Abstract
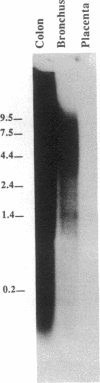
The amino acid and sugar composition of mucins from various organs is similar but not identical. This could arise by one or more of the following: organ-specific processing of a single core protein, organ-specific splicing of a single mucin mRNA, or organ-specific expression of various mucin genes. To begin to investigate the source of this variability, we examined (a) immunological cross-reactivity and (b) cDNA cross-hybridization, among several mucin-secreting organs of the human body. Peptide-directed antibodies raised against both nondeglycosylated (LS) and deglycosylated (HFB) intestinal mucin strongly stained mucous cells in the bronchial epithelium and submucosal glands, indicating homology between mucins of the bronchus and intestine at the peptide level. By screening a bronchus cDNA library with an intestinal mucin cDNA, SMUC-41, we isolated a bronchus mucin cDNA, HAM-1. This cDNA is 96% homologous to the first repeat of SMUC-41. HAM-1 hybridized to restriction fragments of human genomic DNA identical to those hybridizing to SMUC-41 on Southern blots. SMUC-41 also hybridized to polydisperse transcripts in the bronchus, cervix, gall bladder, and mammary gland, indicating mucin homology among all these organs at the RNA level. We conclude that the bronchus and intestine express a common mucin gene, which is likely co-expressed by at least several other mucin-secreting organs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa T., Shimura S., Sasaki H., Takishima T., Yaegashi H., Takahashi T. Morphometric analysis of intraluminal mucus in airways in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989 Aug;140(2):477–482. doi: 10.1164/ajrccm/140.2.477. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Nardelli J., Siddiqui B., Kim Y. S. Isolation and characterization of colon cancer mucin from xenografts of LS174T cells. Cancer Res. 1988 Dec 1;48(23):6678–6685. [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace K. V., Flux M., Sachdev G. P. Comparison of physicochemical properties of purified mucus glycoproteins isolated from respiratory secretions of cystic fibrosis and asthmatic patients. Biochemistry. 1985 Dec 3;24(25):7334–7341. doi: 10.1021/bi00346a047. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Finkbeiner W. E., Basbaum C. B. Monoclonal antibodies directed against human airway secretions. Localization and characterization of antigens. Am J Pathol. 1988 May;131(2):290–297. [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Burchell J. M., Duhig T., Lamport D., White R., Parker M., Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Gilks C. B., Reid P. E., Clement P. B., Owen D. A. Histochemical changes in cervical mucus-secreting epithelium during the normal menstrual cycle. Fertil Steril. 1989 Feb;51(2):286–291. doi: 10.1016/s0015-0282(16)60492-2. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Shin S. S., Manderino G. L., Rittenhouse H. G., Tomita J. T., Gooch G. T. Selective expression of a novel mucin-type glycoprotein in human tumors: immunohistochemical demonstration with Mab A-80. Hum Pathol. 1988 Jun;19(6):623–627. doi: 10.1016/s0046-8177(88)80167-9. [DOI] [PubMed] [Google Scholar]
- Griffiths B., Bobrow L. G., Happerfield L., Swallow D. M. Expression of the hypervariable PUM locus in normal and malignant lung: the tumor-associated epitopes are present but masked in normal tissue. Dis Markers. 1988 Jul-Sep;6(3):195–202. [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Kuan S. F., Byrd J. C., Basbaum C. B., Kim Y. S. Characterization of quantitative mucin variants from a human colon cancer cell line. Cancer Res. 1987 Nov 1;47(21):5715–5724. [PubMed] [Google Scholar]
- Lange P., Nyboe J., Appleyard M., Jensen G., Schnohr P. Ventilatory function and chronic mucus hypersecretion as predictors of death from lung cancer. Am Rev Respir Dis. 1990 Mar;141(3):613–617. doi: 10.1164/ajrccm/141.3.613. [DOI] [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Lafitte J. J., Houdret N., Pruvot F. R., Lamblin G., Slayter H. S., Roussel P. Peptides of human bronchial mucus glycoproteins. Size determination by electron microscopy and by biosynthetic experiments. Biochem J. 1987 Nov 15;248(1):189–195. doi: 10.1042/bj2480189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini J. M., Marianne T., Lafitte J. J., Lamblin G., Roussel P., Mazzuca M. Use of an antiserum against deglycosylated human mucins for cellular localization of their peptide precursors: antigenic similarities between bronchial and intestinal mucins. J Histochem Cytochem. 1989 Jun;37(6):869–875. doi: 10.1177/37.6.2470810. [DOI] [PubMed] [Google Scholar]
- Peto R., Speizer F. E., Cochrane A. L., Moore F., Fletcher C. M., Tinker C. M., Higgins I. T., Gray R. G., Richards S. M., Gilliland J. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis. 1983 Sep;128(3):491–500. doi: 10.1164/arrd.1983.128.3.491. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Stranahan P. L., Due C., Rønne E., Sørensen H. R., Olsson L. Glycoproteins distinguishing non-small cell from small cell human lung carcinoma recognized by monoclonal antibody 43-9F. Cancer Res. 1987 Feb 15;47(4):1161–1169. [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Timpte C. S., Eckhardt A. E., Abernethy J. L., Hill R. L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J Biol Chem. 1988 Jan 15;263(2):1081–1088. [PubMed] [Google Scholar]
- Trump B. F., McDowell E. M., Glavin F., Barrett L. A., Becci P. J., Schürch W., Kaiser H. E., Harris C. C. The respiratory epithelium. III. Histogenesis of epidermoid metaplasia and carcinoma in situ in the human. J Natl Cancer Inst. 1978 Aug;61(2):563–575. [PubMed] [Google Scholar]
- Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- Woodward H. D., Ringler N. J., Selvakumar R., Simet I. M., Bhavanandan V. P., Davidson E. A. Deglycosylation studies on tracheal mucin glycoproteins. Biochemistry. 1987 Aug 25;26(17):5315–5322. doi: 10.1021/bi00391a015. [DOI] [PubMed] [Google Scholar]
- Woodward H., Horsey B., Bhavanandan V. P., Davidson E. A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982 Feb 16;21(4):694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]