Abstract
Activation of the transforming growth factor (TGF) α/epidermal growth factor receptor (EGFR)-mediated signaling pathway is a common mechanism for dysregulated growth of head and neck squamous cell carcinoma (HNSCC). c-Cbl-interacting protein of 85 kDa (CIN85) is an adaptor protein that facilitates EGFR internalization. Little is known, however, about a role of CIN85 in EGFR signaling as well as its relevance to tumor development and progression of HNSCC. Here, we demonstrate that CIN85 is highly expressed in HNSCC tumor samples compared with adjacent normal tissues, and this overexpression is significantly correlated with advanced clinical stage. The experiments using CIN85-overexpressing and knockdown HNSCC cell lines showed that CIN85 promotes HNSCC growth and facilitates EGFR internalization without apparently affecting phosphorylation of EGFR. Moreover, CIN85 promoted TGF-α-induced activation of Ras and phosphorylation of downstream molecules such as c-Raf, MEK, and extracellular signal-regulated kinase, leading to expression of c-Myc that is critical for sustained proliferation of HNSCC. Taken together, these findings suggest that CIN85 not only controls EGFR internalization but also promotes the EGFR-mediated tumor development and progression, and thus, CIN85 may serve as a potential therapeutic target in a subset of HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common form of cancer worldwide. Despite recent advancements in therapeutic strategies including surgery, radiotherapy, and chemotherapy, the prognosis of HNSCC patients in advanced stages remains largely unsatisfactory [1]. In this regard, growing interests are currently being focused on the development of molecular-targeted therapies for this group of patients. Notably, overexpression of epidermal growth factor receptor (EGFR) and its ligand transforming growth factor (TGF) α is commonly observed in a majority (80%–100%) of HNSCC and is associated with poor prognosis of patients [1,2]. However, molecular-targeted therapies with inhibitors of EGFR alone or in combination with conventional treatments have thus far shown only limited efficacy [1].
A possible explanation for this failed response of HNSCC to EGFR inhibitors is that downstream signaling pathways could be activated as well by surrogate growth factors or cytokines. Nevertheless, the most tantalizing issue that precludes us from developing alternative strategies to overcome such insensitivity would be a relative paucity of our understanding of downstream signaling pathways of EGFR that are critical for the maintenance of malignant phenotypes of HNSCC. These signaling cascades include Ras/Raf/extracellular signal-regulated kinase (ERK), signal transducers and activators of transcription (STATs) and PI3K/Akt [1,3–6]. We and other investigators demonstrated that STAT3 activation plays critical roles in downstream signaling of TGF-α/EGFR in diverse steps, and this activation is profoundly associated with the development and progression of HNSCC [5–7]. STAT3 activation is, however, observed in less than 50% of HNSCC, suggesting that TGF-α/EGFR can also transmit its growth signals by using STAT3-independent downstream pathways. The Ras/Raf/ERK pathway is activated and required for malignant transformation in a variety of human malignancies by regulating cell cycle progression and cellular survival (i.e., inhibition of apoptosis). Indeed, enhanced ERK activation in HNSCC is associated with advanced regional lymph node metastasis [4]. However, mechanisms of enhanced ERK activation in HNSCC are not clearly elucidated, although high expression levels of K-ras in HNSCC only partly account for enhanced ERK activation [8]. Collectively, the entire molecular circuitry downstream of EGFR has not yet been elucidated in HNSCC. In this context, more precise mechanistic study on the EGFR signaling pathways would be an urgent task for developing novel therapeutic strategies for HNSCC.
CIN85, c-Cbl-interacting protein of 85 kDa, is a widely expressed multifunctional adaptor protein consisting of three N-terminal SH3 domains, a centrally located proline-rich motif and a C-terminal coiled-coil domain [9,10]. CIN85 can interact with numerous proteins, and the list of these partner proteins is rapidly growing [11]. One of characteristic functions of CIN85 is the regulation of ligand-induced internalization of receptor tyrosine kinases (RTKs) including EGFRs [12,13]. Little is known, however, about a role of CIN85 in EGFR signaling pathways as well as its relevance to the tumor development and progression of HNSCC.
In the present study, we demonstrate that CIN85 is highly expressed inHNSCC tumor samples compared with adjacent normal tissues, and this overexpression is significantly correlated with advanced clinical stage. In vitro experiments showed that CIN85 not only facilitates EGFR internalization but also promotes HNSCC growth. In addition, CIN85 potentiated TGF-α-induced activation of Ras and phosphorylation of downstream molecules such as c-Raf, MEK, and ERK, culminating in expression of c-Myc that is critical for HNSCC tumorigenesis. These findings suggest that CIN85 not only controls EGFR internalization but also promotes the EGFR-mediated cancer cell growth pathway that is crucial for the tumor development and progression, and thus, CIN85 may serve as a potential therapeutic target in a subset of HNSCC.
