Abstract
Prolonged seizures (status epilepticus) are associated with brain region-specific regulation of apoptosis-associated signaling pathways. Bcl-2 homology domain 3-only (BH3) members of the Bcl-2 gene family are of interest as possible initiators of mitochondrial dysfunction and release of apoptogenic molecules after seizures. Previously, we showed expression of the BH3-only protein Bim increased in the rat hippocampus but not neocortex following focal-onset status epilepticus. Here, we examined Bim expression in mice and compared seizure-damage between wild-type and Bim-deficient animals. Status epilepticus induced by intra-amygdala kainic acid caused extensive neuronal death within the ipsilateral hippocampal CA3 region. Hippocampal activation of factors associated with transcriptional and post-translational activation of Bim, including CHOP and c-Jun NH(2)-terminal kinases, was significant within 1 h. Up-regulation of bim mRNA was evident after 2 h and Bim protein was increased from 4–24 h. Hippocampal CA3 neurodegeneration was reduced in Bim-deficient mice compared to wild-type animals following seizures in vivo, and short interfering RNA molecules targeting bim reduced cell death following kainic acid treatment of hippocampal organotypic cultures. In contrast, neocortical Bim expression declined after status epilepticus and neocortex damage in Bim-deficient mice was comparable to wild-type animals. These results demonstrate region-specific differential contributions of Bim to seizure-induced neuronal death.
Keywords: Apoptosis, Bax, Bcl-2, FKHR, Hippocampus, JNK, Neuroprotection, Temporal lobe epilepsy
Signaling pathways associated with the orchestration of apoptosis may contribute to neuronal death after experimental seizures.1 Both caspase-dependent and –independent apoptosis pathways have been implicated.1 The Bcl-2 gene family are critical upstream regulators of the mitochondrial (intrinsic) apoptotic pathways.2 Prolonged seizures (status epilepticus), and glutamate excitotoxicity in certain models, cause mitochondrial dysfunction and release of apoptogenic molecules, including cytochrome c,3 and apoptosis inducing factor (AIF).4 Support for involvement of Bcl-2 family members comes from several observations. Seizures cause multi-domain pro-apoptotic Bax to cluster around mitochondria at the time of cytochrome c release,5 and Bax-deficient neurons have been reported to be abnormally resistant to excitotoxicity.6 Moreover, over-expression of anti-apoptotic Bcl-xl reduces cell death after seizure-like insults in vivo,7 and, conversely, mice deficient in anti-apoptotic Bcl-w are abnormally vulnerable to hippocampal damage after seizures.3 However, a functional role for Bax in excitotoxicity has been excluded by some studies.4 Also, calcium exposure of mitochondria alone,8 and calpain activity,9 can trigger release of apoptogenic factors from mitochondria in neurons independently of Bcl-2 family proteins.
Members of the Bcl-2 homology (BH) domain 3-only subfamily are critical initiators of intrinsic apoptosis signaling and function by activating Bax/Bak either through direct binding or indirectly by binding and inhibiting anti-apoptotic Bcl-2 family members.2 Bcl-2 interacting mediator of cell death (Bim) is amongst the most potent of the BH3-only proteins, because unlike some BH3-only proteins (e.g. Bad) it avidly binds all anti-apoptotic Bcl-2 members.10 Expression of Bim is detectable at low levels in normal (unstressed) brain.11 Bim expression is controlled by a complex series of transcription factors, including forkhead transcription factors belonging to the FoxO subfamily,12,13 c-Jun,14 and CHOP (CCAAT/enhancer-binding protein-homologous protein, also known as GADD153).15 Bim activity is also regulated post-translationally. The c-Jun NH(2)-terminal kinases (JNK) can phosphorylate Bim and thereby potentiate its activity,16,17 while phosphorylation by mitogen-activated protein kinases leads to Bim inactivation,18 and/or ubiquitin-mediated degradation.19,20 Bim-deficient cells are resistant to certain apoptotic stimuli, including deregulated calcium flux,21 and Bim has also been reported to function in caspase-independent pathways in neurons by stimulating AIF release.22
We previously demonstrated that Bim is rapidly induced in the rat hippocampus following focal-onset status epilepticus.23 Bim levels were not altered in the neocortex, while Bim was down-regulated following repeated brief, non-damaging seizures; a model of epileptic tolerance.23 Using an in vitro model of seizure-like injury, Bim upregulation was linked to the FoxO1 transcription factor and antisense targeting of bim mRNA reduced neuronal death.23 Despite these findings, the relationship between seizures, Bim activation and regional vulnerability remains uncertain, and a causal role in vivo remains unproven. In the present study, we used a combination of in vivo and in vitro mouse models and cells deficient or depleted of Bim to characterize the expressional response of Bim and to determine whether Bim is important for seizure-induced neuronal death.
Results
Hippocampal seizures and subfield-specific damage after focal-onset status epilepticus
To examine the role of Bim during seizure-induced neuronal death we used a model of focal-onset status epilepticus induced by intra-amygdala microinjection of the glutamate analogue kainic acid (KA), followed by application of lorazepam to limit damage and mortality.24,25 First, we performed in vivo intra-hippocampal depth electrode recordings from the ipsilateral CA3 subfield during seizures. These recordings confirmed that intra-amygdala KA microinjection elicited high amplitude and high frequency electrographic seizure discharges within the hippocampus (Figure 1A). Next, we examined hippocampal injury using Fluoro-Jade B to stain degenerating neurons. Confirming previous reports,24,25 there was hippocampal neuronal death 24 h after seizures that particularly affected the ipsilateral CA3 and hilar region (Figure 1B). In addition, minor and variable neuronal death was found in the CA1 subfield as previously observed.24 No neuronal death was observed in the hippocampus from vehicle-injected control mice (Figure 1B) or in the contralateral hippocampus of mice that underwent status epilepticus (data not shown).
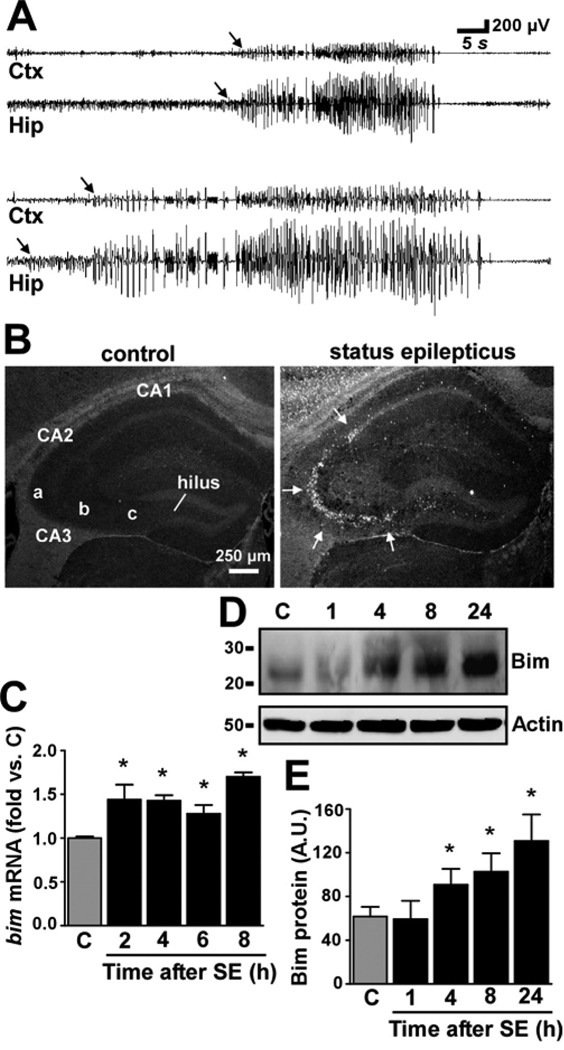
Figure 1. Upregulation of Bim in the hippocampus during seizure-induced neuronal death.
