DG, Farias LL, Ferreira HC, Picanco MR, Amitrano D, Lapa e Silva JR, Negri EM, Mauad T, Carnielli D, Silva LFF. et al. Lung Parenchyma Remodeling in a Murine Model of Chronic Allergic Inflammation. Am J Respir Crit Care Med. 2005;171(8):829–837. doi: 10.1164/rccm.200408-997OC. [DOI] [PubMed] [Google Scholar]
- Henderson WR JR, Tang LO, Chu SJ, Tsao SM, Chiang GKS, Jones F, Jonas M, Pae C, Wang H, Chi EY. A Role for Cysteinyl Leukotrienes in Airway Remodeling in a Mouse Asthma Model. Am J Respir Crit Care Med. 2002;165(1):108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. American Journal Of Respiratory Cell And Molecular Biology. 2002;27(5):526–535. doi: 10.1165/rcmb.2002-0048OC. [DOI] [PubMed] [Google Scholar]
- Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenzie S, Broxmeyer HE, Moore MA. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the Sca-1 positive cell fraction. Stem Cells. 2008. stemcells.2008-0866. [DOI] [PubMed]
- Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- Blazsek I, Chagraoui J, Péault B. Ontogenic emergence of the hematon, a morphogenetic stromal unit that supports multipotential hematopoietic progenitors in mouse bone marrow. Blood. 2000;96:3763–3771. [PubMed] [Google Scholar]
- Kémoun P, Laurencin-Dalicieux S, Rue J, Vaysse F, Roméas A, Arzate H, Conte-Auriol F, Farges JC, Salles JP, Brunel G. Localization of STRO-1, BMP-2/-3/-7, BMP receptors and phosphorylated Smad-1 during the formation of mouse periodontium. Tissue Cell. 2007;39:257–266. doi: 10.1016/j.tice.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, Rosset P, Sensebé L, Layrolle P, Häupl T. et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Bentley JK, Deng H, Linn MJ, Lei J, Dokshin GA, Fingar DC, Bitar KN, Henderson WR, Hershenson MB. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3{beta} phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L176–184. doi: 10.1152/ajplung.90376.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes & Development. 2002;16(21):2749–2754. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Ibanez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7(8):831–837. doi: 10.1038/sj.embor.7400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga Ae. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133(22):4463–4473. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- Deng H, Dokshin GA, Lei J, Goldsmith AM, Bitar KN, Fingar DC, Hershenson MB, Bentley JK. Inhibition of Glycogen Synthase Kinase-3{beta} Is Sufficient for Airway Smooth Muscle Hypertrophy. J Biol Chem. 2008;283(15):10198–10207. doi: 10.1074/jbc.M800624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JS, Serlin D, Li X, Whitley G, Hayes J, Rishikof DC, Ricupero DA, Liaw L, Goetschkes M, O'Regan AW. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1311–1318. doi: 10.1152/ajplung.00394.2003. [DOI] [PubMed] [Google Scholar]
- Kohan M, Breuer R, Berkman N. Osteopontin Induces Airway Remodeling and Lung Fibroblast Activation in a Murine Model of Asthma. Am J Respir Cell Mol Biol. 2009;41(3):290–296. doi: 10.1165/rcmb.2008-0307OC. [DOI] [PubMed] [Google Scholar]
- Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN. Lung Resident Mesenchymal Stem Cells Isolated from Human Lung Allografts Inhibit T Cell Proliferation via a Soluble Mediator. J Immunol. 2008;181(6):4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. PNAS. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman R, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, DuTreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J Immunol. 2007;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Nilsen-Hamilton MHR, Alvarez-Azaustre E. Relationship between mitogen-regulated protein (MRP) and proliferin (PLF), a member of the prolactin/growth hormone family. Gene. 1987;51(2):163–170. doi: 10.1016/0378-1119(87)90304-0. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Shao Lj, Ain R, Dai G, Soares MJ. The Mouse Prolactin Gene Family Locus. Endocrinology. 2003;144(1):313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- Fassett JT, Nilsen-Hamilton M. Mrp3, a Mitogen-Regulated Protein/Proliferin Gene Expressed in Wound Healing and in Hair Follicles. Endocrinology. 2001;142(5):2129–2137. doi: 10.1210/en.142.5.2129. [DOI] [PubMed] [Google Scholar]
- Corbacho A, Martinez De La Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J Endocrinol. 2002;173(2):219–238. doi: 10.1677/joe.0.1730219. [DOI] [PubMed] [Google Scholar]
- Saha S, Gonzalez J, Rosenfeld G, Keiser H, Peeva E. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis & Rheumatism. 2009;60(6):1743–1752. doi: 10.1002/art.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional selected genes upregulated in sorted lung MSCs relative to lung fibroblasts. This file contains a Table, Table S1, listing additional genes upregulated in sorted lung MSCs relative to total lung fibroblasts