Materials and Methods
Clinical Samples
Twenty-nine cancer tissues along with adjacent normal tissues were obtained from untreated patients with oral squamous cell carcinoma who underwent surgical resection at Kyushu University Hospital between March 1997 and March 2008. The tissues were snap-frozen in liquid nitrogen immediately after resection and stored at -80°C until further use.
Cell Lines, Cell Culture, and Reagents
The human oral SCC cell line KB was generously provided by Dr Michihiko Kuwano (Kurume University, Fukuoka, Japan), the hypopharyngeal SCC cell line YCU-H891 was provided by DrMamoru Tsukuda (Yokohama City University, Kanagawa, Japan), and SCC-9 was purchased from the American Type Culture Collection (Manassas, VA). Cell lines were maintained in a 5% CO2 atmosphere at 37°C in RPMI-1640 (Invitrogen Corporation, Carlsbad, CA) for YCU-H891, Dulbecco's modified Eagle medium (Invitrogen Corporation) for KB and SCC-9 with 10% fetal bovine serum (Life Technologies, Grand Island, NY) and antibiotics, unless specified otherwise. For TGF-α (R&D Systems, Minneapolis, MN) stimulation assays, cell lines were grown in serum free medium for 24 hours before treatments. Gefitinib was provided by AstraZeneca (Wilmington, DE), and chlorpromazine (CPZ) was purchased from Sigma-Aldrich Corporation (St Louis, MO). Primary antibodies (Abs) were diluted 1:2000, and secondary Abs were diluted 1:15,000. Rabbit anti-EGFR sera were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-phospho-EGFR (Y1068) monoclonal Abs (mAbs), mouse anti-MEK1/2 mAbs, rabbit anti-phospho-c-Raf (S338), rabbit anti-phospho-MEK1/2 (S221), rabbit anti-phospho-ERK1/2 (T202/Y204), rabbit anti-phospho-Stat3 (Y705), rabbit anti-IκBα, rabbit anti-phospho-IκBα (S32/S36), rabbit anti-phospho-NF-κB p65 (S536), rabbit anti-c-Raf, and rabbit anti-ERK1/2 sera were from Cell Signaling Technology (Beverly, MA). Mouse anti-c-Myc mAbs were from Calbiochem (EMD Biosciences, Darmstadt, Germany). Mouse anti-c-Cbl mAbs were from BD Immunocytometry (San Jose, CA). Rabbit anti-phospho-Cbl (Y774) mAbs were from Epitomics (Burlingame, CA). Mouse anti-CIN85 mAbs were from Upstate Biotechnology (Lake Placid, NY). Mouse anti-V5 mAbs were from Invitrogen. Mouse anti-α-actin mAbs were fromSigma. Goat antirabbit and antimouseHRP-conjugated IgGs were from Jackson ImmunoResearch Laboratories (West Grove, PA).
RNA Interference
The pSUPER-based strategy was adopted to knock down CIN85 expression. To generate CIN85 small hairpin RNA (shRNA), a 19-nucleotide sequence (CAGCAATGACATTGACTTA) selected from human CIN85 complementary DNA was annealed and ligated into GFP-pSUPER knockdown vector.
Stable Transfection of HNSCC Cell Lines with an shRNA Construct or Wild-type CIN85 Construct
Full-length CIN85 expression vector was previously described [14]. KB cells were transfected with 20 µg of CIN85 knockdown vectors (designated as KB/KD cells), full-length CIN85 vectors (designated as KB/OE cells), or corresponding control vectors by electroporation methods and cultured for 48 hours. Clonal cell lines stably expressing these constructs were then selected in medium containing 1 µg/ml G418. We also generated clonal lines of YCU-H cells stably expressing either a CIN85 shRNA construct or a full-length CIN85 construct.
RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA extraction and quantitative reverse transcription-polymerase chain reaction (RT-PCR) assays were carried out essentially as described previously [15]. TaqMan target mixes for human-CIN85, c-Myc and 18S rRNA (internal control) were purchased from Applied Biosystems (Foster City, CA). Preliminary experiments showed that 18S rRNA internal control works best in our quantitative RT-PCR assays. Triplicate data were analyzed with Sequence Detector software (Applied Biosystems). Data were expressed as a fold change compared with the minimal gene expression score (taken as 1.0) in each experiment.
Proliferation Assays
Two thousand cells were cultured in 60-mm dishes for 96 hours, and thereafter, the number of cells in each plate was counted in triplicate assays every other day using a Coulter counter (Beckman Coulter, Fullerton, CA). The mean values were used to generate growth curves.
Cell Viability Assays
Cell viability was determined using a Cell Proliferation Reagent WST-1 (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions.
Protein Extraction and Immunoblot Analysis
Protein extractions and immunoblot analyses were carried out essentially as described previously [15]. The signal intensity of specific bands was quantified using the ImageGauge software (Fuji Film, Tokyo, Japan).