(A) Traces of two high amplitude high frequency seizure burst events captured using combined intra-hippocampal-cortical EEG. Arrows demark point of onset. Note that seizure activity is detected in the hippocampus (Hip) before the cortex (Ctx). (B) Photomicrographs (4× lens) show representative Fluoro-Jade B staining at 24 h in hippocampus of control mice and mice that underwent status epilepticus. Arrows denote cells (white) undergoing degeneration. (C) Graph showing quantification of mRNA levels for bim corrected to β-actin in control (C, 2 h) and seizure-damaged mice at various time points after lorazepam was given. Data are from four independent experiments. (D) Representative Western blot (n = 2 per lane) showing increased expression of Bim from 4–24 h after seizures compared to control and 1 h post-seizure. Actin is shown as a control for protein loading. (E) Graph showing semi-quantification of Bim protein levels. Data are from three independent experiments. A.U., arbitrary units. Molecular weight markers are depicted to the left. *p < 0.05 compared to control.
Upregulation of Bim in the hippocampus following focal-onset status epilepticus
Quantitative real-time PCR determined seizures caused an early (~2 h) increase in hippocampal levels of bim mRNA and these levels remained elevated until at least 8 h later (Figure 1C). Western blot analysis of extracts from the seizure-damaged hippocampus revealed a significant increase in Bim protein levels at 4 h which continued to increase at later time-points examined (8 h and 24 h respectively) (Figure 1D, E). The molecular weight of the Bim band corresponded to the BimEL isoform (~26 kDa).10,11 Bim induction was dependent on mice experiencing high amplitude, high frequency polyspike seizure discharges, because hippocampal Bim levels at 24 h in mice injected with KA that did not develop status epilepticus (n = 2) were similar to those seen in control brains (data not shown).
Seizures activate FoxO transcription factors, CHOP and JNKs in hippocampus
In an effort to determine the pathways involved in up-regulation of Bim in the hippocampus after seizures, we performed Western blot analysis of known transcription factors and modifiers of Bim activity. FoxOs and CHOP are known transcriptional inducers of bim 12,13,15,23. Analysis of seizure-damaged mouse hippocampus revealed that the levels of the inactive (phosphorylated) form of FoxO1 and FoxO3 were significantly reduced following seizures (Figure 2A,B and data not shown). Somewhat preceding the FoxO response, expression of CHOP was found to be significantly elevated within 1 h of seizures (Figure 2C, D). We next examined the phosphorylation (activation) state of the JNK/c-Jun pathway which can directly upregulate bim mRNA or function post-transcriptionally to increase Bim activity by phosphorylation.14,16,17 Phosphorylation of JNK1/2/3 in the hippocampus was significantly increased 1 h following seizures, returning to baseline thereafter (Figure 2C, E). No significant change was detected in total JNK1/2/3 protein levels (Figure 2C and data not shown).
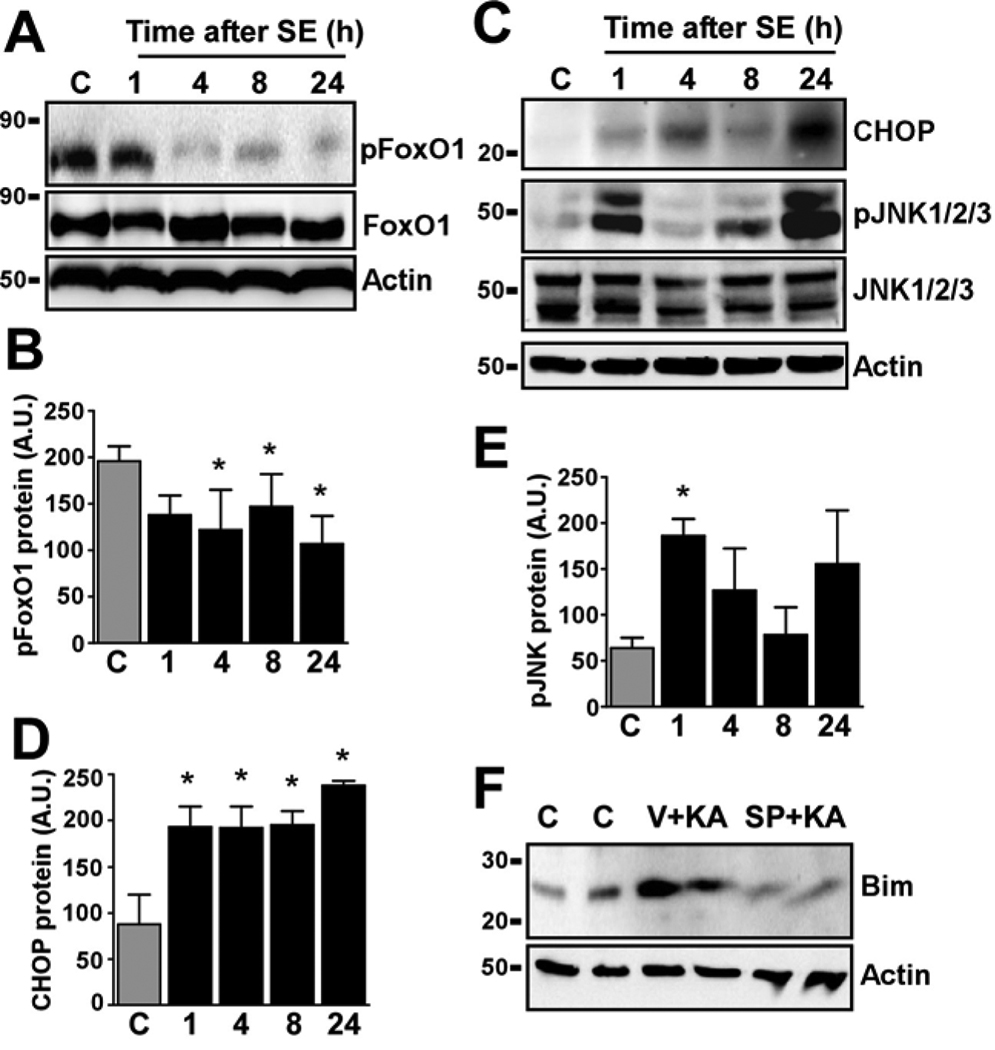
Figure 2. Activation of transcription factors linked to Bim regulation in the hippocampus after status epilepticus.
(A) Representative Western blots (n = 2 per lane) showing expression of phosphorylated (p)FoxO1 declined following seizures. Blot below shows the non-phosphorylated form and Actin as a protein loading control. (B) Graph showing semi-quantitative analysis of pFoxO1 levels in the hippocampus. (C) Representative Western blots (n = 2 per lane) showing expression of CHOP and JNK1/2/3 isoforms. Note increased CHOP and pJNK levels. (D) Graph showing semi-quantitative analysis of CHOP levels in the hippocampus. (E) Graph showing semi-quantitative analysis of pJNK1/2/3. Data are from three independent experiments. *p < 0.05 compared to control (C, 4 h). (F) Representative Western blots (n = 1 per lane) showing hippocampal Bim expression at 24 h in control and KA-treated mice given either vehicle (V) or the JNK inhibitor SP600125 (SP). Note reduced Bim induction in seizure mice treated with SP600125. Actin is included as a protein loading control.
JNK inhibitor SP600125 reduces seizure-induced Bim upregulation
To gain insight into whether any of these pathways functionally contributed to Bim induction after seizures we examined the effect of blocking JNK in vivo using the specific inhibitor SP600125.26 Bim expression was analyzed in the ipsilateral hippocampus from control mice and from KA-injected seizure mice given either intracerebroventricular vehicle or SP600125 (25 µM) at 24 h (n = 4 per group).
