Abstract
The FOXP3 (forkhead box P3) gene is a member of forkhead winged helix family transcription factors and functions as both a transcriptional activator and a repressor. FOXP3 dysfunction is responsible for an X-linked autoimmune syndrome: immune dysregulation, polyendopathy, enterophathy, X-linked syndrome. In addition to its role as an essential transcription factor in regulatory T cells, the FOXP3 gene is an epithelial cell-intrinsic tumor suppressor for breast and prostate cancers. We will focus on the FOXP3 signalling pathway in epithelial cells and discuss how genetic and/or epigenetic inactivation of the FOXP3 contributes to the malignant transformation of cells.
Keywords: FOXP3, epithelial cell, X-linked tumor suppressor gene, breast cancer, prostate cancer
1. Introduction
The FOXP3 gene is a member of the forkhead winged helix family transcription factors and is essential for the development and function of CD4+CD25+ regulatory T cells (Treg) (reviewed in (Liu et al., 2010)). In addition, FOXP3 is expressed in epithelial cells from various organs such as breast, thymus, prostate and lung (Chang et al., 2005; Chen et al., 2008). Importantly, mice that are heterozygous for Foxp3 mutations spontaneously develop mammary carcinomas at a high frequency (Zuo et al., 2007b). Genetic analyses in both mice and humans revealed that FOXP3 is an important X-linked tumor suppressor in breast cancers (Liu et al., 2009a; Zuo et al., 2007a; Zuo et al., 2007b). Moreover, we recently found that FOXP3 also functions as a tumor suppressor in prostate cancer (Wang et al., 2009).
Despite the importance of FOXP3 in mammary and prostate carcinogenesis pathways, the signalling networks of FOXP3 in normal and/or malignant epithelial cells have not yet been fully elucidated. In this review, we will focus on the function of the FOXP3 as a tumor suppressor in epithelial cells, and discuss how its inactivation contributes to the malignant transformation of cells. We will also discuss how reactivation of FOXP3 in tumor samples may be explored for cancer therapy.
2. Functions and Pathologic Context
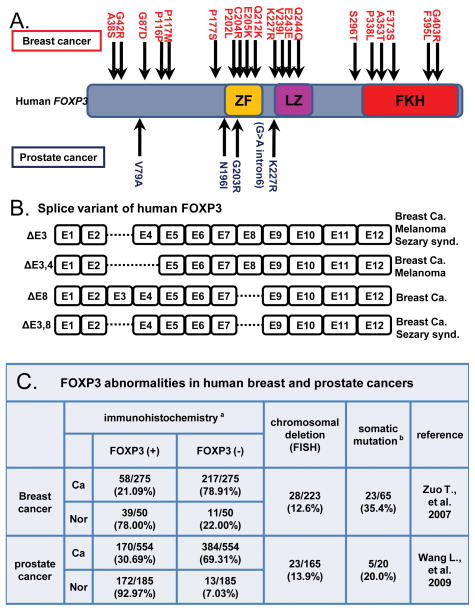
FOXP3 is a member of the FOXP family which has at least four members, FOXP1-4. All contain highly conserved c-terminal tetramerization domains composed of zinc-finger and leucine zipper domains and a DNA binding forkhead box domain (Fig. 1) (Lopes et al., 2006). FOXP3 localizes in the nucleus, and it functions as a sequence-specific transcription factor. The DNA binding forkhead domain of FOXP3 binds to specific DNA sequences in gene promoters: 5′-RYMAAYA-3′ in which R=A/G, Y=C/T, M=A/C.
Figure 1.
A. Diagram of the human FOXP3 and its somatic mutations found among human breast and prostate cancers. ZF: zing finger domain. LZ: leucine-zipper domain. FKH: forkhead domain. B. Splice variants of the FOXP3 that are predominantly expressed in human cancers. “Δ” represents any exons that is/are deleted in the variant forms of FOXP3. Breast Ca: Breast cancer. Sezary synd.: Sezary syndrome. C. FOXP3 aberrations found in human breast and prostate cancers. aSamples with nuclear FOXP3 staining were scored as positive. bSixty-five breast cancer samples and 20 prostate cancer samples were sequenced. Four out of five somatic mutations of prostate cancer were missense mutations, while the remaining one was found in an intron. Ca: cancer tissue. Nor: normal tissue. FISH: fluorescent in situ hybridization.