Ras Activation Assay
The amounts of active form of Ras (Ras-GTP) were measured using Ras activation assay kit (Upstate Biotechnology) according to the manufacturer's instructions.
Confocal Immunofluorescence Microscopy
Cells were grown on poly-l-lysine-coated glass-bottom dish (Matsunami, Osaka, Japan) for 24 hours in serum-free medium and then cultured in the absence or presence of 100 ng/ml of TGF-α for 5 minutes. Cells were subsequently fixed with 3.7% formaldehyde in PBS for 10 minutes, permeabilized in PBS containing 0.1% Triton X-100 for 5 minutes, and blocked in PBS containing 1% bovine serum albumin for 30 minutes. Cells were then incubated with mouse anti-EGFR mAbs (1:50; Santa Cruz Biotechnology) for 45 minutes, followed by a secondary Alexa Flour 594 goat antimouse Ab (1:500; Molecular Probes/Invitrogen) for 30 minutes. Samples were analyzed by confocal microscopy (Nikon A1; Nikon, Tokyo, Japan).
Statistical Analysis
Statistical analyses were carried out by the Mann-Whitney U test. P < .05 was considered statistically significant.
Results
CIN85 Is Highly Expressed in HNSCCs in Advanced Clinical Stage
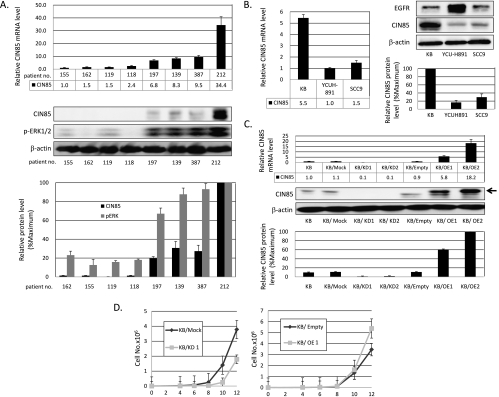
To test a role of CIN85 in the progression of HNSCC, we measured the expression levels of CIN85 messenger RNA (mRNA) in both tumor and adjacent normal tissues obtained from 29 untreated patients with oral squamous cell carcinoma. In 40% of tumor samples, more than two-fold higher levels of CIN85 mRNA were observed, compared with adjacent normal tissues obtained from the respective patient. The mean value of relative CIN85 mRNA expression levels was higher in tumor tissues (69.9 ± 4.4) than normal tissues (49.3 ± 3.7), albeit not statistically significant. Notably, increased levels of CIN85 mRNA in tumor samples significantly correlated with the existence of nodal metastases (P = .0044) and advanced clinical stages (P = .028; Table 1). No differences were observed between the expression levels of CIN85 and age, sex, tumor recurrence, or smoking habits (Table 1). We found a positive correlation between CIN85 mRNA and protein levels in tumor samples from different patients (Figure 1A). These results strongly suggest that CIN85 is highly expressed in HNSCC samples and that its expression levels are significantly associated with tumor progression indicated by high frequencies of nodal metastases and advanced clinical stage.
Table 1.
Relationship between CIN85 Expression and Clinicopathologic Parameters.
| n | Mean Score of Relative Expression of CIN85 mRNA | P | |
| T stage | |||
| I and II | 1 | 886.72 ± 37.36 | .79 |
| III and IV | 11 | 42.25 ± 8.07 | |
| Nodal metastasis | |||
| Negative | 10 | 21.84 ± 6.53 | .0044 |
| Positive | 19 | 95.12 ± 35.47 | |
| Clinical stage | |||
| I and II | 8 | 24.11 ± 8.04 | .028 |
| III and IV | 21 | 87.28 ± 31.66 | |
| Recurrence | |||
| Negative | 11 | 58.47 ± 29.91 | .34 |
| Positive | 16 | 79.48 ± 37.87 | |
| Age (years) | |||
| <60 | 16 | 76.52 ± 38.01 | .79 |
| >60 | 13 | 61.64 ± 25.23 | |
| Sex | |||
| Male | 21 | 56.16 ± 15.67 | .59 |
| Female | 8 | 105.79 ± 76.79 | |
| Smoking | |||
| Brinkman score <400 | 13 | 97.06 ± 4.45 | .31 |
| Brinkman score >400 | 16 | 68.73 ± 9.91 |
Figure 1.