All vehicle- and SP600125-treated mice developed status epilepticus after KA and seizure durations were not significantly different between groups (data not shown). As before, Bim expression was significantly increased in the hippocampus after seizures (to 174 ± 63 % of non-seizure control levels) (Figure 2F). In contrast, induction of Bim was strongly reduced in seizure mice treated with SP600125 (125 ± 45 % of control levels) (Figure 2F). SP600125 also blocked induction of CHOP after status epilepticus (Supplementary Figure 1). Levels of pFoxO1 were not different between vehicle- and SP600125-treated mice 24 h after status epilepticus (data not shown).
Bim expression declines in neocortex following focal-onset status epilepticus
We next analyzed cell death and Bim expression within the ipsilateral neocortex. In agreement with our previous report,25 small numbers of degenerating neurons were found within the temporal, ectorhinal, perirhinal and piriform cortex 24 h after status epilepticus in mice (Figure 3A). The sparse cell death was mainly confined to cortical layers IV–VI, with layers I–III displaying little or no cell death (Figure 3A). Thus, the neocortex represents a recruited brain region that undergoes less extensive cell death compared to hippocampal CA3.
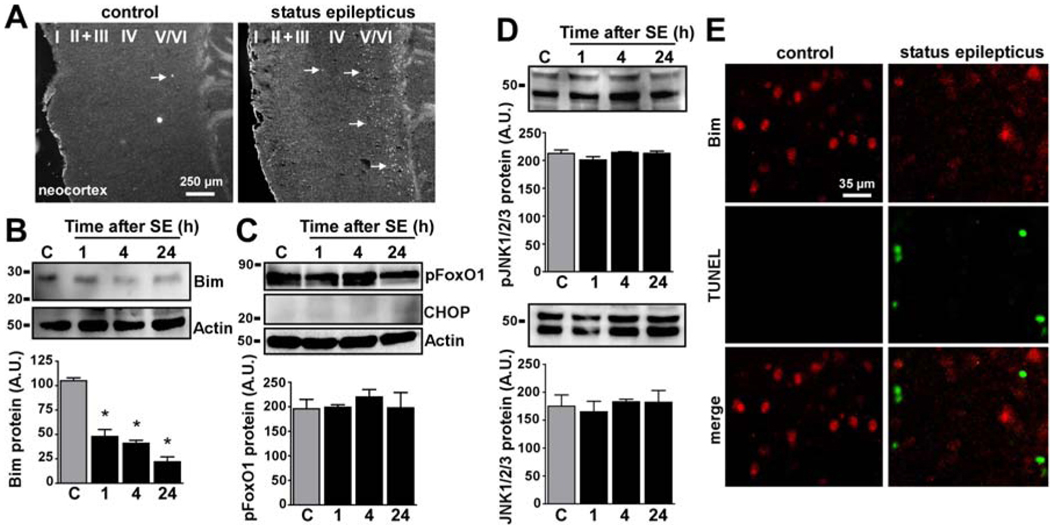
Figure 3. Bim expression declines in the less-damaged neocortex following seizures.
(A) Photomicrographs (4× lens) show representative Fluoro-Jade B staining at 24 h in the neocortex of control mice and mice that underwent status epilepticus. Arrows denote cells (white) undergoing degeneration. Cortical layers are indicated. (B) Representative Western blot (n = 1 per lane) showing decreased expression of Bim after seizures. Actin is shown as a control for protein loading. Graph below shows semi-quantification of Bim protein levels in the neocortex. *p < 0.05 compared to control (C, 4 h). (C) Representative Western blots (n = 1 per lane) for pFoxO1, CHOP and actin and corresponding semi-quantification of protein levels for pFoxO1 in the neocortex. (D) Representative Western blots (n = 1 per lane) and graphs showing (top) pJNK1/2/3 and (bottom) total-JNK1/2/3 in the neocortex. All graph data are from three independent experiments. (E) Panels show representative 40× lens photomicrographs of Bim and TUNEL staining in control and neocortex 24 h after status epilepticus. Note decline in Bim staining and presence of scattered TUNEL-positive cells after seizures.
Western blot analysis of Bim in samples from the neocortex determined that moderate levels of Bim were detected in control mice (Figure 3B). In mice subject to status epilepticus cortical expression of Bim underwent a rapid and significant decline (Figure 3B).
We next examined responses of transcriptional and post-translational regulators of Bim expression. Phosphorylation of FoxO1 was largely unchanged in the neocortex following seizures (Figure 3C). Similarly, no increased CHOP was evident in the neocortex (Figure 3C). No change in expression of phosphoJNK1/2/3 or total JNK1/2/3 was found at any time after seizures in neocortex (Figure 3D).
To further explore the relationship between Bim regulation and cell death in the neocortex we performed double-label fluorescence microscopy experiments on tissue sections. Bim immunoreactivity could be detected at low level in many cells in control mouse neocortex (Figure 3E). Reduced Bim immunoreactivity was evident in neocortex 24 h after status epilepticus (Figure 3E). Cells positive for DNA fragmentation detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) showed minimal Bim immunoreactivity (Figure 3E).
Normal brain architecture in Bim-deficient mice
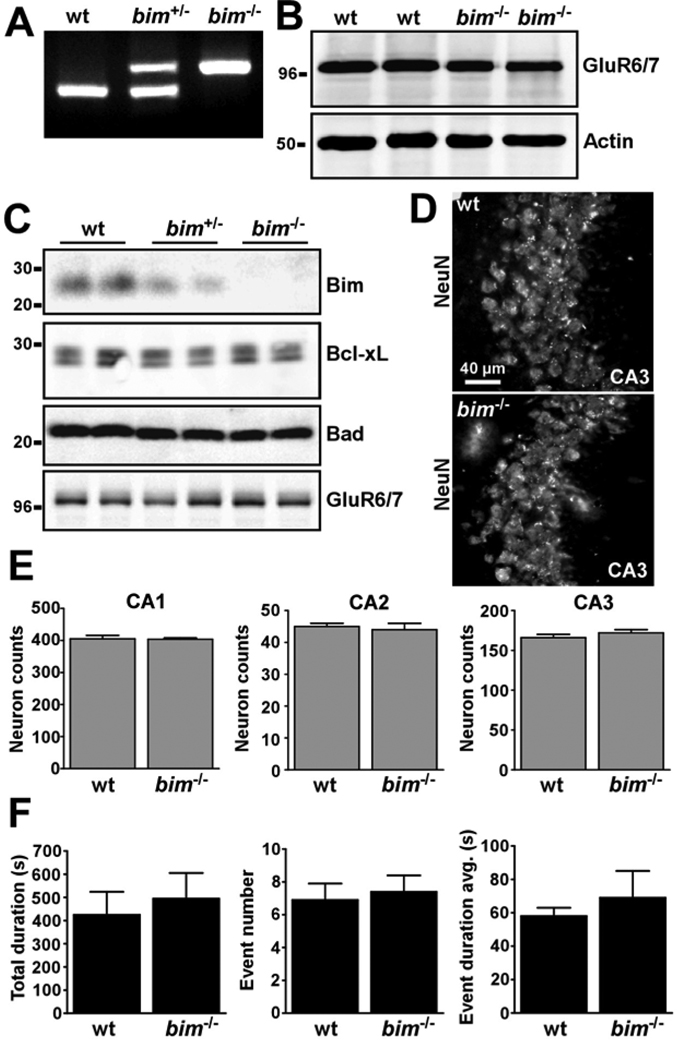
To determine whether Bim is required for seizure-induced neuronal death in either the hippocampus or the neocortex in this model, we compared seizure-damage between wildtype (bim+/+) and Bim-deficient (bim−/−) mice. Representative genotyping of wildtype, heterozygous (bim+/−) and Bim-deficient mice is shown in Figure 4A. To examine whether bim−/− mice display any developmental abnormalities which might render them more or less prone to seizures or damage, we investigated expression of a variety of marker genes in the amygdala (site of seizure elicitation) and hippocampus (site of major damage). Expression of the KA receptor GluR6/7 was not different between wild-type and bim−/− mice (Figure 4B). Likewise, GluR6/7 expression in the hippocampus was comparable between both genotypes (Figure 4C). Hippocampal expression of Bad (another BH3-only protein) and Bcl-xL (an anti-apoptotic Bcl-2 family member) were similar between both genotypes, while, as expected, Bim protein was not detected in brain lysates from bim−/− mice (Figure 4C). Moreover, under low- and high- power magnification no gross hippocampal or cortical abnormalities were evident in bim−/− mouse brains (Figure 4D and data not shown). Counts of NeuN-stained hippocampal subfields from wild-type and bim−/− mice revealed similar numbers of neurons in all major hippocampal subfields – CA1, CA2 and CA3 (Figure 4E). Finally, we subjected groups of wild-type and bim−/− mice to seizures induced by intra-amygdala KA microinjection. Analysis of seizure EEG between injection and lorazepam administration confirmed no differences between the two genotypes of mice with respect to time to seizure onset, total duration of high amplitude high frequency bursts, number of events recorded or duration of individual events (Figure 4F and data not shown). Taken together, these data confirm suitability of the bim−/− mice for analysis of seizure damage.