We observed that mice with germline Foxp3 mutations are substantially more prone to developing mammary carcinomas, either spontaneously or carcinogen-induced (Zuo et al., 2007b). The role of the Foxp3 gene in the mammary carcinogenesis has been supported by several lines of evidence (Fig. 1). The Foxp3 gene is expressed in normal breast epithelia but is down-regulated in mammary cancer (Zuo et al., 2007b). Ectopic expression of Foxp3 in variety of breast cancer cell lines resulted in cell cycle arrest; cessation of cell growth (Zuo et al., 2007b). Moreover, Foxp3 directly regulates transcription of ErbB2, Skp2 and p21 (Liu et al., 2009a; Zuo et al., 2007a; Zuo et al., 2007b). Furthermore, our analyses of clinical human breast cancers also supported that FOXP3 plays an important role in mammary carcinogenesis (Fig. 1). Frequent chromosomal deletions and somatic mutations of the FOXP3 gene were detected in cancer samples (Zuo et al., 2007b). By immunohistochemistry (IHC) onto tissue microarrays, we found down-regulation of FOXP3 in cancer cells compared to normal breast epithelia (Liu et al., 2009a; Zuo et al., 2007a; Zuo et al., 2007b). Surprisingly, a recent study showed the expression of FOXP3 in over 60% of breast cancer (Merlo et al., 2009). However, this study included cancer cells expressing FOXP3 in its cytoplasmic form, which may well be mutant FOXP3 (Wang et al., 2009).
The FOXP3 gene also plays an important role in prostate epithelia (Fig. 1) (Wang et al., 2009). Among human prostate cancers, we found frequent chromosomal deletions, somatic mutations and epigenetic silencing of the FOXP3 gene (Fig. 1) (Wang et al., 2009). Since the FOXP3 gene is located on the X chromosome, a genetic/epigenetic single-hit results in inactivation of this gene in males. IHC revealed that FOXP3 expression was significantly down-regulated in cancer cells when compared to normal prostate glands (Wang et al., 2009). Moreover, mice with prostate-specific ablations of Foxp3, Foxp3fl/y;PB-Cre+, developed prostatic hyperplasia and prostatic intraepithelial neoplasm (PIN), putative pre-cancerous lesions of the prostate (Wang et al., 2009). In human samples, FOXP3 expression in PINs were already down-regulated compared to adjacent normal prostate glands, which suggests that the inactivation of the FOXP3 gene plays an important role in the initial stage of prostatic carcinogenesis (Wang et al., 2009). Importantly, we identified c-MYC to be directly repressed by FOXP3 in prostate epithelia (Wang et al., 2009).
A recent study reported that among 26 ovarian cancer samples, the expression of the FOXP3 was significantly decreased as compared to normal ovarian epithelia (Zhang and Sun, 2010). Another interesting aspect of FOXP3 abnormalities is that some types of cancers predominantly express splice variants of the FOXP3 protein. As shown in figure 1B, among breast and ovarian cancers, malignant melanomas and malignant T cells of Sezary syndrome, specific splice variants of the FOXP3, such as Δ3, Δ3-4, Δ3/8 and Δ8, were reported to be preferably expressed (Ebert et al., 2008; Krejsgaard et al., 2008; Zhang and Sun, 2010; Zuo et al., 2007b). The Δ3-4 splice variant resulted in a truncated FOXP3 with a premature stop codon, and therefore might contribute to the malignant progression of cells.