CIN85 upregulates the growth of HNSCCs. (A) CIN85 mRNA and protein expression in representative clinical samples. Total RNA and proteins were extracted from the tissues of untreated patients with oral squamous cell carcinoma. CIN85 mRNA and protein expression and phospho-ERK1/2 expression were evaluated by quantitative RT-PCR and Western blot analysis. CIN85 mRNA expression in no. 155 sample was taken as 1.0. In Western blots, β-actin served as a loading control. The intensity of bands was quantified and normalized with respect to those of corresponding β-actin. The resulting values were expressed as the percentage in reference to that of no. 212. (B) CIN85 mRNA and protein expressions and EGFR protein expression in three HNSCC cell lines (KB, YCU-H891, and SCC9) by quantitative RT-PCR and Western blot assays. Total RNA and proteins were extracted from exponentially growing cells and subjected to quantitative RT-PCR analysis and Western blots. In quantitative RT-PCR analysis (left panel), the level of CIN85 mRNA expression in YCU-H891 was taken as 1.0. In Western blot analysis (right panel), the resulting values were expressed as the percentage in reference to that of KB cells. (C) Establishment of stable CIN85-overexpressing and CIN85 knockdown KB cells. Either an shRNA construct for CIN85 or a full-length CIN85 construct was transfected with KB cells. Independent clonal lines were designated as KB/KD1, KB/KD2, KB/OE1, and KB/OE2. Expression levels of CIN85 mRNA (upper panel) and proteins (lower panel) in parental, vector ,control and transfected cells were measured by quantitative RT-PCR or Western blots. In quantitative RT-PCR analysis, the level of CIN85 mRNA expression in parental KB cells was taken as 1.0. In Western blot analysis, the resulting values were expressed as the percentage in reference to that of KB/OE2 cells. Note that a full-length CIN85 transgene is V5-tagged, and thus, its product migrates slower than endogenous CIN85 on a gel (arrow). (D) Growth curves of control and transfected cells. Two thousands cells of the indicated cell lines were cultured for 96 hours and counted every other day. The results are representative of three independent experiments. The values at each point indicate the average of triplicate dishes; bars, SD.
CIN85 Upregulates the Growth Potential of HNSCC Cells
High levels of CIN85 expression in clinical samples prompted us to determine whether CIN85 regulates cell growth potential using HNSCC cell lines. To this end, we first tested expression levels of EGFR and CIN85 in three HNSCC cell lines (Figure 1B). All three HNSCC cell lines were positive for EGFR protein, albeit with considerable variation in expression levels. Conversely, KB cells significantly express CIN85 at both mRNA and protein levels, compared with SCC9 and YCU-H891 cells. This expression profile suggests that KB cells express higher levels of CIN85 and thus mimic a subset of HNSCC with progressive phenotypes.
We next generated independent clonal KB cell lines of CIN85 knockdown and full-length CIN85 overexpression (designated as KB/KD1, KB/KD2, KB/OE1, and KB/OE2 cells), as described in Materials and Methods. As shown in Figure 1C, expression levels of CIN85 mRNA and protein were significantly decreased in KB/KD cells, whereas they were markedly increased in KB/OE cells, compared with parental and control cells.
Using these stable KB lines, we monitored the growth of parental KB, vector control KB, KB/KD1, and KB/OE1 cells (Figure 1D). Because parental and vector control cells displayed similar proliferation patterns, representative data of vector control cells are shown hereafter. KB/KD1 cells demonstrated a lower rate of proliferation compared with control cells, and the differences of growth at days 10 and 12 were statistically significant (P < .05). In contrast, enforced overexpression of CIN85 protein further enhanced the growth of these cells, and the difference of growth at day 12 was statistically significant (P < .05). These results suggest that CIN85 upregulates the growth of HNSCC cells, at least in part accounting for progressive phenotypes of the clinical samples with high CIN85 expression.
CIN85 Facilitates EGFR Internalization without Affecting Its Phosphorylation
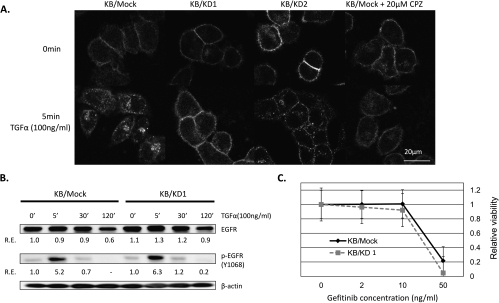
One of the characteristic functions of CIN85 is the regulation of ligand-induced internalization of receptor tyrosine kinases (RTKs) including EGFRs [12,13]. We thus determined whether CIN85 knockdown affects EGFR internalization in KB cells after TGF-α stimulation (Figure 2A). Confocal microscopic analyses demonstrated that TGF-α-induced EGFR internalization (TIEI) was clearly observed in KB/mock cells. In contrast, CIN85 knockdown (KB/KD1 and KB/KD2 cells) significantly inhibited TIEI. To directly test whether this inhibition is not secondary to CIN85 knockdown, we reintroduced full-length CIN85 gene into KB/KD1 cells and found that TIEI gets normalized in these cells (Figure W1). Treatment of KB/mock cells with 20 µM of CPZ, an inhibitor of clathrin-mediated RTK internalization [16], also partially inhibited TIEI. Consistent with previous reports [12,13], these results suggest that CIN85 facilitates EGFR internalization in HNSCC cells.
Figure 2.
