Abstract
Evidence indicates that diets enriched in n-3 polyunsaturated fatty acids (n-3 PUFAs) reduce the risk of prostate cancer, but biochemical mechanisms are unclear. Syndecan-1 (SDC-1), a transmembrane heparan sulfate proteoglycan, supports the integrity of the epithelial compartment. In tumor cells of epithelial lineage, SDC-1 is generally downregulated. This may result in perturbation of homeostasis and lead to progression of malignancy. Our studies have shown that the n-3 PUFA species, docosahexaenoic acid (DHA), increases SDC-1 expression in prostate tissues of Pten knockout (PtenP-/-) mice/cells and human prostate cancer cells. We have now determined that DHA-mediated up-regulation of SDC-1 induces apoptosis. Bovine serum albumin-bound DHA and exogenous human recombinant SDC-1 ecotodomain were delivered to PC3 and LNCaP cells in the presence or absence of SDC-1 small interfering (si)RNA. In the presence of control siRNA, both DHA and SDC-1 ectodomain induced apoptosis, whereas SDC-1 silencing blocked DHA-induced but not SDC-1 ectodomain-induced apoptosis. Downstream effectors of SDC-1 signaling linked to n-3 PUFA-induced apoptosis involved the 3′-phosphoinositide-dependent kinase 1 (PDK1)/Akt/Bad integrating network. A diet enriched in n-3 PUFA decreased phosphorylation of PDK1, Akt (T308), and Bad in prostates of PtenP-/- mice. Similar results were observed in human prostate cancer cells in response to DHA and SDC-1 ectodomain. The effect of DHA on PDK1/Akt/Bad signaling was abrogated by SDC-1 siRNA. These findings define a mechanism by which SDC-1-dependent suppression of phosphorylation of PDK1/Akt/Bad mediates n-3 PUFA-induced apoptosis in prostate cancer.
Introduction
Prostate cancer is the most frequently diagnosed cancer and a leading cause of male cancer-related death in the United States. Epidemiological studies that show geographical variations in prostate cancer incidence and mortality [1,2] and migration-associated changes in risk [3,4] have provided evidence that environmental and lifestyle-related factors, including diet, may contribute to this disease. Of particular interest are the long-chain marine n-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA, [20:5, n-3]), and docosahexaenoic acid (DHA, [22:6, n-3]), which are reported to be lower in plasma [5] and prostate tissue [6,7] of prostate cancer patients compared with cancer-free men. Moreover, cell culture and animal models have indicated that higher levels of n-6 PUFA drive prostate cancer growth, whereas n-3 PUFA are protective [8–10]. Although a number of biologic pathways have been implicated in the cancer-promoting and cancer-protective properties of n-6 PUFA and n-3 PUFA, respectively (reviewed in Larsson et al. [11] and Berquin et al. [12]), the molecular mechanisms remain poorly understood.
Syndecan-1 (SDC-1) is a transmembrane heparan sulfate proteoglycan known to play a role in the regulation of cell-cell and cell-matrix interactions, cell migration, adhesion, differentiation, and tumorigenesis [13–18]. A biologically active ectodomain of SDC-1 can be shed from the cell through the action of matrix-metalloproteinases [19–21]. Low levels or a complete loss of SDC-1 in tumor cells is associated with tumor progression and aggressive phenotypes of various cancers [22–25], whereas re-expression of SDC-1 restores the epithelial morphology and suppresses hormone-induced growth of tumor cells [26]. In human prostate cancer, recent studies show that there is an inverse correlation between SDC-1 expression and Gleason grade of prostate cancer [27,28]. SDC-1 is uniformly expressed in the basolateral membrane of normal epithelia, but its distribution shifts from the plasma membrane to the cytoplasm in prostate cancer cells [27]. In contrast, enhanced expression of SDC-1 also corresponds with unfavorable phenotypes in some cancers including ovarian, breast, pancreatic, endometrial cancers, and glioma [29–33]. Furthermore, mounting evidence implicates discrepant effects of epithelial and stromal cell SDC-1 [24], but how SDC-1 as a key regulator of epithelial-stromal interaction modifies the tumor microenvironment and affects tumor progression is unknown. In addition, SDC-1 extracellular domain shedding is believed to contribute to diverse pathophysiological events including cancer [34], although the spatial regulation of SDC-1-extracellular matrix engagement through shedding needs to be further investigated.
Our previous studies have shown that n-3 PUFAs increase SDC-1 expression in prostate cancer cells through a transcriptional pathway involving peroxisome proliferator-activated receptor γ (PPARγ) [35]. In the present study, we have used prostate-specific Pten (phosphatase and tensin homolog deleted on chromosome 10) knockout mice and human prostate cancer cells to explore functional outcomes of SDC-1 upregulation by n-3 PUFA. We found that n-3 PUFA regulated an SDC-1-dependent pathway that involved inhibition of 3′-phosphoinositide-dependent kinase 1 (PDK1)/Akt/Bad phosphorylation to result in apoptosis induction.
Materials and Methods
Materials
Anti-Akt, anti-phospho-Akt (S473), anti-phospho-Akt (T308), anti-phospho-PDK1 (S243), anti-PDK1, anti-phospho-Bad (S112), anti-Bad, anti-poly(ADP-ribose) polymerase (PARP), and anti-HRP-conjugated secondary antibody against rabbit were purchased from Cell Signaling Technology (Danvers, MA). Anti-SDC-1 (H-174) antibody and normal rabbit lgG were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). CellTiter 96 Aqueous One Solution Cell Proliferation Assay and Caspase-Glo 3/7 Assay were from Promega (Madison, WI). Wortmannin was purchased from Sigma-Aldrich Company Ltd (Allentown, PA). Hoechst 33342 was from Invitrogen (Eugene, OR). EPA and DHA were from Sigma Chemical Co (St Louis, MO) and prepared as 600-µM stocks complexed to bovine serum albumin (BSA) as described [36]. Low-density lipoproteins (LDLs) were isolated from plasma of adult vervet monkeys fed n-3 PUFA (fish oil)-enriched diets for more than 3 years. LDL isolation and characterization procedures were previously described [36].