3. Cascades and Key Molecules
3.1. HER2/neu (ERBB2)
HER2 is a member of the transmembrane receptor tyrosine kinases and is involved in the regulations of various cellular functions such as cell growth and survival (Spector and Blackwell, 2009). The cytoplasmic portion of HER2 is phosphorylated at conserved tyrosine residues and these phosphorylated tyrosines can serve as binding sites for adaptors which link HER2 to its downstream pathways or targets such as PI3K-Akt and MAPK-Erk (Spector and Blackwell, 2009). Both HER2 gene amplification and loss of nuclear FOXP3 contributed to HER2 overexpression in breast cancer samples (Zuo et al., 2007b). FOXP3 can repress the transcription of HER2 in human breast cancers by binding directly to ERBB2 gene promoter (Fig. 2) (Zuo et al., 2007b). Since in vitro HER2 overexpression nullifies the ability of FOXP3 to inhibit cell growth (Zuo et al., 2007b), repression of HER2 may be critical for the tumor suppressor function of FOXP3 in the breast epithelial cells.
Figure 2.
A schematic view of the signalling networks of the FOXP3 in epithelial cells.
3.2. MYC
Overexpression of c-MYC contributes to more aggressive and poorly differentiated cancer phenotypes (Pelengaris et al., 2002). c-MYC is a sequence specific transcription factor and an important player of various cellular processes including cell cycle and apoptosis; processes which are also dysregulated in cancer cells with high level c-MYC expressions (Pelengaris et al., 2002). c-MYC directly activates CDK4 and CCND2 expression, while indirectly represses CDK inhibitors such as CDKN1A (p21) and CDKN2B (p15) expression (Pelengaris et al., 2002). Moreover, c-MYC directly up-regulates eIF4E and eIF2α; both of which are the rate-limiting effectors of cell cycle (Pelengaris et al., 2002). In prostatic epithelial cells, FOXP3 directly represses c-MYC gene expression (Fig. 2) (Wang et al., 2009). Importantly, mice with prostate-specific ablation of the Foxp3 gene exhibited elevated expression of c-Myc, and in human prostate cancers mRNA expressions of FOXP3 and c-MYC showed an inverse relationship (p=6.29×10−6) (Wang et al., 2009).
3.3. p21 (CDKN1A, CIP1, WAF1)
p21 is a universal CDK inhibitor and plays an important role in regulating cell cycle progression, specifially at the G1-checkpoint (Abukhdeir and Park, 2008). It is frequently implicated in multiple malignancies including breast cancer (Abukhdeir and Park, 2008). Cancer cells with low levels of p21 can escape from G1 arrest, and thus cells acquire a growth advantage during tumor development. We have reported that in breast epithelial cells, FOXP3 occupies and activates the p21 promoter (Fig. 2) (Liu et al., 2009a). In various breast cancer cell lines, p21 expression was significantly upregulated after FOXP3 induction (Liu et al., 2009a). IHC of human breast cancer tissue microarray revealed that the protein expression of FOXP3 and p21 showed a positive correlation (p=0.011) (Liu et al., 2009a). Moreover, cell growth suppression by FOXP3 transduction was rescued by shRNA silencing of the p21 gene (Liu et al., 2009a). The data strongly suggests that the activation of p21 contributes to FOXP3’s tumor suppressor function.
3.4. SKP2
High levels of expression of SKP2 has been reported in a wide variety of cancers (Nakayama and Nakayama, 2006). SKP2 is an important player in the ubiquitin dependent degradation of p27KIP1, a CDK inhibitor especially of Cyclin-E/CDK2 and Cyclin-A/CDK2 (Nakayama and Nakayama, 2006). SKP2 is robustly expressed during S and G2 phases of cell cycle and regulates p27 degradation. Thus SKP2 facilitates progression of the cell cycle. In human cancers, overexpression of SKP2 is frequently observed and is correlated with poor prognoses of breast cancers (Nakayama and Nakayama, 2006). We found that FOXP3 directly represses SKP2 expression in human and mouse mammary epithelial cells (Fig. 2) (Zuo et al., 2007a). Foxp3 occupies the Skp2 promoter and represses promoter activity of the locus (Zuo et al., 2007a). Among human breast cancers, expression of FOXP3 negatively correlated with SKP2 levels (p=0.0016) (Zuo et al., 2007a). As in the case of p21, FOXP3 directly regulates key molecules of cell cycle regulation, which further supports the notion that FOXP3 is an important tumor suppressor.