Figure 4. Genotype and phenotype analysis of bim−/− mice.
(A) Representative PCR showing genotyping of mice. (B) Representative Western blot (n = 2 per lane) confirming normal expression of KA receptor GluR6/7 in the mouse amygdala between wild-type (wt) and bim knockout mice. Actin is shown as a control for protein loading. (C) Representative Western blots of hippocampal samples confirming Bim deficiency in bim−/− mice, while levels of a selection of other proteins are normal. (D) Representative photomicrographs of NeuN-stained CA3 subfields from wild-type and bim−/− mice. (E) Graphs showing counts of NeuN-positive cells in various hippocampal fields. No differences were evident between mice of the two genotypes (n = 3 per group). (F) Graphs showing EEG data on seizure parameters between wild-type and bim−/− mice following intra-amygdala KA microinjection. No differences were found between mice of the two genotypes (n = 9–10 per group).
Decreased hippocampal cell death in bim−/− mice compared to wild-type animals
Fluoro-Jade B staining of tissue sections was performed 72 h after status epilepticus. This time-point, rather than 24 h, was selected to ensure we could be confident that any damage difference between wild-type and bim−/− animals was lasting. In wild-type mice, status epilepticus caused a typical lesion encompassing the ipsilateral CA3 subfield of the hippocampus (Figure 5A,C, E). In comparison, neurodegeneration after status epilepticus in bim−/− mice was ~45 % less (Figure 5B, D, F). Counts of Fluoro-Jade B positive cells in CA3 confirmed that there were significantly lower number of damaged/dying cells in bim−/− compared to wild-type animals (Figure 5G).
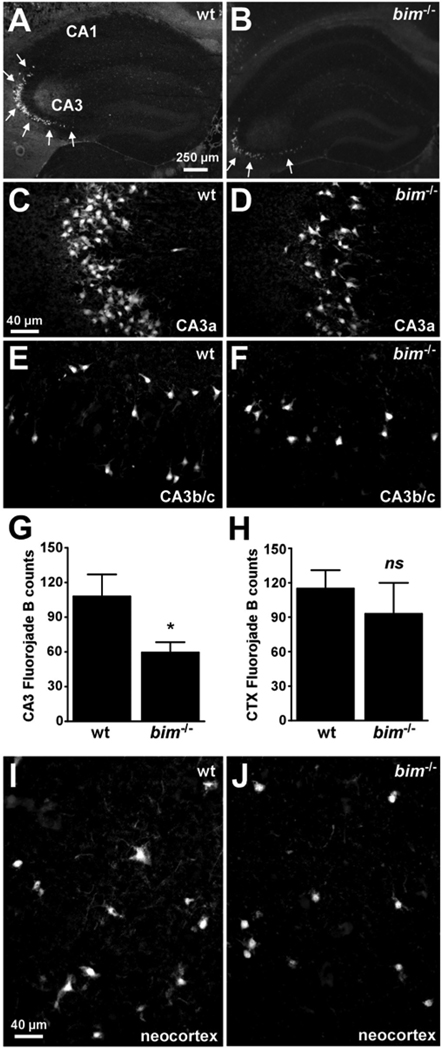
Figure 5. Hippocampal but not neoortical seizure damage is reduced in bim−/− mice.
(A, B) Representative photomicrographs (4× lens) of Fluoro-Jade B staining within the ipsilateral hippocampus 72 h after status epilepticus in wild-type (wt) and bim−/− mice. Note the reduction in numbers of damaged/dying cells within CA3 in bim−/− mice compared to wild-type animals. (C–F) Representative photomicrographs (40× lens) of Fluoro-Jade B staining within the ipsilateral hippocampal CA3a and CA3b/c regions 72 h after status epilepticus in wild-type and bim−/− mice. Note, the reduced numbers of Fluoro-Jade B positive cells in bim−/− mice compared to wild-type animals. (G, H) Graphs quantifying numbers of Fluoro-Jade B stained cells in the hippocampal CA3 and the neocortex for each genotype of mice. Data are derived from n = 6–10 per group. *p < 0.05. ns, not significant. (I, J) Representative photomicrographs (4× lens) of Fluoro-Jade B staining within the ipsilateral neocortex 72 h after status epilepticus in wild-type and bim−/− mice.
In contrast to the hippocampus, there were no significant difference in counts of Fluoro-Jade B positive cells in the ipsilateral neocortex between wild-type and bim−/− mice (Figure 5H–J). Bim was also not required for KA-induced cortical neuronal death in vitro, since cultures of primary cortical neurons from wild-type and Bim-deficient mice were similarly vulnerable to KA (Supplementary Figure 2).
Bim knockdown is protective against KA-induced hippocampal cell death in vitro
To support our in vivo data, we examined the role of Bim in neuronal death in vitro using a model of seizure-like injury to mouse organotypic hippocampal cultures.27 In this model, KA (5 µM) treatment for 24 h results in neuronal death within the CA3 and CA1 subfields (Figure 6A,C,E,F). Western blot analysis of lysates from KA-treated hippocampal cultures revealed an increase in Bim expression levels compared to controls (Figure 6D). To determine whether Bim contributed to neuronal death in this model, we used a short interfering RNA (siRNA) approach to knock-down Bim expression. Hippocampal cultures were pre-treated for 24 h with bim-targeting or a non-targeting scrambled siRNA and neuronal death was assessed 24 h after KA administration. Western blot analysis confirmed that siRNA targeting of bim reduced Bim protein expression following KA treatment (Figure 6D). In contrast, cultures treated with the scrambled siRNA showed normal induction of Bim after KA administration (Figure 6D). As a further control, we verified that treatment of hippocampal cultures with siRNA had no significant effect on the expression of KA receptor GluR6/7 (Figure 6D). Analysis of cell death 24 h after KA revealed typical hippocampal CA1 and CA3 damage in cultures treated with scrambled siRNA (Figure 6B,C,E,F). In contrast, hippocampal cultures treated with the siRNA targeting bim displayed significantly less cell death in both CA1 and CA3 when treated with KA, compared to KA-treated cultures treated with scrambled siRNA subfields (Figure 6B,C,E,F).