Cell Lines and Cell Culture
PC3, LNCaP, and DU145 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained in advanced Dulbecco's modified Eagle medium (Invitrogen, Grand Island, NY) containing 1% fetal bovine serum (FBS; PC3 cells), RPMI 1640 medium (Invitrogen) with 10%FBS (LNCaP cells), or Eagle minimum essential medium with Earl's salts medium (Invitrogen) containing 10%FBS (DU145 cells). Normal human prostate epithelial cells (PrEC) were purchased from Clonetics (San Diego, CA) and were maintained in Clonetics Prostate Epithelial Cell Medium. Mouse prostate epithelial cells derived from PtenP+/+ and PtenP-/- mice were provided by Dr. Scott D. Cramer (Wake Forest University School of Medicine) and maintained in advanced Dulbecco's modified Eagle medium containing 1% FBS. Cell Pten status was confirmed by Western analysis.
Mice
As previously described [10,37,38], prostate-specific Pten-knockout mice were generated by crossing Pten loxP/loxP mice with mice of the ARR2Probasin-cre transgenic line PB-cre4, wherein the Cre recombinase is under the control of a modified rat prostate-specific probasin promoter. For simplicity, PtenloxP/loxPPB-cre4-/- and PtenloxP/+PB-cre4-/- are referred to as PtenP+/+ and PtenloxP/loxP PB-cre4T/- as PtenP-/-. PtenP+/+ and PtenP-/- mice were used in this study. Mice were fed a high n-3 (n-6: n-3 ratio = 1:1) or a high n-6 (n-6: n-3 ratio = 40:1) diet for 8 weeks. Diet composition, body weights, fatty acid ratios in food, blood, and prostate tissues and dissection of prostate lobes have been described [10]. Animal care was conducted in compliance with the state and federal Animal Welfare Acts and standards and policies of the Department of Health and Human Services. The protocol was approved by our Institutional Animal Care and Use Committee.
Preparation of Recombinant SDC-1 Ectodomain
The preparation of SDC-1 ectodomain was previously described [39]. Briefly, SDC-1 ectodomain expression construct was designed to encode a COOH-terminal polyhistidine fusion protein and was created using a two-step cloning process as follows. The SDC-1 ecto-domain complementary DNA (cDNA) was amplified by polymerase chain reaction (PCR) using the SDC-1 plasmid (OriGene Technologies, Inc, Rockville, MD). The resulting cDNA was cut with BamHI and EcoRI restriction endonucleases and ligated into the pTcam4 expression plasmid. In the second round of PCR, 5′-gcaaagcttgaattcggcagcatgaggcgcgcg-3′ (forward) and 5′-gcaaagcttgaattcggcagcatgaggcgcgcg-3′ (reverse) primers were used to amplify the SDC-1 ectodomain cDNA. The amplified cDNA was treated with HindIII and XhoI and subsequently ligated into the pCEP4 (Invitrogen) expression plasmid, then transfected into 293-EBNA cells. The stable expressing cells selected by the addition of 200 µg/ml hygromycin B were cultured, and the conditioned medium was used to purify recombinant SDC-1 ectodomain byMillipore Pellicon 2 tangential flow system (Millipore Corp, Billerica, MA) and anion exchange chromatography.
Cell Growth Assay
Prostate cancer cells were plated in 96-well plates at a density of 2 x 103 cells per well in 100 µl of medium. After treatment with DHA or SDC-1 ectodomain, cell growth was measured using a CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega) based on the manufacturer's protocol. Data represent the mean absorbance of three wells, and these are presented relative to control.
Western Blot Assay
Mouse prostate tissues were homogenized and lysed in ice-cold lysis buffer (25 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1 mg/ml phenylmethanesulfonyl fluoride) with 1x proteinase and 1x phosphatase inhibitors (Roche Applied Science, Indianapolis, IN). Lysates of human prostate cancer cells were similarly prepared. For the analysis of SDC-1 protein, lysates were dialyzed against 100 mM Tris and 30 mM sodium acetate, pH 8.0 for 24 hours at 4°C, and then digested by chondroitinase ABC (Seikagaku, Ijamsville, MD) and heparinase III (Sigma-Aldrich) at 37°C overnight. Protein extracts were prepared for Western blot analysis as described earlier using indicated antibodies [40]. Band densities on photographic films were analyzed using Image J 1.37v (National Institutes of Health, Bethesda, MD).
SDC-1 Gene Silencing by Small Interfering RNA
Two SDC-1 small interfering RNA (siRNA) specific for human SDC-1 gene were purchased from Ambion (catalog no. AM16708A [SDC-1 siRNA I] and catalog no. AM16708 [SDC-1 siRNA III]; Austin, TX). Cells were plated in six-well plates at a density of 2.0 x 105 cells per well, and transfected with siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Control cells received a negative control siRNA with no known target. At 6 hours after transfection, wells were supplemented with medium containing 1% FBS for 24 hours. The most effective siRNA (AM16708A, SDC-1 siRNA I) in silencing SDC-1 gene expression was used for further experiments.
Quantitative Real-time PCR
Quantitative real-time PCR (RT-PCR) was performed as described [39,41]. Briefly, total cell RNA was prepared. Amplification reactions were performed in triplicate in Applied Biosystems 7500 Real-Time PCR System. The primers used for mouse SDC-1 were 5′-tggagaacaagacttcacctttg-3′ (forward) and 5′-ctcccagcacttccttcct-3′ (reverse), and those for human SDC-1 were 5′-ggagcaggacttcacctttg (forward) and 5′-ctcccagcacctctttcct (reverse). Primers for mouse peptidylprolylisomerase B housekeeping gene were 5′-gcccaaagtcaccgtcaa (forward) and 5′-tccgaagagaccaaagatcac (reverse), and those for human were 5′-gcccaaagtcaccgtcaa (forward) and 5′-tccgaagagaccaaagatcac (reverse). Data were normalized to the housekeeping control peptidylprolylisomerase B, and these are presented relative to control.