3.5. Molecular mechanisms by which FOXP3 regulates the expression of its target genes
Previous reports have revealed that FOXP3 forms complexes with Rel family transcription factors NFAT and NFκB, and FOXP3 blocks their ability to activate Il-2 and INFγ transcription (Bettelli et al., 2005; Holmes et al., 2008). By making a repressive FOXP3:NFAT complex, FOXP3 inhibits NFAT:AP-1 complex at the Il-2 promoter (Holmes et al., 2008; Wu et al., 2006). Another report showed that FOXP3 could also weaken DNA binding activity of AP-1 (Lee et al., 2008). AML1/RUNX1, which activates endogenous Il-2 and IFNγ expression in CD4+ T cells, is reported to make a complex with FOXP3 (Ono et al., 2007). AML1/RUNX1 could bind to the Il-2 enhancer with FOXP3 and exert optimal repression of the Il-2 in Tregs (Ono et al., 2007).
We and others have found that FOXP3 can dramatically change histone modifications at its binding loci (Liu et al., 2009a; Zheng et al., 2007). In this case, FOXP3 functions as a scaffolding molecule which recruits other co-factors to the FOXP3-bound loci. So far, a couple of histone modification enzymes such as TIP60, HDACs 7 and 9, and EOS/CtBP1 have been found to be physically associated with FOXP3 to form a repressor complex (Li et al., 2007; Pan et al., 2009). What FOXP3 complexes are involved in FOXP3-mediated gene activation remains elusive.
3.6. Regulation of FOXP3 expression
In Tregs, a couple of molecules and pathways have been identified to activate Foxp3 gene expression. TGFβ-Smad, Il-2 and INFγ signals are known to activate Foxp3 expression in the development of Tregs (Lal and Bromberg, 2009). SMAD, NFAT and Rel family molecules are known to occupy Foxp3 enhancer and/or promoter loci and to activate Foxp3 transcription (Ruan et al., 2009; Tone et al., 2008). In mammary epithelial cells, ATF-2 is essential for FOXP3 expression (Liu et al., 2009b). In cancer cells, doxolubicine, which induces activation of p53 via DNA damage response pathway, significantly upregulates FOXP3 expression (Jung et al., 2010).
4. Therapeutic Implications
The FOXP3 gene has important functions as a tumor suppressor of breast and prostate cancers. Thus an interesting issue is whether the FOXP3 gene is also a tumor suppressor for other malignancies. Our investigation of heterozygous Scurfy mice Foxp3sf/+ revealed that they also harbored spontaneous lymphoma, hepatoma and sarcoma although their incidences were lower than that of mammary tumors (Zuo et al., 2007b). In addition, aberrant FOXP3 expression and/or splicing have also been reported in melanoma, malignant T cell, ovarian cancer and pancreatic cancer (Ebert et al., 2008; Hinz et al., 2007; Krejsgaard et al., 2008; Zhang and Sun, 2010). However, molecular biological functions of the FOXP3 in these malignancies have not been fully investigated.
As discussed in greater detail elsewhere (Liu et al., 2010), in female cancers, X-inactivation inactivates only one of the FOXP3 alleles, and therefore it might be possible to reactivate the silenced FOXP3 gene from this allele for therapeutic purposes. Although molecular mechanisms have not been clarified yet, some agents have been reported to activate FOXP3 in cancer cells. We reported that anisomycin could induce the transcription of FOXP3 in various breast cancer cell lines, and induced FOXP3 significantly repressed cell growth in vitro and tumor formations of implanted cancer cells in vivo (Liu et al., 2009b). In breast and colon cancer cell lines, FOXP3 expression is directly regulated by p53, and doxolubicine which activates p53 dramatically activated FOXP3 transcription in vitro (Jung et al., 2010). These reports further supports a promising hypothesis that the restoration of the FOXP3 function in tumor cells could have great potential to be a novel therapeutic strategy against human malignancies. Additional studies are needed to determine whether induction of FOXP3 can be explored for cancer therapy. And it is also substantially important to explore any methodologies and devices to re-activate the FOXP3 gene specifically in malignant cells.