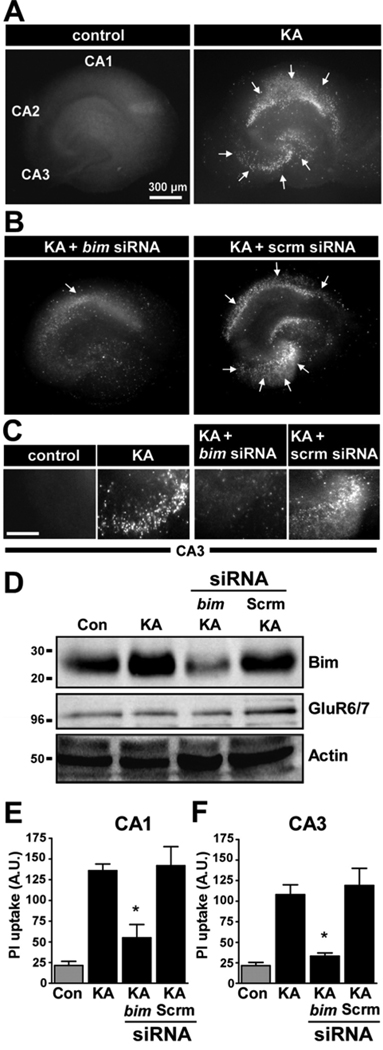
Figure 6. Bim depletion by short interfering RNA reduces KA-induced neuronal death in hippocampal organotypic cultures.
(A) Representative photomicrographs (4× lens) show control and KA-treated cultures stained with propidium iodide (PI) at 24 h. Note, the significant cell death within hippocampal CA1 and CA3 (arrows). (B) Representative PI staining in KA-treated cultures at 24 h. Note, cell death is reduced in the cultures transfected with short interfering RNA targeting bim (bim siRNA) compared to cultures treated with a scrambled sequence (scram siRNA). (C) Representative PI staining in CA3 for each group. (D) Representative Western blots (n = 3 per lane) showing expression of Bim following KA treatment increases, and that bim siRNA reduces Bim protein levels. Blots below show levels of the KA receptor were not altered by the treatments, and probing for actin is provided as a control for equal protein loading. Data are derived from three independent experiments. (E, F) Graphs show quantification of cell death in CA1 and CA3 24 h after treatment. *p < 0.05 for comparison between cultures treated with KA plus bim siRNA compared to KA-treated cultures treated with scrambled siRNA.
Discussion
The main findings of the present study are: (1) focal-onset status epilepticus in mice is associated with rapid induction of multiple transcription factors linked to bim regulation, upregulation of bim mRNA and increased expression of Bim protein in the hippocampus; (2) deficiency in Bim confers significant protection against hippocampal cell death after seizures in vivo and against KA-induced damage in vitro; (3) Bim expression declines in the neocortex after seizures and this region is not protected in bim−/− mice. These studies demonstrate a differential and region-specific involvement of Bim in seizure-induced neuronal death thereby providing novel insight regarding gene-based mechanisms of seizure-induced neuronal death.
The present study was undertaken to determine whether there is a causal role for Bim in seizure-induced neuronal death. Causal roles for Bim in neuronal death have previously been shown in models of neonatal hypoxia-ischemia,28 and in motor neuron disease.29 Functional roles for other Bcl-2 family members as killers or protectors in seizure-induced and excitotoxic neuronal death have been demonstrated, including for Bax or Bcl-xL and Bcl-w, respectively.3,6,7 However, a requirement for Bcl-2 family members in mitochondrial dysfunction in these settings has been questioned by several reports. Excitotoxic neuronal death has been shown to occur normally in Bax-deficient neurons.4 Also, calcium exposure of mitochondria alone,8 and calpain activity,9 can trigger release of apoptogenic factors from neuronal mitochondria. Functional redundancy among BH3-only proteins has been observed in various contexts.2 The studies here provide evidence for Bim involvement in the molecular mechanism of seizure-induced neuronal death. The mouse model used is associated with neuronal death and hallmarks of the intrinsic apoptosis pathway including cytochrome c release, caspase activation and double-stranded DNA fragmentation with nuclear clumping of fragmented chromatin in one third of cells.3,24 Data presented here show that Bim expression is increased at both the mRNA and protein levels in the injured hippocampus. Induction of Bim appears to be temporally compatible with a causal role in mitochondrial dysfunction because we have previously reported that cytochrome c is not yet released 1 h after status epilepticus in this model, but is evident by 4 h.3 These data extend our previous studies performed in rats subject to focal-onset status epilepticus in which Bim protein was also found to be up-regulated at 4 h.23 Our data are also in agreement with findings of increased Bim levels following pilocarpine-induced status epilepticus.30 However, our data contrast the report by Korhonen and co-workers who found that hippocampal Bim expression declined after status epilepticus induced by intracerebroventricular KA injection.31 The increase in hippocampal Bim protein that we detected is rather greater than would be expected from the relatively modest increase in bim mRNA levels. This may indicate that increased expression of Bim is a combination of transcriptional as well as post-translational activation mechanisms. Consistent with this hypothesis we observed activation of transcription factors linked to bim induction (e.g. CHOP) as well as post-translational activators of Bim (e.g. JNK). Both of these events preceded the increase in Bim protein. Since SP600125 blocked Bim induction and also CHOP after seizures, the JNK pathway may be most critical. It remains, however, possible that other transcription factors and/or post-translational regulators may also play a role in Bim regulation in status epilepticus. Indeed, seizure model data from rats previously linked FoxO1 to Bim induction.23 Also, CHOP has been shown to trigger Bim expression in at least certain cell types following endoplasmic reticulum stress,15 and endoplasmic reticulum stress is a feature of both seizure-induced neuronal death,32 and chronic human TLE.33
The present study demonstrates that Bim has a causal role in the status epilepticus-induced cell death process because neurodegeneration was reduced in bim−/− mice. These are the first data showing that deficiency of a BH3-only protein can reduce seizure damage in vivo. Our analysis of damage was undertaken three days after status epilepticus, probably excluding an observation regarding the absence of Bim delaying rather than blocking cell death, as reported for Bim in other models.34 Nevertheless, neurodegeneration following status epilepticus can continue for several weeks.35 bim depletion by siRNA was also found to protect against hippocampal cell death following KA treatment in vitro. Since Bim deficiency was not fully protective in vivo, it appears likely that other BH3-only proteins or other mechanisms are also involved in neuronal death after seizures in this model. Our data contrast findings by Theophilas and co-workers,36 who reported that in the hippocampus hippocampal damage was not different between wild-type and Bim-deficient mice following intra-hippocampal KA injection. The explanation for this discrepancy is unclear. However, Theofilas et al. used a model of direct intra-hippocampal KA injection, where dissociation of seizure effects versus direct KA toxicity is not possible. In contrast, our model uses KA to elicit status epilepticus from a site remote from the target hippocampus and we could confirm recruitment of the hippocampus in seizures using intracerebral recordings. Thus, hippocampal damage in the present model results from local glutamatergic and other mechanisms, not from direct toxic effects of KA on hippocampal cells. That said, we found that Bim reduction by siRNA was also neuroprotective in vitro where seizure-related injury is not dissociable from direct toxic effects of KA. Theofilas et al. also did not report data on hippocampal CA3 damage comparing bim−/− and wild-type mice, nor did they report Bim expression in their model. Since we observed that in locations where Bim is not induced (i.e. neocortex) deficiency of Bim confers no protective advantage, this remains a possible explanation for the differences between the results from these two studies. Regardless, these data suggest Bim is not always required for seizure-induced neuronal death. We emphasise the importance of interpreting our data within the context of the model used and caution in extrapolating findings to the human condition.
The present studies may have implications for Bim involvement in other acute neurologic insults featuring an excitotoxic component, such as ischemia. Indeed, ischemia induces Bim expression,37 and neonatal hypoxia-ischemic injury induced neuronal death in mice is reduced by loss of Bim.28 While the molecular mechanism of Bim-induced neuronal death was not a focus of our study, we have previously shown that Bim binds to anti-apoptotic Bcl-w in vivo after seizures in rats and mice.3,23 However, other mechanisms of action are possible. Bim has been reported to contribute to release of AIF in neurons,22 and AIF mediates a component of seizure-induced neuronal death.4
The present study represents an important compliment to findings on anti-apoptotic Bcl-2 family proteins in seizure-induced neuronal death. Over-expression of Bcl-xL protects hippocampus against seizure-like insults in vivo.7 In our earlier report on Bcl-w using the same model applied presently, we found that Bcl-w was targeted by Bim during seizure-induced neuronal death and Bcl-w deficiency led to significantly enhanced hippocampal damage from seizures.3 However, bcl-w−/− mice displayed earlier seizure onset than wild-type animals, implying an endogenous function for that gene that when disrupted leads to greater seizure susceptibility.3 We observed no seizure abnormalities in bim−/− mice, which is in agreement with EEG recordings in bim−/− mice after intra-hippocampal KA.36 This implies that basal Bim expressed in neurons may have limited roles in normal neuronal physiology. These observations emphasise potential value in targeting certain pro-apoptotic BH3-only proteins.