Detection of Apoptosis
PC3 and/or LNCaP cells transfected with SDC-1 siRNA or control siRNA were treated with DHA or SDC-1 ectodomain. Apoptosis was measured by cleaved PARP usingWestern blot assay or caspase 3 activity using Caspase-Glo 3/7 assay following the manufacturer's protocol and/or Hoechst 33342 staining.
Statistical Analysis
Data are expressed as mean and SD or SE. Statistical analysis was performed using one-way analysis of variance. P < .05 was considered statistically significant.
Results
Apoptosis Is Induced by n-3 PUFA in Human Prostate Cancer Cells
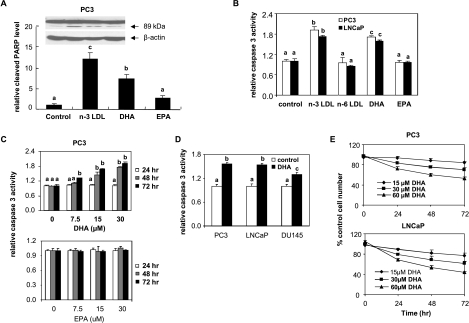
Our previous studies had shown that LDL isolated from monkeys fed an n-3 PUFA-enriched diet contained two major species of n-3 PUFA, namely, EPA and DHA, and both were effectively delivered to the cells by the LDL [35,36]. To determine the effects of the individual n-3 PUFA in apoptosis induction, PC3 cells were incubated with the n-3 PUFA-enriched LDL, BSA-bound DHA, or BSA-bound EPA for 48 hours, and apoptosis was measured by PARP cleavage. The n-3 LDL and DHA significantly increased cleaved PARP levels compared with control cells, whereas EPA was ineffective (Figure 1A). Apoptosis was confirmed in PC3 and LNCaP treated with n-3 LDL and DHA by Caspase-Glo 3/7 Assay (Figure 1, B and C). As shown in Figure 1C, apoptosis induction by DHA was dose-dependent, whereas no apoptotic effect was observed with BSA-bound EPA and n-6 PUFA-enriched LDL at similar doses in cells treated for up to 72 hours (Figure 1, A–C). In addition, DU145 cells were slightly less sensitive to DHA-induced apoptosis than PC3 and LNCaP cells (Figure 1D). Consistent with the induced apoptosis, DHA inhibited cell growth in a dose- and time-dependent manner in PC3 and LNCaP cells (Figure 1E).
Figure 1.
Effects of n-3 PUFA on apoptosis in human prostate cancer cells. (A) PC3 cells were treated with n-3 PUFA-enriched LDL (100 µg/ml), DHA-BSA, or EPA-BSA (30 µM) for 48 hours. Protein extracts were used for Western blot analysis of cleaved PARP and β-actin. Values representing the cleaved PARP/β-actin mean ratios compared with controls (n = 3) with different letters are significantly different (P < .05). (B) PC3 and LNCaP cells were treated with n-3 and n-6 PUFA-enriched LDL (100 µg/ml), DHA-BSA, or EPA-BSA (30 µM) for 48 hours. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05). (C) PC3 cells were treated with indicated concentrations of DHA-BSA or EPA-BSA for indicated times. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05). (D) PC3, LNCaP, and DU145 cells were treated with DHA-BSA (30 µM) for 48 hours. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05). (E) PC3 and LNCaP cells were treated with indicated concentrations of DHA-BSA. Cell growth was measured using MTS assay and compared with that of controls (untreated cells) at the same time point. Means ± SD (n = 3) are presented.
SDC-1 Is Upregulated by n-3 PUFA In Vivo and In Vitro
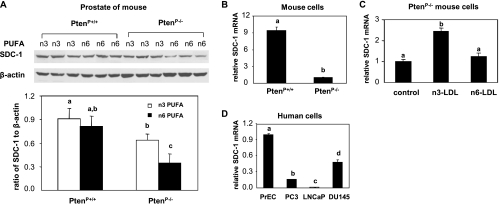
Our previous studies have identified SDC-1 expression in prostate tissue of C57BL/6J mouse and in human prostate cancer cells. SDC-1 gene expression was increased when mice were fed an n-3 PUFA-enriched diet or when cells were treated with n-3 LDL or BSA-bound DHA [35]. In the present study, Pten deletion (PtenP-/-) and wild-type (PtenP+/+) mice were used to further test the effect of n-3 PUFA on SDC-1 protein expression. The PtenP-/- genotype develop hyperplasia at 4 weeks and progress through carcinoma in situ to invasive adenocarcinoma by 16 weeks. Mice were fed an n-3 PUFA- and n-6 PUFA-enriched diet and killed at 8 weeks. Prostate lobes were dissected and homogenized, and lysates were prepared for measurement of SDC-1 protein by Western blot analysis. SDC-1 level was lower in PtenP-/- compared with PtenP+/+ mice (Figure 2A). Whereas the SDC-1 levels were similar in PtenP+/+ mice fed n-3 PUFA or n-6 PUFA diets, SDC-1 protein was significantly elevated in PtenP-/- mice fed an n-3 PUFA diet compared with those fed an n-6 PUFA diet. Cultured cells were derived from the prostate tissue of the two mouse genotypes. Consistent with data from the mouse tissues, expression of SDC-1 was markedly lower in PtenP-/- compared with PtenP+/+ cells (Figure 2B), and expression of SDC-1 in PtenP-/- cells was increased by n-3 PUFA-enriched LDL (Figure 2C). In addition, we demonstrated that endogenous SDC-1 expression was lower in three human prostate cancer cell lines compared with nontumorigenic prostate epithelial cells (Figure 2D). It is of interest to note that, as in the mouse PtenP-/- cells, SDC-1 expression was lower in the two human Pten-negative cell lines, PC3 and LNCaP, than in Pten-positive DU145 and PrEC cells (Figure 2D).
Figure 2.