Signalling network Facts.
FOXP3 is expressed not only in regulatory T cells but also in epithelial cells from various organs.
FOXP3 acts both as a transcriptional activator and repressor. FOXP3 directly regulates the expression of both oncogenes and tumor suppressor genes, including ERBB2, SKP2, c-MYC, p21 and other important cancer-related genes.
Genetic and/or epigenetic inactivation of the FOXP3 gene in breast and prostate epithelial cells plays an important role in the development and progression of cancers.
Because many important cancer-related signalling pathways are regulated by FOXP3, it may be a potential therapeutic target for human malignancies.
Acknowledgments
We apologize to any authors whose work could not be referenced due to strict limitation in citation numbers. We thank Drs. Lizhong Wang and Runhua Liu for valuable discussions about this manuscript, and Ms. Chun-Shu Wong for editorial assistance. This work was supported by grants from National Institute of Health and Department of Defense, the United States of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. doi: 10.1017/S1462399408000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A. 2005;102:5138–43. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Gao JX, Jiang Q, Wen J, Seifers N, Su L, et al. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med. 2005;202:1141–51. doi: 10.1084/jem.20050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: Broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol. 2008;180:5163–6. doi: 10.4049/jimmunol.180.8.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–9. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- Holmes D, Jiang Q, Zhang L, Su L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol Res. 2008;41:248–66. doi: 10.1007/s12026-008-8037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DJ, Jin DH, Hong SW, Kim JE, Shin JS, Kim D, et al. Foxp3 expression in p53-dependent DNA damage responses. J Biol Chem. 2010;285:7995–8002. doi: 10.1074/jbc.M109.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejsgaard T, Gjerdrum LM, Ralfkiaer E, Lauenborg B, Eriksen KW, Mathiesen AM, et al. Malignant Tregs express low molecular splice forms of FOXP3 in Sezary syndrome. Leukemia. 2008;22:2230–9. doi: 10.1038/leu.2008.224. [DOI] [PubMed] [Google Scholar]
- Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–35. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Gao B, Fang D. FoxP3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA-binding activity of AP-1. Blood. 2008;111:3599–606. doi: 10.1182/blood-2007-09-115014. [DOI] [PubMed] [Google Scholar]
- Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–6. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang L, Chen G, Katoh H, Chen C, Liu Y, et al. FOXP3 up-regulates p21 expression by site-specific inhibition of histone deacetylase 2/histone deacetylase 4 association to the locus. Cancer Res. 2009a;69:2252–9. doi: 10.1158/0008-5472.CAN-08-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Zheng P. X-linked tumor suppressors: perplexing inheritance, a unique therapeutic opportunity. Trends Genet. 2010 doi: 10.1016/j.tig.2010.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Li W, Zheng P, Liu Y. Activating transcription factor 2 and c-Jun-mediated induction of FoxP3 for experimental therapy of mammary tumor in the mouse. Cancer Res. 2009b;69:5954–60. doi: 10.1158/0008-5472.CAN-09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, Ochs HD, et al. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol. 2006;177:3133–42. doi: 10.4049/jimmunol.177.5.3133. [DOI] [PubMed] [Google Scholar]
- Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–52. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–9. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–6. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–76. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–40. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–47. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, et al. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell. 2009;16:336–46. doi: 10.1016/j.ccr.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Sun H. Up-regulation of Foxp3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 2010;287:91–7. doi: 10.1016/j.canlet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, et al. FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest. 2007a;117:3765–7s3. doi: 10.1172/JCI32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007b;129:1275–86. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]