Abstract
Objectives
To comprehensively examine cardiovascular reserve function with exercise in patients with heart failure and preserved ejection fraction (HFpEF).
Background
Optimal exercise performance requires an integrated physiologic response, with coordinated increases in heart rate, contractility, lusitropy, arterial vasodilatation, endothelial function and venous return. Cardiac and vascular responses are coupled, and abnormalities in several components may interact to promote exertional intolerance in HFpEF.
Methods
Subjects with HFpEF (n=21), hypertension without heart failure (n=19) and no cardiovascular disease (control, n=10) were studied before and during exercise with characterization of cardiovascular reserve function by Doppler echocardiography, peripheral arterial tonometry and gas exchange.
Results
Exercise capacity and tolerance were reduced in HFpEF compared with hypertensives and controls, with lower VO2 and cardiac index at peak, and more severe dyspnea and fatigue at matched low-level workloads. Endothelial function was impaired in HFpEF and in hypertensives as compared with controls. However, blunted exercise-induced increases in chronotropy, contractility and vasodilation were unique to HFpEF and resulted in impaired dynamic ventricular-arterial coupling responses during exercise. Exercise capacity and symptoms of exertional intolerance were correlated with abnormalities in each component of cardiovascular reserve function, and HFpEF subjects were more likely to display multiple abnormalities in reserve.
Conclusion
HFpEF is characterized by depressed reserve capacity involving multiple domains of cardiovascular function, which contribute in an integrated fashion to produce exercise limitation. Appreciation of the global nature of reserve dysfunction in HFpEF will better inform optimal design for future diagnostic and therapeutic strategies.
Keywords: Heart Failure, Contractility, Endothelial Function, Exercise, Hypertension, Vasodilation
INTRODUCTION
Exercise intolerance is a defining symptom in patients with heart failure (HF) and preserved ejection fraction (HFpEF), yet its mechanisms remain poorly understood(1). Reductionist strategies to studying human disease are predicated on the concept that a single unifying process causes a specific disease phenotype. However, HFpEF is principally a disease of the elderly(2), and in geriatric medicine, it is more likely that multiple processes and age-related comorbidities coexist in the same patient(3). These processes interact synergistically to produce a clinical phenotype. Because exercise requires coordinated changes in ventricular function, arterial tone, endothelial function, venous return and autonomic signaling, it would be expected that abnormalities in many such components exist and interact to promote subjective and objective exercise limitation in HFpEF(4,5).
Accordingly, the present study sought to examine multiple components of exercise reserve responses in patients with HFpEF, including assessment of chronotropic, preload, contractile, endothelial and global vascular reserve functions and importantly, ventricular-arterial coupling reserve responses. Because population-based studies have shown that patients with HFpEF are typically older, hypertensive and female(2), and because each of these features may independently affect cardiovascular function, we compared reserve responses in HFpEF to a predominantly female, elderly hypertensive control group without HF, in addition to an apparently healthy control group free of cardiovascular disease.
METHODS
Study Population
Subjects with HFpEF (n=21) confirmed by Framingham criteria(5) and EF>50% were studied in an outpatient, compensated state. Exclusion criteria included valvular or pericardial disease; infiltrative or hypertrophic cardiomyopathy; cor pulmonale; pulmonary disease; unstable coronary disease; atrial fibrillation; pregnancy; primary renal or hepatic disease; and inability to exercise or to suspend cardiovascular medicines. Hypertensive control subjects without HF (n=19, defined by history of blood pressure>140/90mmHg and treatment with ≥1 antihypertensive medication) were identified from medical chart review and contacted for participation. Healthy controls without cardiovascular disease or diabetes (n=10) were recruited by advertisement. Because population-based studies have shown that HFpEF patients are predominantly older-aged and female(2), we sought to enroll controls with similar demographics during screening. The study was approved by the Mayo Institutional Review Board. The authors had access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Study Design
Cardiovascular medicines were withheld for 24 hours prior to study. Subjects were studied in a compensated, fasting state in a quiet, temperature-controlled room (21°C). Transthoracic echo-Doppler/tissue Doppler study acquired at rest and during the final 1.5 minutes of each 3 minute graded exercise stage (GE Vivid 7, GE Healthcare, Chalfont St. Giles, United Kingdom). Endothelial function was measured using peripheral arterial tonometry (PAT). All data were interpreted off-line in a blinded fashion. HF symptoms were assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). B-type natriuretic peptide (BNP) levels were assessed by enzymatic immunoassay (Beckman Instruments, Chaska, MN). Glomerular filtration rate was estimated by the modified Cockroft-Gault formula. Daily dose of β-blocker was expressed as units of metoprolol (total daily milligrams of metoprolol=atenololx2=carvedilolx4)(5). Brachial blood pressure (BP) was obtained by auscultation by a single investigator during rest and each stage of exercise. Mean (MBP; diastolic pressure + pulse pressure/3) and end-systolic (ESP; 0.9*systolic BP) BP were calculated as previously described(6).
Exercise Metabolic Performance
Subjects underwent maximal-effort upright cycle exercise testing starting at 20 Watt (20W) workload, increasing by 20W every 3 minutes until exhaustion. Oxygen consumed (VO2), carbon dioxide produced (VCO2), minute ventilation (VE), and respiratory exchange ratio (RER =VCO2/VO2) were measured (MedGraphics, St. Paul, MN) throughout exercise to quantify exercise performance(5) Subjective symptoms of fatigue and dyspnea were recorded at each workload by the Borg effort (6–20) and dyspnea scores (0–10), where higher values indicate more severe symptoms(7).