SDC-1 up-regulation by n-3 PUFA in prostates of PtenP-/- mice/cells and in human prostate cancer cells. (A) Protein extracts from the prostates of 8-week-old PtenP+/+ and PtenP-/- mice fed n-3 PUFA or n-6 PUFA diets were dialyzed and treated with heparinase III plus chondroitin ABC lyase for 18 hours at 37°C. SDC-1 was measured by Western blot assay and normalized to β-actin. Values representing the mean and SD (n = 3) for SDC-1/β-actin ratios with different letters are significantly different (P < .05). (B) Cultured prostate cells from PtenP+/+ and PtenP-/- mice were harvested at approximately 70% confluence, and (C) PtenP-/- prostate cells were incubated with n-3 or n-6 PUFA-enriched LDL (100 µg/ml) for 8 hours. Total RNA was isolated, and SDC-1 mRNA was determined by real-time PCR. Values expressed relative to controls (n = 3) with different letters are significantly different (P < .05). (D) Human prostate cells (normal prostate epithelial cells [PrEC] and prostate cancer cells [PC3, LNCaP, and DU145]) were harvested at approximately 70% confluence. Total RNA was isolated, and SDC-1 mRNA was determined by real-time PCR. Values, expressed relative to controls (n = 3), with different letters are significantly different (P < .05).
Apoptosis Is Induced by Exogenous SDC-1 Ectodomain in Human Prostate Cancer Cells
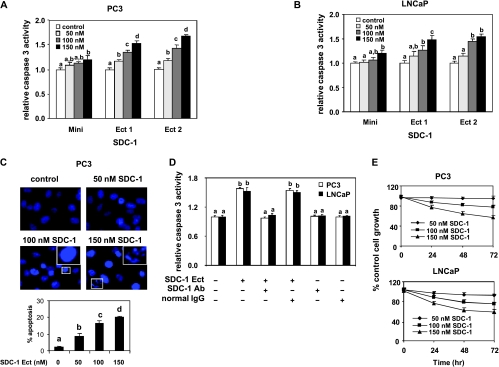
Given that human prostate cancer cells had lower expression of SDC-1 (Figure 2D), the consequence of providing the cells with exogenous SDC-1 was evaluated by measurement of apoptosis. PC3 and LNCaP cells were incubated for 48 hours with two similar preparations of SDC-1 ectodomain (Ect 1 and Ect 2) and a truncated ectodomain (Mini) of human recombinant SDC-1. Ect 1 and Ect 2 significantly increased caspase 3 activities in a dose-dependent manner in both cell lines, whereasMini had little ability to induce caspase 3 activation (Figure 3, A and B). Hoechst staining demonstrated that PC3 cells treated with SDC-1 ectodomain (Ect 2) exhibited typical apoptotic nuclear morphology characterized by chromatin condensation and DNA fragmentation (Figure 3C, upper). The quantitative analysis for apoptotic cells confirmed that the cellular apoptosis elicited by SDC-1 ectodomain was concentration-dependent, resulting in up to 20% apoptotic cells at 150 nM SDC-1 (Figure 3C, lower). Furthermore, the effect of SDC-1 ectodomains on apoptosis was blocked by an SDC-1 antibody but not by a control IgG (Figure 3D). Consistent with apoptosis induction, SDC-1 ectodomain (Ect 1) treatment led to a dose- and time-dependent decrease in cell number of PC3 and LNCaP cells (Figure 3E).
Figure 3.
SDC-1 ectodomain induces apoptosis in human prostate cancer cells. PC3 (A) and LNCaP (B) were treated with 50, 100, and 150 nM SDC-1 mini or ectodomain (Ect) preparations 1 or 2 for 48 hours. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values expressed relative to controls (n = 3) with different letters are significantly different (P < .05). (C) PC3 cells were treated with SDC-1 Ect (50, 100, and 150 nM) for 48 hours. Cell morphologic changes were examined by Hoechst 33342 staining and observed by fluorescence microscopy at a magnification of x40. Representative photographs were obtained from three separate experiments, in which apoptotic cells with condensed nuclei and fragmented chromatin are indicated by insets with high-magnification images of the areas in the white rectangles exposed to 100 and 150 nM SDC-1, respectively. The percentages of apoptotic cells are expressed in the graph as means ± SD of three individual experiments. Values with different letters are significantly different (P < .05). (D) PC3 and LNCaP cells were treated with SDC-1 Ect (150 nM) and SDC-1 antibody (SDC-1 Ab, 5 nM) or nonspecific lgG (5 nM) as a negative control for 48 hours. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values expressed relative to controls (n = 3) with different letters are significantly different (P < .05). (E) PC3 and LNCaP cells were treated with indicated concentrations of SDC-1 Ect. Cell growth was measured using MTS assay and compared with that of controls (untreated cells) at the same time point. Means ± SD (n = 3) are presented.
Apoptosis Induction by n-3 PUFA Is SDC-1-Dependent
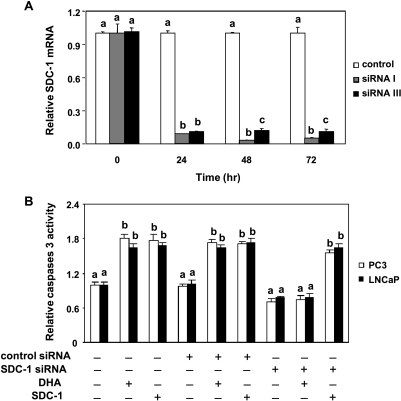
To test whether SDC-1 is a mediator in n-3 PUFA-induced apoptosis, two SDC-1 siRNA (SDC-1 siRNA I and SDC-1 siRNA III) were transfected into PC3 and LNCaP cells. Both effectively inhibited SDC-1 messenger RNA (mRNA) more than 90% for up to 72 hours after transfection in PC3 cells (Figure 4A). Similar data were obtained with LNCaP cells (not shown). On the basis of these effects, SDC-1 siRNA Iwas used for further experiments. Cells transfected for 24 hours with either control siRNA (no known target) or SDC-1 siRNA were exposed to 30 µM DHA for 48 hours, and caspase 3 activity was measured. As shown in Figure 4B, both DHA and SDC-1 ectodomain significantly increased caspase activity in nontransfected and control siRNA-transfected cells. However, SDC-1 siRNA effectively blocked the ability of DHA to increase caspase 3 activity in both PC3 and LNCaP cells. Caspase 3 induction by SDC-1 ectodomain was not blocked by SDC-1 siRNA in either cell line. These data imply that DHA induces apoptosis through an SDC-1-dependent mechanism.