CIN85 facilitates EGFR internalization without affecting its phosphorylation. (A) Confocal microscopic assays for EGFR internalization in KB/mock and KB/KD cells. The indicated cells were grown on poly-l-lysine-coated glass-bottom dishes (IWATANI) for 24 hours in serum-free medium, and half of the dishes were stimulated with 100 ng/ml of TGF-α for 5 minutes. For inhibition of clathrin-mediated EGFR internalization, KB/mock cells were treated with 20 µM of CPZ for 30 minutes before TGF-α stimulation (right panel). Cells were then fixed, and the localization of EGFR was visualized with anti-EGFR mAb and a secondary Alexa Flour 594-conjugated antimouse Ab. The results are representative of three independent experiments. (B) Western blot assays for EGFR and phospho-EGFR (p-EGFR) protein in KB/mock and KB/KD1 cells. The exponentially growing cells were stimulated with 100 ng/ml of TGF-α for the indicated times, and cell lysates were prepared. β-Actin was served as a loading control. Relative expression (R.E.) was calculated as the ratio to the level of unstimulated KB/mock cells (taken as 1.0). (C) Effect of CIN85 expression on cell sensitivity for gefitinib. Cells were treated with the indicated concentrations of gefitinib for 72 hours, and then cell viability was determined by cell proliferation assays. The values at each point indicate the mean of triplicate wells; bars, SD.
We next questioned whether EGFR internalization facilitated by CIN85 affects the expression levels of EGFR and phospho-EGFR in KB cells (Figure 2B). Immunoblot assays of EGFR and phospho-EGFR demonstrated that, on TGF-α stimulation, the levels of total cellular EGFR proteins remained unchanged for 30 minutes and slightly decreased at 120 minutes in both KB/mock and KB/KD1 cells to a similar extent, probably due to a partial degradation of the EGFR protein. The levels of basal and TGF-α-stimulated phospho-EGFR proteins were comparable between KB/mock and KB/KD1 cells. These suggest that CIN85 facilitates EGFR internalization without altering the levels of total EGFR protein and the phosphorylation of EGFR in HNSCC cells. Thus, growth inhibition in KB/KD1 (Figure 1D) was not simply due to decreased phosphorylation of EGFR. A possible explanation for the lack of CIN85's effect on EGFR phosphorylation could be that a majority of TGF-α-bound EGFRs are processed through the recycling back pathway [12]. We treated KB/mock cells and KB/KD1 cells with gefitinib, an EGFR-specific inhibitor, and assessed their survival (Figure 2C). Control and KB/KD1 cells exhibited similar sensitivity to gefitinib, again suggesting that CIN85 does not affect TGF-α-induced phosphorylation of EGFR (i.e., signal intensity of EGFR) in HNSCC cells.
CIN85 Promotes Activation of the Ras-ERK Pathway Required for Cell Growth of HNSCCs
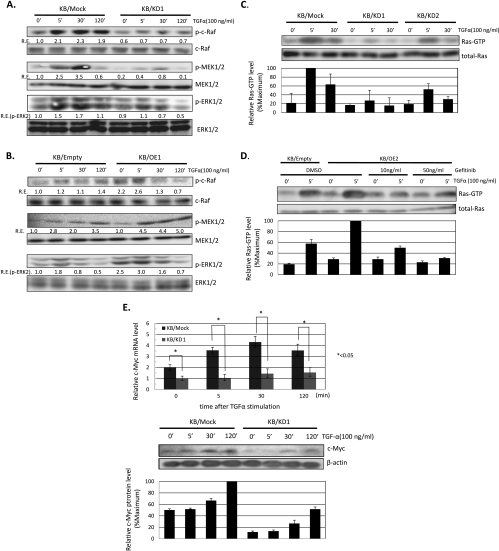
To further elucidate the molecular mechanism by which CIN85 enhances proliferation of KB cells, we analyzed the phosphorylation of signaling molecules downstream of EGFR including c-Cbl, mitogen-activated protein kinases (MAPKs), and NF-κB byWestern blot assays. No significant differences were observed in the expression levels of IκBα, phospho-IκBα, phospho-p65 NFκB, c-Cbl, and phospho-c-Cbl between control, KB/OE1, and KB/KD1 cells regardless of TGF-α stimulation (data not shown). Conversely, the levels of phospho-c-Raf, -MEK1/2, and -ERK1/2 were significantly inhibited in KB/KD1 cells, whereas they were enhanced in KB/OE1 cells (Figure 3, A and B). Similar data were also obtained using KB/KD2 and KB/OE2 cells (Figure W2).
Figure 3.