The second finding of significance in the present study was that Bim was down-regulated in the less damaged neocortex after status epilepticus. Furthermore, the transcriptional and post-translational pathways associated with Bim induction in the hippocampus were not recruited in the neocortex. These data may provide evidence for seizure-induced tolerance mechanisms in the neocortex. Indeed, we previously showed that Bim expression is down-regulated in the rat hippocampus after repeated brief, non-harmful electroshock seizures,23 a model of epileptic tolerance. These findings may constitute the experimental correlate of human data showing lower Bim levels in brains from patients with intractable epilepsy.23 Such adjustments to pro-apoptotic Bcl-2 family proteins have been reported elsewhere,38 and may represent endogenous mechanisms to reduce vulnerability to seizure-induced neuronal death. The mechanism of Bim down-regulation in the neocortex is currently unknown. The rapid run-down excludes a simple block on new mRNA production. Indeed, the half-life of Bim in neurons is estimated at 2.8 h,20 and we detected Bim levels were below 50 % in samples 1 h after status epilepticus. More likely, Bim is degraded, for example via ubiquitination that is triggered by mitogen-activated protein kinases or other mediators.20 An alternative mechanism may lie with protein kinase B/Akt.39 We showed, using a model in the rat, that Akt is activated in the neocortex in response to status epilepticus, but when this is blocked, Bim up-regulation occurs resulting in increased neocortical cell death.5,23 Finally, our in vitro data raise the intriguing possibility of intrinsic differences between hippocampal and cortical neurons in the importance of Bim for seizure- and excitotoxin-induced cell death.
In conclusion, the present study demonstrates a complex relationship between Bim expression, seizures and neuronal death in vivo but provides further evidence that Bcl-2 family genes play a significant role in seizure-induced neuronal death. Bim is induced in a temporally-compatible pattern with hippocampal neuronal death and significant, albeit moderate, neuroprotection could be demonstrated in its absence. Since Bim expression is also regulated in the hippocampus from patients with intractable temporal lobe epilepsy 23,33, the present data may be relevant not just for neuroprotection in the wake of status epilepticus but also in intractable epilepsy where patients may be at risk of progressive damage over time to involved brain structures.
Materials and methods
In vivo model of focal-onset status epilepticus in mice
Animal experiments were carried out in accordance with the principals of the European Communities Council Directive (86/609/EEC) and National Institute of Health’s Guide for the Care and Use of Laboratory Animals. Procedures were reviewed and approved by the Research Ethics Committee of the Royal College of Surgeons in Ireland, under license from the Department of Health, Dublin, Ireland.
Studies were performed according to previously described techniques.25 Mice (C57Bl/6 adult male, 8–10 weeks old, 20–25 g) were obtained from Harlan, UK. Bim wildtype mice (bim+/+) and bim knockout (bim−/−) mice, originally generated on a mixed C57BL/6x129SV background using 129SV-derived ES cells, backcrossed for at least eight generations on a C57BL/6 genetic background have previously been described.21 Mice were anesthetized using isoflurane (3–5%) and maintained normothermic by means of a feedback-controlled heat blanket (Harvard Apparatus Ltd, Kent, England). A catheter was inserted into the femoral vein for administration of anticonvulsant. Mice were next placed in a stereotaxic frame and following a midline scalp incision three partial craniectomies were performed and mice were affixed with cortical electrodes (Bilaney Consultants Ltd, Sevenoaks, UK) to record surface EEG. Electrodes were placed above dorsal hippocampus and a third over frontal cortex. EEG was recorded using a Grass Comet digital EEG (Medivent Ltd, Lucan, Ireland). For combined intra-hippocampal with cortical recordings additional mice were were anaesthetized and stereotaxically implanted with a twisted wire bipolar electrode (Plastics One Inc), into the dorsal CA3 subfield of the hippocampus while cortical EEG was recorded by means of two skull-mounted recording electrodes (Plastics One Inc) placed over the frontal cortex and cerebellum and secured with dental cement. A guide cannula was affixed (coordinates from Bregma: AP = −0.94; L = −2.85 mm) and the entire skull assembly fixed in place with dental cement. Anesthesia was then discontinued and freely moving mice were placed in a clear Perspex recording chamber. EEG recordings were commenced and after establishing baseline EEG an injection cannula was lowered through the guide cannula to 3.75mm below the dura for microinjection of KA (Ocean Produce International, Nova Scotia, Canada) (0.3 µg in 0.2 µL phosphate-buffered saline, PBS) into the basolateral amygdala nucleus. Non-seizure control mice received the same volume of intra-amygdala vehicle. Forty minutes following microinjection of KA or vehicle, mice received intravenous lorazepam (6 mg/kg) and the EEG monitored for up to 1 h thereafter. Administration of lorazepam was used to stop seizures thereby reducing mortality, morbidity and restricting the extent of damage, as described previously.24 Mice were euthanized 1, 2, 4, 6, 8, 24 or 72 h following treatment with lorazepam and perfused with saline to remove intravascular blood components. Brains were either flash-frozen whole in 2-methylbutane at −30°C for histopathology or hippocampus and neocortex microdissected and processed for mRNA and protein analysis, as described below.
In vivo effect of JNK inhibitor SP600125
Additional groups of control and seizure mice were instrumented for intracerebroventricular administration of vehicle or the JNK inhibitor SP600125.26 Briefly, mice were implanted with a second cannula ipsilateral to the side of KA injection. Coordinates from Bregma were: AP = −0.3 mm, L = −1.0 mm, V = −2.0 mm. Mice received 1.75µL infusions of either vehicle (10 % DMSO in artificial cerebrospinal fluid) or SP600125 (0.5 mM stock; resultant cerebrospinal fluid concentration approximating 25 µM), 15 minutes before KA and again 1 h after KA. Duration of high frequency high amplitude seizures following KA up to time of lorazepam administration was monitored by EEG and quantified for all mice. A separate group of non-seizure control mice received vehicle into both ventricle and amygdala. Mice were killed 24 h later and the ipsilateral hippocampi removed and processed for Western blotting as described below.
Genotyping
Genotyping was performed according to previously described methods.21 Genomic DNA was extracted from tail snips. A triple primer protocol was used to identify genotypes. Primer 1, CATTCTCGTAAGTCCGAGTCT; primer 2, CTCAGTCCATTCATCAACAG; primer 3, GTGCTAACTGAAACCAGATTA. The amplified fragments, detected by DNA agarose electrophoresis were 400 bp for wild-type (bim+/+), 550 bp + 400 bp for heterozygote (bim+/−) and 550 bp only for knockout (bim−/−).