Figure 4.
SDC-1 knockdown blocks DHA-induced apoptosis in human prostate cancer cells. (A) PC3 cells were transfected with a control siRNA or two SDC-1 siRNA (I and III). At the indicated times after transfection, total RNA was isolated, and SDC-1 mRNA was determined by real-time PCR. Both SDC-1 siRNA had similar effects on SDC-1 gene expression. Data shown are the levels of SDC-1 gene expression relative to control (n = 3). Values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05). (B) PC3 and LNCaP cells were transfected with control siRNA or SDC-1 siRNA (I) for 6 hours, then supplemented with growth medium containing 1% FBS for 24 hours. Cells were treated with DHA-BSA (30 µM) or SDC-1 ectodomain (100 nM) for 48 hours. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05).
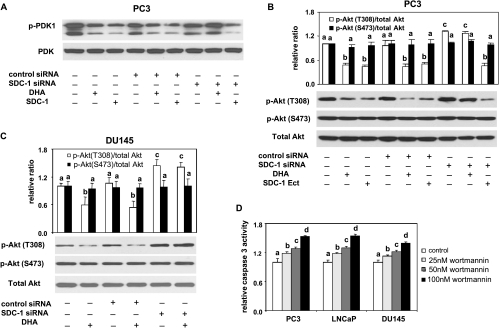
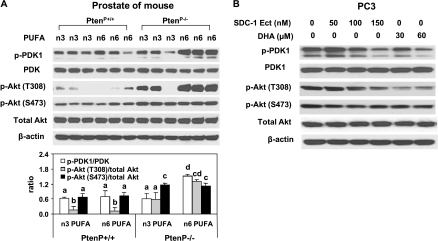
Suppression of PDK1/Akt Activation by n-3 PUFA Is Regulated by SDC-1
Akt is a critical survival kinase that controls programmed cell death. Full activation of Akt requires phosphorylation on two highly conserved residues, T308 and S473 [42]. T308 is phosphorylated by protein serine/threonine kinase PDK1, whereas S473 is primarily phosphorylated by the mammalian target of rapamycin [43,44]. Phosphorylation on T308 is essential for Akt kinase activity [45]. To investigate possible downstream signaling related to n-3 PUFA-induced apoptosis, Akt and PDK1 status were examined byWestern blot assay in prostates of PtenP+/+ and PtenP-/- mice fed an n-3 and n-6 PUFA-enriched diet. No difference in phosphorylation of either Akt or PDK1 (ratios of p-Akt [T308 or S473]/total Akt and p-PDK1/PDK1) was detected between PtenP+/+ mice fed an n-3 and an n-6 PUFA diet (Figure 5A). There was also no change in phosphorylation of Akt at S473 in prostates of PtenP-/- mice fed an n-3 PUFA diet (Figure 5A), which is consistent with our previous report [10]. However, we previously found an increase in apoptosis [10], and here we show a significant decrease in phosphorylation of Akt at T308 and reduced phosphorylation of PDK1 in prostates of PtenP-/- mice fed an n-3 PUFA-enriched diet (Figure 5A). Similar results were observed in PC3 cells in response to DHA and human SDC-1 ectodomain (Figure 5B). The effect of DHA on phosphorylation of Akt at T308 and phosphorylation of PDK1 was abrogated by SDC-1 siRNA (Figure 6, A and B). In addition, SDC-1 knockdown led to activation of Akt at T308 in either PC3 or DU145 cells (Figure 6, B and C). An Akt inhibitor, wortmannin, increased caspase 3 activity to a similar extent as DHA (Figure 6D). LY294002, another Akt inhibitor, also demonstrated a dose-dependent induction of apoptosis in all three cell lines (not shown). These data show that n-3 PUFA up-regulation of SDC-1 results in the suppression of phosphorylation of PDK1 and Akt followed by an induction of apoptosis.
Figure 6.
SDC-1 knockdown blocks the effect of DHA on phosphorylations of PDK1/Akt, and an Akt inhibitor, wortmannin, induces apoptosis. (A and B) PC3 cells were transfected with control siRNA or SDC-1 siRNA for 6 hours then supplemented with growth medium containing 1% FBS for 24 hours. Cells were treated with DHA (60 µM) or SDC-1 ectodomain (150 nM) for 48 hours before harvest. PDK1, p-PDK1 (S243) (A), total Akt, and p-Akt (T308 and S473) (B) were measured by Western blot assay. All data shown are representative of three independent experiments. Relative ratios and SEM are shown in graph B. Values with different letters are significantly different (P < .05). (C) DU145 cells were transfected with control siRNA or SDC-1 siRNA for 6 hours and then supplemented with growth medium containing 1% FBS for 24 hours. Cells were treated with DHA (60 µM) for 48 hours before harvest. Total Akt and p-Akt (T308 and S473) were measured by Western blot assay. All data shown are representative of three independent experiments. Relative ratios and SEM are shown. Values with different letters are significantly different (P < .05). (D) PC3, LNCaP, and DU145 cells were treated with an Akt inhibitor, wortmannin, at the indicated concentration for 48 hours. Caspase 3 activity was measured with the Caspase-Glo 3/7 assay. Values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05).
Figure 5.
SDC-1 and n-3 PUFA reduce PDK-1 and Akt phosphorylation in vivo and in vitro. (A) Protein extracts from the prostates of 8-week-old PtenP+/+ and PtenP-/- mice fed n-3 PUFA- and n-6 PUFA-enriched diets were used for Western blot analysis of p-PDK1(S243), PDK1, p-Akt (S473 and T308), total Akt, and β-actin. The mean ± SD for ratios of P-PDK1 to PDK1 and p-Akt (S473 and T308) to Akt are shown in the bottom graph. (B) PC3 cells were treated with SDC-1 ectodomain (50, 100, and 150 nM) and DHA-BSA (30 and 60 µM) for 48 hours. Protein extracts were used for Western blot analysis of p-PDK1, PDK1, p-Akt (S473 and T308), total Akt, and β-actin.