CIN85 preferentially promotes activation of the Ras-ERK pathway critical for the growth of HNSCCs. (A and B) Western blot assays for phospho-c-Raf, -MEK1/2, and -ERK1/2 in KB/mock, KB/KD1, KB/empty, and KB/OE1 cells. Cells were treated with 100 ng/ml of TGF-α for the indicated times, and cell lysates were prepared. Relative expression (R.E.) was calculated as described in Figure 2. The results are representative of three independent experiments. (C) Effect of CIN85 expression on Ras activation in KB/mock and KB/KD cells. Cells were treated with 100 ng/ml of TGF-α for the indicated times, and the abundance of the activated forms of Ras (Ras-GTP) and total cellular Ras proteins was determined. The levels of specific bands were quantified, and those of Ras-GTP were normalized with respect to those of corresponding Ras. The resulting values were expressed as the percentage in reference to that of KB/mock stimulated with TGF-α for 5 minutes. The results are representative of two independent experiments. (D) Gefitinib blocks CIN85-induced enhancement of Ras activation. Cells were treated with the indicated concentrations of gefitinib for 30 minutes before TGF-α stimulation, and the abundance of activated forms of Ras (Ras-GTP) and total cellular Ras proteins was determined. The resulting values were expressed as the percentage in reference to that of KB/OE2 stimulated with TGF-α for 5 minutes. The results are representative of two independent experiments. (E) Effect of CIN85 expression on c-Myc expression in KB/mock and KB/KD cells. Cells were treated with 100 ng/ml of TGF-α for the indicated times, and total RNA and proteins were extracted. Relative expression of c-Myc mRNA was determined by quantitative RT-PCR assays (upper panel). The values are the mean ± SD of three independent experiments. *P < .05. The levels of c-Myc protein were detected by Western blot assays (lower panel). β-Actin was served as a loading control. The resulting values were expressed as the percentage in reference to that of KB/mock stimulated with TGF-α for 120 minutes.
To further determine whether CIN85 functions more upstream of the MAPK cascade, we analyzed the activities of Ras in KB/mock, KB/KD1, and KB/KD2 cells (Figure 3C). On TGF-a stimulation, the levels of Ras-GTP (activated form of Ras) were significantly inhibited in KB/KD cells, suggesting that CIN85 promotes Ras activation downstream of EGFR in HNSCC cells. We next tested whether an EGFR inhibitor, gefitinib, could cancel CIN85-induced enhancement of Ras activation in KB/OE1 cells. As a result, we found that gefitinib significantly suppressed Ras activation in KB/OE1 cells (Figure 3D), suggesting that CIN85's effect on activation of the Ras/MAPK pathway absolutely requires EGFR stimulation.
Finally, we determined the expression levels of c-Myc mRNA and protein between KB/mock and KB/KD1 cells (Figure 3E) because c-Myc is one of the important targets of the Ras-ERK pathway that is critical for tumor progression in a variety of tumors including HNSCCs [17,18]. Consistent with the current results that CIN85 promotes activation of the Ras-ERK pathway, expression levels of c-Myc were significantly decreased in KB/KD1 cells with or without TGF-α, compared with KB/mock cells.
Discussion
To date, there are only two reports examining the expression levels of CIN85 in the clinical samples of human cancer. In human gliomas, CIN85 is expressed at higher levels than in normal brain [19]. In human melanoma, expression levels of CIN85 are higher in tumors compared with adjacent normal tissues [20]. These results are thus consistent with our present study on HNSCC and indicate that CIN85 is involved in the tumor development of certain types of human malignancies. In our study, we further analyzed the correlation between the levels of CIN85 expression and clinicopathologic parameters and found that CIN85 expression is more pronounced in HNSCC tumor tissues obtained from patients in advanced clinical stages, providing the first evidence that CIN85 expression is associated with tumor progression in human cancer. Considering the limited number of samples used in our study, further accumulation of data is required to consolidate the significance of CIN85 expression in HNSCC, especially as a prognostic marker.
A functional role of CIN85 in tumorigenesis has thus far been poorly characterized. A previous article showed that CIN85 is critical for the maintenance of invasive phenotype in breast cancer cells [21]. In the present study using HNSCC cell lines, we found that CIN85 upregulates the growth of HNSCCs through potentiating EGFR-mediated Ras-ERK activation. In addition, ERK phosphorylation was more pronounced in clinical samples expressing high levels of CIN85 (Figure 1A). Notably, a previous study showed that ERK activation is enhanced in HNSCCs with advanced regional lymph node metastasis [4], consistent with our result that increased CIN85 expression is associated with nodal metastases (Table 1). These findings suggest that CIN85 is considered as a critical mediator of EGFR-induced ERK activation and consequent tumor progression in HNSCC. We have thus unraveled a new function of CIN85: a mediator of EGFR signaling to the Ras-ERK pathway.