In vitro models of seizure-like injury
Organotypic hippocampal slice cultures were prepared and maintained as previously described.27 Briefly, 10 day old C57BL/6 mice of either sex were decapitated, the brains removed and placed in dissection media containing Hanks Balanced Salt Solution (Gibco, Carlsbad, CA), 20 mM Hepes (Gibco), 100 U/mL Penicillin/Streptomycin (Sigma-Aldrich, Dublin, Ireland) and 6.5 mg/mL D-Glucose (Sigma-Aldrich). Hippocampi were then dissected out and cut into 450 µm-thick sections. The slices were then transferred into fresh dissection media and were selected based on the presence of intact hippocampal subfields (CA1, CA3 and the dentate gyrus). The slices were then transferred onto the porous (0.4 µm) membrane of a Millcell insert (Millipore, Cork, Ireland). These inserts were then placed into 24 well tissue culture plates, with each well containing 300 µL of culture medium consisting of 50 % Minimal Essential Medium (MEM) (Sigma-Aldrich), 25 % Horse Serum (Gibco), 4 mM L-Glutamine, 6 mg/ml D-Glucose, 2% B27 and 50 U/mL Penicillin/Streptomycin. The slices were then incubated in a humidified chamber with 5 % CO2 at 35°C. Cultures were maintained for up to 12 days and medium was changed every 3–4 days. To model seizure-like injury, cultures were treated with KA (5 µM) (Sigma-Aldrich) for 24 h at 35°C. Control slices were incubated with medium treated with vehicle. RNA interference (RNAi) was used for modulating expression of Bim in vitro. Briefly, hippocampal cultures were transiently transfected with a mouse-specific pool of 3 target-specific 20–25 nt short interfering RNAs (siRNA) to deplete bim (sc-29803, Santa Cruz Biotechnology, Santa Cruz, CA). The siRNA duplexes were resuspended in 330 µL of RNAse-free water at a stock concentration of 10 µM and transfections performed for 24 h using a siRNA transfection reagent (sc-29528) with the siRNA at a concentration of 0.8 µM (Santa Cruz Biotechnology) in culture media. Control cultures were treated with a scrambled sequence that does not lead to specific degradation of any known cellular mRNA (sc-37007) (Santa Cruz Biotechnology). Cell death was assessed by propidium iodide (PI) uptake.27 Briefly, PI (5 µg/mL) was introduced into cultures for 40 min and then washed out through medium change. Fluorescence from dead cells which take up the dye in the nucleus was visualized using a Hamamatsu Orca 285 camera attached to a Nikon 2000s epifluorescence microscope under 540 to 580/600 to 660nm (red). Analysis of the optical density of PI staining was carried out using Image J (http://rsb.info.nih.gov/ij/). PI staining was determined for each sub-region of the hippocampus as the average of five field views of 1 µm2. Data were averaged from three similarly treated slices and at least three independent experiments run for each condition.
For primary cortical cultures, wildtype or bim−/− mouse embryos (E16–E18) were isolated by hysterectomy and transferred to a dissection medium (PBS with 0.25 % glucose, 0.3 % BSA) on ice. The cerebral cortex from each embryo was isolated and then incubated with trypsin-EDTA (0.25 %) at 37°C for 15 min. The tissue was then dissociated in 5 ml of plating medium (MEM containing 5% fetal calf serum, 5% horse serum, 100 U/ml Pen/strep, 0.5 mM L-glutamine, 6% glucose). Following dissociation, the neurons were pelleted by centrifugation. The media was aspirated and the neurons resuspended in fresh plating medium and plated at 7×105 cells per ml on poly lysine coated plates. Cells were incubated at 37°C, 5 % CO2 in feeding media (NBM-embryonic containing 100 U/ml of Pen/Strep, 2 % B27 and 0.5 mM L-glutamine and 1 µM Cytosine arabinofuranoside). Media was changed every 2–3 days until days in vitro 6 (DIV 6) and cortical neurons exposed to varying concentrations of KA (3–30 µM) on DIV 7. Cell death was assayed by counts of Hoechst 33258-stained cells displaying nuclear pyknosis.
mRNA analysis
Real-time quantitative PCR analysis of bim mRNA levels was undertaken as previously described (Jimenezs-Mateos et al. 2008). Primers were designed using Primer3 software (http://frodo.wi.mit.edu) and verified by BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Forward: CAACACAAACCCCAAGTCCT
Reverse: CATTTGCAAACACCCTCCTT. One microgram total RNA from control (2 h) and seizure hippocampus (2–8 h) was used to generate cDNA by reverse transcription using Superscript II Reverse Transcriptase enzyme (Invitrogen Corporation, Carlsbad, CA, USA). Quantitative real-time PCR was performed using a LightCycler 1.5 (Roche Diagnostics, Indianapolis, IN, USA) in combination with QuantiTech SYBR Green PCR kit (Qiagen Ltd, Crawley, England) as per manufacturer's protocol and 1.25 µM of primer pair used. Data were analyzed by LightCycler 1.5 software with data normalized to expression of β-actin.
Western blot analysis
Western blotting was performed as previously described.3,27 Whole hippocampus or pools of 3–4 organotypic slices were homogenized in buffer containing a protease inhibitor cocktail. Following determination of total protein concentration, 20 µg samples were boiled in gel-loading buffer and separated on 12–15% SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes by electroblotting and blots incubated with the following antibodies: β-actin, CHOP (Santa Cruz Biotechnology), Bim (Stressgen, Victoria, BC, Canada), Bad, JNK, phospho-JNK, FoxO1, phospho-FoxO1, FoxO3 and phospho-FoxO3 (Cell signaling Technology, Beverly, MA), Bcl-xL (BD Biosciences, Oxford, UK) and GluR6/7 (Millipore). Membranes were next incubated with horseradish peroxidise-conjugated secondary antibodies (Jackson ImmnuoResearch, Plymouth, PA) and protein bands visualized using Supersignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). Images were captured using a Fuji-film LAS-3000, densitometry performed using AlphaEaseFC4.0 software and data expressed as change relative to control.
Histopathology and immunohistochemistry
Brains were sectioned at −20°C on a Leica cryostat and 12 µm sections collected at the level of dorsal hippocampus (AP from Bregma; −1.82 mm according to a mouse stereotaxic atlas.40 Neuronal damage was assessed using Fluoro-Jade® B (FjB), a polyanionic fluorescein derivative which is a sensitive and specific marker of degenerating neurons. Methods were as previously described (Mouri et al. 2008). Briefly, sections were air-dried and post-fixed in 10% formalin. Next, sections were immersed in 100% ethanol (3 min), followed by immersion in 70% ethanol (1 min) then rinsed in distilled water (1 min). Sections were next transferred to fresh 0.006% potassium permanganate solution for 15 min with gentle shaking. Sections were then rinsed again and transferred to FjB solution (0.001% in 0.1% acetic acid) (Chemicon Europe Ltd, Chandlers Ford, UK). After staining, sections were rinsed again, dried, cleared and mounted in DPX (Sigma-Aldrich). For Bim immunohistochemistry, sections were fixed and permeabilized, blocked in goat serum and then incubated overnight with anti-Bim followed by incubation with goat anti-rabbit AlexaFluor568 (Molecular Probes, Eugene, OR). Next, sections were stained for DNA fragmentation using a fluorescein-based TUNEL kit (Promega U.S., Madison, WI) as previously described.3,5 Sections were examined using a Nikon 2000s epifluorescence microscope (Micro-optica, Dublin, Ireland) under Ex/Em wavelengths of 472/520 nm (green) and imaged using a Hamamatsu Orca 285 camera. Hippocampal FjB positive cells were the average of two adjacent sections for the CA3 subfield. Counts of FjB-positive cells in neocortex were the average sum of ten 40× lens fields from two adjacent sections within the ectorhinal, perirhinal and piriform cortices. Counts were by an observer blinded to experimental treatment.
For analysis of the naïve brains of wild-type compared to bim−/− mice, whole brain or micro-dissected hippocampus were subject to assessment of gene expression or NeuN counts, as described (Murphy et al. 2007). For neuron counts, coronal mouse brain sections were first air dried and post-fixed in 10 % formalin for 30 min, followed by washes in PBS. Sections were then blocked with 5 % goat serum and incubated overnight at 4°C with antibodies against NeuN (Millipore). Sections were washed in PBS and then incubated for 2 h at room temperature in a 1:1000 dilution of goat anti-mouse AlexaFluor568 (Molecular Probes). Counts of NeuN-stained hippocampal CA1, CA2 and CA3 from naïve wild-type and bim−/− mice were the mean from two adjacent sections at the level of dorsal hippocampus. Expression of Bim, Bad, Bcl-xl (as control Bcl-2 family proteins) and GluR6/7 (KA receptor subunits) was also performed as described above by Western analysis.