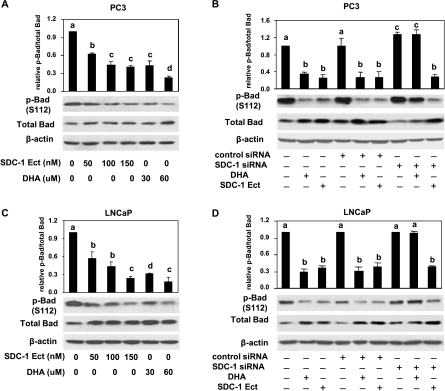
Phosphorylation of Bad Is Inhibited by DHA through SDC-1
Bad plays an important role in mediating the apoptotic signal in cells. Phosphorylation of Bad is necessary to protect prostate cancer cells from apoptosis [46]. Our previous studies showed lower levels of phosphorylated Bad in prostate tumors of mice fed an n-3 PUFA-enriched diet, and Bad knockdown in vitro eliminated the ability of n-3 PUFA to induce cell death [10]. In this study, the involvement of SDC-1 in the suppression of Bad phosphorylation by DHA was investigated in PC3 and LNCaP cells. As shown in Figure 7, A and C, human SDC-1 ectodomain and DHA decreased phosphorylation of Bad in a dose-dependent manner in both cell lines. In addition, when a SDC-1 siRNA was transfected into cells to silence SDC-1 expression, inhibition of Bad phosphorylation by DHA was blocked (Figure 7, B and D), thus suggesting a modulating role of SDC-1 in DHA inhibition of Bad phosphorylation.
Figure 7.
DHA inhibits Bad phosphorylation through SDC-1. PC3 (A) and LNCaP (C) cells were treated with DHA (30 and 60 µM) or SDC-1 ectodomain (50, 100, and 150 nM) for 48 hours. Total proteins were used to measure p-Bad (S112) and total Bad by Western blot assay. PC3 cells (B) and LNCaP cells (D) were transfected with control siRNA or SDC-1 siRNA for 6 hours and then supplemented with growth medium containing 1% FBS for 24 hours. Cells were treated with DHA (60 µM) or SDC-1 ectodomain (150 nM) for 48 hours before harvest. Total Bad and p-Bad (S112) were measured by Western blot assay. All data shown are representative of three independent experiments. Relative ratios and SEMare shown in graphs, and values representing the mean ± SD (n = 3) with different letters are significantly different (P < .05).
Discussion
Apoptosis represents an effective way to eliminate cancer cells. Unfortunately, advanced prostate tumors eventually progress to develop resistance to current apoptosis-mediated therapeutic approaches [47]. However, increasing evidence from epidemiological, animal, and in vitro studies indicates that n-3 PUFAs inhibit the promotion and progression of prostate cancer. In the present studies, we have demonstrated that n-3 PUFAs induce apoptosis and present the novel finding that apoptosis induction by the n-3 PUFA, DHA, is dependent on its ability to increase the expression of the proteoglycan, SDC-1. In addition, we have shown that the PDK1/Akt/Bad signaling pathway is affected by SDC-1 to result in apoptosis induction. This finding may pave the way for a new mechanism-based chemoprevention of n-3PUFA in prostate cancer.
SDC-1, a multifunctional proteoglycan, plays a key role in support of homeostasis of the epithelial compartment of normal prostate and nonmalignant hormone-responsive and well-differentiated prostate tumors [48]. Therefore, alteration in SDC-1 content or structure may disrupt homeostasis. Our studies show that basal SDC-1 expression was lower in human prostate cancer cells and mouse Pten knockout cells when compared with human normal prostate epithelial cells and mouse Pten wild-type cells, respectively. Moreover, similar to the mouse cells, SDC-1 expression was lower in the human Pten-null cell lines than in the Pten-positive ones. The n-3 PUFA, DHA, stimulated SDC-1 expression in both mouse and human Pten-null cells. Because recombinant SDC-1 ectodomain was effective in inducing apoptosis and concomitantly inhibiting the growth of the prostate cancer cells, restoration of SDC-1 by DHA could be an effective way to restore a lost homeostatic mechanism and thereby to impede the progression of prostate cancer.
Epidemiological studies suggest that increased intake of n-3 PUFA-rich fish or fish oil reduces prostate cancer progression, recurrence, and mortality [49–52]. Serum levels of n-3 PUFA were reported to be significantly lower in patients with benign prostate hyperplasia and prostate cancer, and n-6 PUFA levels were higher in patients with prostate cancer [5]. In prostate tissues, the percentage of total PUFA was shown to be significantly lower in the presence of perineural invasion, seminal vesicle involvement, and stage T3 tumor [53]. In addition, increased levels of n-6 PUFA and the ratio of n-6/n-3 PUFA were associated with an increased mean prostate-specific antigen level and risk of prostate cancer [54]. Recently, we have shown that n-3 PUFA-enriched diets inhibit tumor growth and increase survival in Pten knockout mice, which develop prostate cancer with 100% penetration, whereas an n-6 PUFA-enriched diet had the opposite effect [10]. Our present studies in vitro further show that at concentrations slightly lower than those found in fasted human plasma, n-3 PUFA-enriched LDL induced apoptosis and inhibited the growth of both androgen-dependent and androgen-independent human prostate cancer cells. This suggests that increasing the ambient n-3 PUFA in prostate tissue could retard progression to androgen-independent growth. Analysis of the role of the two major marine n-3 PUFA species EPA and DHA found that DHA but not EPA was effective in the induction of apoptosis. This is consistent with our earlier study showing that DHA but not EPA upregulated SDC-1 expression in human prostate cancer cells [35]. Furthermore, SDC-1 knockdown by specific SDC-1 siRNA blocked the stimulatory effect of DHA on apoptosis. These data strongly support a critical role of SDC-1 in DHA-induced apoptosis in prostate cancer cells.