The precise molecular mechanisms by which CIN85 regulates EGFR-mediated Ras activation remain to be elucidated. Nevertheless, a couple of possibilities could be envisioned. Consistent with previous studies [12,13], CIN85 facilitated ligand-induced EGFR internalization in HNSCC cells (Figure 2A). Because of the classic conceptual framework, EGFR internalization was considered to downregulate EGFR signaling and cause tumor suppression. However, accumulating evidence suggests that EGFR continues to signals in cytoplasm as well as on plasma membrane. In this process, the endosome functions as a critical compartment that enables the propagation of signaling downstream of EGFR. Signals generated at endosomes are sufficient for the growth of epithelial cells presumably through promoting ERK activation [22]. Intriguingly, ERK is colocalized with activated Ras on rasosomes that are Ras-enriched small cytosolic nanoparticles [23]. Thus, a provocative possibility is that CIN85 plays a key role in connecting early endosomes with rasosomes and contributes to activation of the Ras-ERK pathway. This possibility, taken together with the fact that in HNSCC the major EGFR ligand, TGF-a, mainly sorts EGFR in the recycling back loop [24], accounts at least in part for the mechanism of CIN85-mediated HNSCC tumorigenesis. Thus, CIN85 facilitates TGF-α-induced internalization of EGFRs, which are constantly supplied in the recycling back-loop. These cytoplasmic EGFRs continue to stimulate the Ras-ERK pathway.
Another possibility is that CIN85 regulates Ras activation by a mechanism independent of EGFR internalization. First, overexpression of focal adhesion kinase (FAK) promotes Ras activity in malignant astrocytoma cells [25]. Because FAK is a binding partner for CIN85 [26], CIN85 may enhance Ras activity through its interaction with FAK in HNSCC cells. Second, Sprouty 2 plays a critical role in tumor formation by H-Ras-transformed human fibroblasts [27]. Given that Sprouty 2 is also a binding partner for CIN85 [28], CIN85 may regulate Ras activity through altering the function of Sprouty 2. Further work is needed to clarify these possibilities.
In contrast to our current study, Tossidou et al. [29] previously suggested a negative regulatory role of CIN85 in ERK phosphorylation in podocytes. Of note, podocytes poorly express CIN85 and instead abundantly express CD2AP, a paralog of CIN85 [30]. In addition, mice deficient in CD2AP die around 6 weeks of age because of renal failure, and podocyte-specific expression of CD2AP corrects this phenotype [30]. Given that CIN85 and CD2AP compete for their binding partners, CD2AP may play a more dominant role in Ras-ERK activation in podocytes. Thus, CD2AP/CIN85 balance could influence their roles in RTK response depending on cell type.
In conclusion, our current findings suggest that CIN85 overexpression is associated with tumor development and progression in a subset of HNSCC. Moreover, we uncovered a novel function of CIN85 as a promoter of the Ras-ERK cascade downstream of TGF-α/EGFR in human cancer. Given that the Ras-ERK pathway is critical for the maintenance of malignant phenotypes of HNSCCs, CIN85 can be a potential therapeutic target in a subset of HNSCC in the future.
Supplementary Material
Footnotes
This study was supported in part by funds from Grants-in-Aid for Scientific Research: 21592195 and 21591267 to Muneyuki Masuda and Hiroaki Niiro, respectively.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Bernier J, Bentzen SM, Vermorken JB. Molecular therapy in head and neck oncology. Nat Rev Clin Oncol. 2009;6:266–277. doi: 10.1038/nrclinonc.2009.40. [DOI] [PubMed] [Google Scholar]
- 2.Grandis JR, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 3.Ongkeko WM, Altuna X, Weisman RA, Wang-Rodriguez J. Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am J Clin Pathol. 2005;124:71–76. doi: 10.1309/BTLN5WTMJ3PCNRRC. [DOI] [PubMed] [Google Scholar]
- 4.Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, et al. Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor α expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001;61:6500–6510. [PubMed] [Google Scholar]
- 5.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 6.Masuda M, Ruan HY, Ito A, Nakashima T, Toh S, Wakasaki T, Yasumatsu R, Kutratomi Y, Komune S, Weinstein IB. Signal transducers and activators of transcription 3 up-regulates vascular endothelial growth factor production and tumor angiogenesis in head and neck squamous cell carcinoma. Oral Oncol. 2007;43:785–790. doi: 10.1016/j.oraloncology.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Masuda M, Wakasaki T, Suzui M, Toh S, Joe AK, Weinstein IB. Stat3 orchestrates tumor development and progression: the Achilles' heel of head and neck cancers? Curr Cancer Drug Targets. 2010;10:117–126. doi: 10.2174/156800910790980197. [DOI] [PubMed] [Google Scholar]