Statistical analysis
Data are presented as mean ± SEM. Comparison of data was performed using one way analysis of variance (ANOVA) followed by post hoc Fisher’s PLSD test or Student’s t-test. Significance was accepted at p < 0.05.
Supplementary Material
Acknowledgements
The authors thank Philippe Bouillet for the original generation of the Bim-deficient mice and for technical support, Aurelien Caballero and Aine Murphy. Funding: Health Research Board, Ireland (RP/2005/24 to DH, PD/2005/35, RP/2008/69 to BM), Science Foundation Ireland (07/SK/B1243A to BM, 08/IN1/B1875 to DH), Wellcome Trust (GR076576 to DH), Irish Research Council for Science Engineering and Technology (to TE), Marie Curie ToK FP-14499 and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NS39016, NS47622 to RS). Additional support (to AS) from NHMRC (Canberra; programs #461221), the Leukemia and Lymphoma Society (SCOR grant #7015), the NIH (CA043540-18 and CA80188-6), the JDRF/NHMRC and the Charles and Sylvia Viertel Charitable Foundation (PB).
References
- 1.Engel T, Henshall DC. Apoptosis, Bcl-2 family proteins and caspases: the ABCs of seizure-damage and epileptogenesis? Int J Physiol Pathophysiol Pharmacol. 2009;1:97–115. [PMC free article] [PubMed] [Google Scholar]
- 2.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 3.Murphy B, Dunleavy M, Shinoda S, Schindler C, Meller R, Bellver-Estelles C, et al. Bcl-w protects hippocampus during experimental status epilepticus. Am J Pathol. 2007;171:1258–1268. doi: 10.2353/ajpath.2007.070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC, et al. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci. 2005;25:1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henshall DC, Araki T, Schindler CK, Lan J-Q, Tiekoter K, Taki W, et al. Activation of Bcl-2-associated death protein and counter-response of Akt within cell populations during seizure-induced neuronal death. J Neurosci. 2002;22:8458–8465. doi: 10.1523/JNEUROSCI.22-19-08458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS. Bax involvement in p53-mediated neuronal cell death. J Neurosci. 1998;18:1363–1373. doi: 10.1523/JNEUROSCI.18-04-01363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju KL, Manley NC, Sapolsky RM. Anti-apoptotic therapy with a Tat fusion protein protects against excitotoxic insults in vitro and in vivo. Exp Neurol. 2008;210:602–607. doi: 10.1016/j.expneurol.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan C, Chen J, Chen D, Minami M, Pei W, Yin XM, et al. Overexpression of the cell death suppressor Bcl-w in ischemic brain: implications for a neuroprotective role via the mitochondrial pathway. J Cereb Blood Flow Metab. 2000;20:620–630. doi: 10.1097/00004647-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. Embo J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, et al. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol. 2000;157:449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 13.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 14.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 15.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 17.Becker EB, Howell J, Kodama Y, Barker PA, Bonni A. Characterization of the c-Jun N-terminal kinase-BimEL signaling pathway in neuronal apoptosis. J Neurosci. 2004;24:8762–8770. doi: 10.1523/JNEUROSCI.2953-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas SC, Greene LA. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J Biol Chem. 2002;277:49511–49516. doi: 10.1074/jbc.M208086200. [DOI] [PubMed] [Google Scholar]
- 20.Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, et al. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem. 2006;281:7429–7436. doi: 10.1074/jbc.M512138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 22.Liou AK, Zhou Z, Pei W, Lim TM, Yin XM, Chen J. BimEL up-regulation potentiates AIF translocation and cell death in response to MPTP. FASEB J. 2005;19:1350–1352. doi: 10.1096/fj.04-3258fje. [DOI] [PubMed] [Google Scholar]
- 23.Shinoda S, Schindler CK, Meller R, So NK, Araki T, Yamamoto A, et al. Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest. 2004;113:1059–1068. doi: 10.1172/JCI19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinoda S, Araki T, Lan JQ, Schindler CK, Simon RP, Taki W, et al. Development of a model of seizure-induced hippocampal injury with features of programmed cell death in the BALB/c mouse. J Neurosci Res. 2004;76:121–128. doi: 10.1002/jnr.20064. [DOI] [PubMed] [Google Scholar]
- 25.Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, et al. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 26.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy N, Bonner HP, Ward MW, Murphy BM, Prehn JH, Henshall DC. Depletion of 14-3-3 zeta elicits endoplasmic reticulum stress and cell death, and increases vulnerability to kainate-induced injury in mouse hippocampal cultures. J Neurochem. 2008;106:978–988. doi: 10.1111/j.1471-4159.2008.05447.x. [DOI] [PubMed] [Google Scholar]
- 28.Ness JM, Harvey CA, Strasser A, Bouillet P, Klocke BJ, Roth KA. Selective involvement of BH3-only Bcl-2 family members Bim and Bad in neonatal hypoxia-ischemia. Brain Res. 2006;1099:150–159. doi: 10.1016/j.brainres.2006.04.132. [DOI] [PubMed] [Google Scholar]
- 29.Hetz C, Thielen P, Fisher J, Pasinelli P, Brown RH, Korsmeyer S, et al. The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ. 2007;14:1386–1389. doi: 10.1038/sj.cdd.4402166. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Huang Y, Yu X, Sun H, Li Y, Deng Y. Erythropoietin preconditioning suppresses neuronal death following status epilepticus in rats. Acta Neurobiol Exp (Wars) 2007;67:141–148. doi: 10.55782/ane-2007-1641. [DOI] [PubMed] [Google Scholar]
- 31.Korhonen L, Belluardo N, Mudo G, Lindholm D. Increase in Bcl-2 phosphorylation and reduced levels of BH3-only Bcl-2 family proteins in kainic acid-mediated neuronal death in the rat brain. Eur J Neurosci. 2003;18:1121–1134. doi: 10.1046/j.1460-9568.2003.02826.x. [DOI] [PubMed] [Google Scholar]
- 32.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto A, Murphy N, Schindler CK, So NK, Stohr S, Taki W, et al. Endoplasmic reticulum stress and apoptosis signaling in human temporal lobe epilepsy. J Neuropathol Exp Neurol. 2006;65:217–225. doi: 10.1097/01.jnen.0000202886.22082.2a. [DOI] [PubMed] [Google Scholar]
- 34.Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, et al. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 35.Nairismagi J, Grohn OH, Kettunen MI, Nissinen J, Kauppinen RA, Pitkanen A. Progression of brain damage after status epilepticus and its association with epileptogenesis: a quantitative MRI study in a rat model of temporal lobe epilepsy. Epilepsia. 2004;45:1024–1034. doi: 10.1111/j.0013-9580.2004.08904.x. [DOI] [PubMed] [Google Scholar]
- 36.Theofilas P, Bedner P, Huttmann K, Theis M, Steinhauser C, Frank S. The proapoptotic BCL-2 homology domain 3-only protein Bim is not critical for acute excitotoxic cell death. J Neuropathol Exp Neurol. 2009;68:102–110. doi: 10.1097/NEN.0b013e31819385fd. [DOI] [PubMed] [Google Scholar]
- 37.Inta I, Paxian S, Maegele I, Zhang W, Pizzi M, Spano P, et al. Bim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemia. J Neurosci. 2006;26:12896–12903. doi: 10.1523/JNEUROSCI.3670-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondratyev A, Sahibzada N, Gale K. Electroconvulsive shock exposure prevents neuronal apoptosis after kainic acid-evoked status epilepticus. Brain Res Mol Brain Res. 2001;91:1–13. doi: 10.1016/s0169-328x(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 39.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 40.Franklin KBJ, Paxinos P. The mouse brain in stereotaxic coordinates. San Diego: Academic Press, Inc; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.