SDC-1 as a heparan sulfate-containing cell surface molecule can simultaneously bind various components of the extracellular matrix and members of the heparin-binding growth factors. Therefore, SDC-1 may act as a coreceptor for growth factors, which could promote or restrict their interaction with growth factor receptors [55–57]. In addition, SDC-1 can be shed from the surface of cancer cells as an intact ectodomain, and this shed SDC-1 serves multiple functions including an induction of apoptosis and inhibition of cell growth in vitro [34,58]. In our study, we found that human normal prostate epithelial cells had higher SDC-1 gene expression than human prostate tumor cells, but comparing those tumor cells, the more aggressive cell line, DU145, had higher basal level of SDC-1 than LNCaP cells. Considering that SDC-1 up-regulation by DHA induces apoptosis, endogenously produced SDC-1 could be modulated by other factors or may need to be shed to exert its apoptotic activity. Different biologic functions of shed and membrane-bound SDC-1 have been reported in other systems [34]. Further experiments are needed to clarify the complexity of biologic consequences from endogenous and shed SDC-1 in the prostate cancer cells.
Akt is a mediator of growth factor-induced survival and has the capacity to drive tumor cell growth, proliferation, metabolism, and survival [59]. The most important upstream activators of Akt are the class I phosphoinositide 3-kinases (PI3K) [42]. The membrane-associated PI3K lipid products bind directly to the plekstrin homology domain of Akt, resulting in its recruitment to the membrane, exposure of its two main phosphorylation sites, and activation on T308 through PDK1, which also has a plekstrin homology domain directing it to membrane PI3K products, and on S473 through primarily mammalian target of rapamycin [45,60]. Our data here show that n-3 PUFA and DHA significantly inhibited phosphorylation of Akt at the T308 site, which was also reflected in a decrease in phosphorylation of PDK1. Exogenous SDC-1 mimicked the effect of DHA on Akt and PDK1 phosphorylation. Abrogation of SDC-1 expression by siRNA abolished the inhibitory effect of DHA. Further, Bad protein is a switch integrating the antiapoptotic effects of multiple pathways that suppress apoptosis in Pten-deficient tumor cells [61]. PI3K/Akt mediates phosphorylation of Bad to suppress cell death and promote cell survival [62]. In the present study, we show that exogenous SDC-1 ectodomain and DHA inhibited Bad phosphorylation in a dose-dependent manner in PC3 and LNCaP cells. SDC-1 knockdown by siRNA completely eliminated the effect of DHA but not of SDC-1 ectodomain. These results indicate that the suppression of phosphorylation of Bad may involve SDC-1 to promote the apoptotic effect of n-3 PUFA.
Taken together, these studies show that a novel mode of action by which n-3 PUFA induces apoptosis in prostate cancer cells is through up-regulating SDC-1 expression followed by concomitant suppressions of PDK1/Akt/Bad phosphorylation. These seminal observations provide insight into how n-3 PUFA may provide chemoprevention of prostate cancer.
Acknowledgments
The authors thank the support of Bioriginal (Saskatoon, Saskatchewan, Canada) and Croda, Inc (Edison, NJ), for providing the omega-3 fatty acid for the animal study.
Abbreviations
- BSA
bovine serum albumin
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FBS
fetal bovine serum
- LDL
low-density lipoprotein
- PARP
poly(ADP-ribose) polymerase
- PPARγ
peroxisome proliferator-activated receptor γ
- Pten
phosphatase and tensin homolog deleted on chromosome 10
- PUFA
polyunsaturated fatty acid
- SDC-1
syndecan-1
Footnotes
This work was supported by P01CA106742 (Y.Q.C., I.J.E., and J.O.) and in part by R01CA115958 (I.J.E.) and R01CA107668 (Y.Q.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977;20:680–688. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 4.Haenszel W, Kurihara M. Studies of Japanese migrants: I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 5.Yang YJ, Lee SH, Hong SJ, Chung BC. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem. 1999;32:405–409. doi: 10.1016/s0009-9120(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 6.Mamalakis G, Kafatos A, Kalogeropoulos N, Andrikopoulos N, Daskalopulos G, Kranidis A. Prostate cancer vs hyperplasia: relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukot Essent Fatty Acids. 2002;66:467–477. doi: 10.1054/plef.2002.0384. [DOI] [PubMed] [Google Scholar]
- 7.Freeman VL, Meydani M, Hur K, Flanigan RC. Inverse association between prostatic polyunsaturated fatty acid and risk of locally advanced prostate carcinoma. Cancer. 2004;101:2744–2754. doi: 10.1002/cncr.20676. [DOI] [PubMed] [Google Scholar]
- 8.Rose DP, Connolly JM. Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate. 1991;18:243–254. doi: 10.1002/pros.2990180306. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, Leung P, Hong-Gonzalez J, Freedland SJ, Said J, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662–4670. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 12.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebersbach BF, Sanderson RD. Expression of syndecan-1 inhibits cell invasion into type I collagen. J Biol Chem. 1994;269:20013–20019. [PubMed] [Google Scholar]
- 14.Liu W, Litwack ED, Stanley MJ, Langford JK, Lander AD, Sanderson RD. Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J Biol Chem. 1998;273:22825–22832. doi: 10.1074/jbc.273.35.22825. [DOI] [PubMed] [Google Scholar]
- 15.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 16.Inki P, Jalkanen M. The role of syndecan-1 in malignancies. Ann Med. 1996;28:63–67. doi: 10.3109/07853899608999076. [DOI] [PubMed] [Google Scholar]