- 8.Hoa M, Davis SL, Ames SJ, Spanjaard RA. Amplification of wild-type K-ras promotes growth of head and neck squamous cell carcinoma. Cancer Res. 2002;62:7154–7156. [PubMed] [Google Scholar]
- 9.Take H, Watanabe S, Takeda K, Yu ZX, Iwata N, Kajigaya S. Cloning and characterization of a novel adaptor protein, CIN85, that interacts with c-Cbl. Biochem Biophys Res Commun. 2000;268:321–328. doi: 10.1006/bbrc.2000.2147. [DOI] [PubMed] [Google Scholar]
- 10.Dikic I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002;529:110–115. doi: 10.1016/s0014-5793(02)03188-5. [DOI] [PubMed] [Google Scholar]
- 11.Havrylov S, Rzhepetskyy Y, Malinowska A, Drobot L, Redowicz MJ. Proteins recruited by SH3 domains of Ruk/CIN85 adaptor identified by LC-MS/MS. Proteome Sci. 2009;7:21. doi: 10.1186/1477-5956-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 14.Narita T, Ando A, Mikami Y, Taniyama T. Overexpression of CIN85 suppresses the growth of herpes simplex virus in HeLa cells. Exp Cell Res. 2005;311:265–271. doi: 10.1016/j.yexcr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Tabrizi SJ, Niiro H, Masui M, Yoshimoto G, Iino T, Kikushige Y, Wakasaki T, Baba E, Shimoda S, Miyamoto T, et al. T cell leukemia/lymphoma 1 and galectin-1 regulate survival/cell death pathways in human naive and IgM+ memory B cells through altering balances in Bcl-2 family proteins. J Immunol. 2009;182:1490–1499. doi: 10.4049/jimmunol.182.3.1490. [DOI] [PubMed] [Google Scholar]
- 16.Vink SR, van der Luit AH, Klarenbeek JB, Verheij M, van Blitterswijk WJ. Lipid rafts and metabolic energy differentially determine uptake of anti-cancer alkylphospholipids in lymphoma versus carcinoma cells. Biochem Pharmacol. 2007;74:1456–1465. doi: 10.1016/j.bcp.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Akervall J, Bockmuhl U, Petersen I, Yang K, Carey TE, Kurnit DM. The gene ratios c-MYC:cyclin-dependent kinase (CDK)N2A and CCND1: CDKN2A correlate with poor prognosis in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2003;9:1750–1755. [PubMed] [Google Scholar]
- 18.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 19.Bogler O, Furnari FB, Kindler-Roehrborn A, Sykes VW, Yung R, Huang HJ, Cavenee WK. SETA: a novel SH3 domain-containing adapter molecule associated with malignancy in astrocytes. Neuro Oncol. 2000;2:6–15. doi: 10.1093/neuonc/2.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayevska O, Shuvayeva H, Igumentseva N, Havrylov S, Basaraba O, Bobak Y, Barska M, Volod'ko N, Baranska J, Buchman V, et al. Expression of adaptor protein Ruk/CIN85 isoforms in cell lines of various tissue origins and human melanoma. Exp Oncol. 2006;28:275–281. [PubMed] [Google Scholar]
- 21.Nam JM, Onodera Y, Mazaki Y, Miyoshi H, Hashimoto S, Sabe H. CIN85, a Cbl-interacting protein, is a component of AMAP1-mediated breast cancer invasion machinery. EMBO J. 2007;26:647–656. doi: 10.1038/sj.emboj.7601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennock S, Wang Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol Cell Biol. 2003;23:5803–5815. doi: 10.1128/MCB.23.16.5803-5815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotblat B, Yizhar O, Haklai R, Ashery U, Kloog Y. Ras and its signals diffuse through the cell on randomly moving nanoparticles. Cancer Res. 2006;66:1974–1981. doi: 10.1158/0008-5472.CAN-05-3791. [DOI] [PubMed] [Google Scholar]
- 24.Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, Willumsen BM, van Deurs B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecker TP, Ding Q, Rege TA, Hanks SK, Gladson CL. Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene. 2004;23:3962–3971. doi: 10.1038/sj.onc.1207541. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt MH, Chen B, Randazzo LM, Bogler O. SETA/CIN85/Ruk and its binding partner AIP1 associate with diverse cytoskeletal elements, including FAKs, and modulate cell adhesion. J Cell Sci. 2003;116:2845–2855. doi: 10.1242/jcs.00522. [DOI] [PubMed] [Google Scholar]
- 27.Lito P, Mets BD, Kleff S, O'Reilly S, Maher VM, McCormick JJ. Evidence that sprouty 2 is necessary for sarcoma formation by H-Ras oncogenetransformed human fibroblasts. J Biol Chem. 2008;283:2002–2009. doi: 10.1074/jbc.M709046200. [DOI] [PubMed] [Google Scholar]
- 28.Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 29.Tossidou I, Kardinal C, Peters I, Kriz W, Shaw A, Dikic I, Tkachuk S, Dumler I, Haller H, Schiffer M. CD2AP/CIN85 balance determines receptor tyrosine kinase signaling response in podocytes. J Biol Chem. 2007;282:7457–7464. doi: 10.1074/jbc.M608519200. [DOI] [PubMed] [Google Scholar]
- 30.Grunkemeyer JA, Kwoh C, Huber TB, Shaw AS. CD2-associated protein (CD2AP) expression in podocytes rescues lethality of CD2AP deficiency. J Biol Chem. 2005;280:29677–29681. doi: 10.1074/jbc.M504004200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.