- 17.Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- 18.Rapraeger AC. Molecular interactions of syndecans during development. Semin Cell Dev Biol. 2001;12:107–116. doi: 10.1006/scdb.2000.0239. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 20.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 21.Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, Gattegno L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16:488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto Y, Skacel M, Adams JC. Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: an immunohistochemical study of clinically annotated tumors. BMC Cancer. 2008;8:185. doi: 10.1186/1471-2407-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchymelike cells. Mol Biol Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loussouarn D, Campion L, Sagan C, Frenel JS, Dravet F, Classe JM, Pioud-Martigny R, Berton-Rigaud D, Bourbouloux E, Masnier JF, et al. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br J Cancer. 2008;98:1993–1998. doi: 10.1038/sj.bjc.6604400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008;283:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppa S, Mali M, Miettinen HM, Jalkanen M. Syndecan expression regulates cell morphology and growth of mouse mammary epithelial tumor cells. Proc Natl Acad Sci USA. 1992;89:932–936. doi: 10.1073/pnas.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras HR, Ledezma RA, Vergara J, Cifuentes F, Barra C, Cabello P, Gallegos I, Morales B, Huidobro C, Castellon E. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial-mesenchymal transition markers, E-cadherin and β-catenin, in prostate cancer. Urol Oncol. doi: 10.1016/j.urolonc.2009.03.018. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Kiviniemi J, Kallajoki M, Kujala I, Matikainen MT, Alanen K, Jalkanen M, Salmivirta M. Altered expression of syndecan-1 in prostate cancer. APMIS. 2004;112:89–97. doi: 10.1111/j.1600-0463.2004.apm1120202.x. [DOI] [PubMed] [Google Scholar]
- 29.Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, Slade RJ, Martin-Hirsch P, Gallagher JT, Jayson GC. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res. 2004;10:5178–5186. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- 30.Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, Veronese S, Caffo O, Lucenti A, Palma D, et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98:474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 31.Conejo JR, Kleeff J, Koliopanos A, Matsuda K, Zhu ZW, Goecke H, Bicheng N, Zimmermann A, Korc M, Friess H, et al. Syndecan-1 expression is upregulated in pancreatic but not in other gastrointestinal cancers. Int J Cancer. 2000;88:12–20. doi: 10.1002/1097-0215(20001001)88:1<12::aid-ijc3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Choi DS, Kim JH, Ryu HS, Kim HC, Han JH, Lee JS, Min CK. Syndecan-1, a key regulator of cell viability in endometrial cancer. Int J Cancer. 2007;121:741–750. doi: 10.1002/ijc.22713. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe A, Mabuchi T, Satoh E, Furuya K, Zhang L, Maeda S, Naganuma H. Expression of syndecans, a heparan sulfate proteoglycan, in malignant gliomas: participation of nuclear factor-κB in upregulation of syndecan-1 expression. J Neurooncol. 2006;77:25–32. doi: 10.1007/s11060-005-9010-3. [DOI] [PubMed] [Google Scholar]
- 34.Nikolova V, Koo CY, Ibrahim SA, Wang Z, Spillmann D, Dreier R, Kelsch R, Fischgrabe J, Smollich M, Rossi LH, et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis. 2009;30:397–407. doi: 10.1093/carcin/bgp001. [DOI] [PubMed] [Google Scholar]
- 35.Edwards IJ, Sun H, Hu Y, Berquin IM, O'Flaherty JT, Cline JM, Rudel LL, Chen YQ. In vivo and in vitro regulation of syndecan 1 in prostate cells by n-3 polyunsaturated fatty acids. J Biol Chem. 2008;283:18441–18449. doi: 10.1074/jbc.M802107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards IJ, Berquin IM, Sun H, O'Flaherty JT, Daniel LW, Thomas MJ, Rudel LL, Wykle RL, Chen YQ. Differential effects of delivery of omega-3 fatty acids to human cancer cells by low-density lipoproteins versus albumin. Clin Cancer Res. 2004;10:8275–8283. doi: 10.1158/1078-0432.CCR-04-1357. [DOI] [PubMed] [Google Scholar]
- 37.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of themurine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 39.Sun H, Berquin IM, Owens RT, O'Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y, Sun H, Owens RT, Wu J, Chen YQ, Berquin IM, Perry D, O'Flaherty JT, Edwards IJ. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia. 2009;11:1042–1053. doi: 10.1593/neo.09760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun H, Berquin IM, Edwards IJ. omega-3 polyunsaturated fatty acids regulate syndecan-1 expression in human breast cancer cells. Cancer Res. 2005;65:4442–4447. doi: 10.1158/0008-5472.CAN-04-4200. [DOI] [PubMed] [Google Scholar]
- 42.Dibble CC, Manning BD. A molecular link between AKTregulation and chemotherapeutic response. Cancer Cell. 2009;16:178–180. doi: 10.1016/j.ccr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 46.Smith AJ, Karpova Y, D'Agostino R, Jr, Willingham M, Kulik G. Expression of the Bcl-2 protein BAD promotes prostate cancer growth. PLoS One. 2009;4:e6224. doi: 10.1371/journal.pone.0006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Kan M, Wang F, Jin C, Yu C, McKeehan WL. A rare premalignant prostate tumor epithelial cell syndecan-1 forms a fibroblast growth factor-binding complex with progression-promoting ectopic fibroblast growth factor receptor 1. Cancer Res. 2001;61:5295–5302. [PubMed] [Google Scholar]
- 49.Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008;88:1297–1303. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 51.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–1242. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–1766. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 53.Freeman VL, Meydani M, Yong S, Pyle J, Flanigan RC, Waters WB, Wojcik EM. Prostatic levels of fatty acids and the histopathology of localized prostate cancer. J Urol. 2000;164:2168–2172. [PubMed] [Google Scholar]
- 54.Ritch CR, Brendler CB, Wan RL, Pickett KE, Sokoloff MH. Relationship of erythrocyte membrane polyunsaturated fatty acids and prostate-specific antigen levels in Jamaican men. BJU Int. 2004;93:1211–1215. doi: 10.1111/j.1464-410X.2004.04841.x. [DOI] [PubMed] [Google Scholar]
- 55.Mali M, Elenius K, Miettinen HM, Jalkanen M. Inhibition of basic fibroblast growth factor-induced growth promotion by overexpression of syndecan-1. J Biol Chem. 1993;268:24215–24222. [PubMed] [Google Scholar]
- 56.Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 57.Deepa SS, Yamada S, Zako M, Goldberger O, Sugahara K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J Biol Chem. 2004;279:37368–37376. doi: 10.1074/jbc.M403031200. [DOI] [PubMed] [Google Scholar]
- 58.Dhodapkar MV, Abe E, Theus A, Lacy M, Langford JK, Barlogie B, Sanderson RD. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91:2679–2688. [PubMed] [Google Scholar]
- 59.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 60.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 61.She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]