Cardiovascular Function and Reserve Analysis
Echo-Doppler measurements represent the mean of ≥3 beats. LV mass was obtained from 2-D measurements of wall thickness and chamber dimension(8). EF was determined from Simpson’s biplane method(8). Stroke volume index (SV) was determined from the LV outflow dimension and pulse-wave Doppler and was indexed to body surface area (SVI). Cardiac index (CI) was determined from the product of HR and SVI.
Chronotropic reserve
Heart rate reserve (HRR) was determined from continuous 12-lead electrocardiogram using standard formulas, with chronotropic incompetence is defined as HRR<0.8, or HRR<0.62 in subjects on beta-adrenergic antagonists(9).
Preload and preload reserve
LV end diastolic volume index (LVEDVI) was determined from the quotient of SVI/EF(8,10). Resting transmitral flow velocities (E and A) and mitral annular tissue-Doppler velocities (E’ and A’) were measured to assess diastolic function. The E/E’ ratio was used to estimate filling pressures at rest(8). Doppler estimation of filling pressures with exercise was not performed.
Contractile function and reserve
Load-independent contractility was determined using 3 separate indices: (1) peak power index (PWRI; determined from product of peak volumetric ejection rate from LV outflow Doppler and SBP, divided by EDV)(10,11), single-beat end-systolic elastance (Ees; determined from BP, SV, EF and pre-ejection and systolic ejection time intervals from LV outflow Doppler)(12), and single-beat preload recruitable stroke work (PRSW; determined from SV, MBP, LV mass and EDV)(13). The change in each parameter was used to characterize contractile reserve.
Vascular function and reserve
Ventricular afterload was measured by systemic vascular resistance (MBP*80/CO) and effective arterial elastance (Ea=ESP/SV)(6,8) at rest, with the change in each during exercise used to characterize global arterial reserve.
Endothelial function
Peripheral arterial tonometry (PAT) was measured using the EndoPAT 2000 system (Itamar-Medical, Caesarea, Israel). Endothelial function was quantified by the reactive hyperemic (RH) change in digital blood flow after arm occlusion(14,15). After 5 minutes baseline recording, a BP cuff was inflated to supra-systolic pressure in the test arm. After 5 minutes of occlusion, the cuff was rapidly deflated, with PAT tracings recorded. RH-PAT response was determined as the ratio of PAT amplitude in the test arm to control arm, averaged in 30 second intervals post cuff deflation, divided by the average PAT ratio measured for the 140 second interval before cuff inflation. The reactive hyperemia index (RHI) was determined as the RH-PAT ratio measured between 60 and 120 seconds post occlusion. Endothelial dysfunction was defined categorically by RHI<2.0. RHI was log-transformed for subsequent analysis(14).
Dynamic peripheral vasodilation was further assessed by changes in PAT amplitude responses during exercise(16). Mean PAT amplitudes were determined from 3 minute recordings obtained at rest and at peak exercise after manually deleting motion artifacts. Exercise PAT responses were normalized to baseline PAT amplitude to create a dimensionless unit, and represent the average of both arms.
Ventricular-vascular coupling and coupling reserve
Ventricular-arterial interaction was assessed by the coupling ratio (Ea/Ees) of arterial to ventricular systolic elastance(6).
Statistical Analysis
Continuous variables are reported as mean±SD. Between-group differences were compared by χ2, 1-way ANOVA or Wilcoxon Rank Sum/Kruskal-Walllis tests. Normality was evaluated by the Shapiro-Wilk W Test. Bonferroni correction was applied for multiple comparisons. The hyperemic changes in PAT amplitude between groups were compared by repeated measures ANOVA assuming a quadratic relationship of PAT ratio over time. Linear regression was performed to test associations between reserve function, symptoms and exercise performance.
RESULTS
Subject Characteristics
Age, sex, race (all but 2 Caucasian) and renal function were similar in all groups, with controls and HFpEF being more obese than hypertensives (Table 1). Coronary disease and diabetes were more common in HFpEF. BNP levels were higher and KCCQ scores lower (more symptomatic) in HFpEF. HFpEF subjects were more likely to be on loop diuretics and lipid lowering therapy. Other medication use was similar in HFpEF and hypertensives, including β-blockers and mean dose of β-blockers (not shown).
Table 1.
Clinical Characteristics and Resting Cardiovascular Function
| Control (n=10) |
Hypertension (n=19) |
HFpEF (n=21) |
p | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age (years) | 62±7 | 65±11 | 67±11 | 0.4 |
| Gender (% Female) | 70 | 74 | 76 | 0.9 |
| Body Mass Index (kg/m2) | 31.2±7.9 | 28.3±3.0 | 34.3±6.6† | 0.004 |
| KCCQ score | 99±4 | 94±16 | 69±18*† | <0.001 |
| Hypertension (%) | 0 | 100* | 86* | <0.001 |
| Coronary Artery Disease (%) | 0 | 11 | 33* | 0.02 |
| Diabetes (%) | 0 | 5 | 43*† | 0.003 |
| Smoking (%) | 0 | 0 | 9 | 0.2 |
| GFR (ml/min) | 87±17 | 81±20 | 81±38 | 0.9 |
| Plasma BNP (pg/ml) | 38±40 | 60±50 | 152±106*† | 0.001 |
| Hemoglobin (gm/dl) | 13.0±2.2 | 14.2±1.5 | 13.0±1.3 | 0.06 |
| β-Blockers (%) | 0 | 42* | 57* | <0.001 |
| ACEI or ARB (%) | 0 | 53* | 67* | <0.001 |
| Loop Diuretic (%) | 0 | 0 | 57*† | <0.001 |
| Lipid Lowering (%) | 40 | 63 | 90* | 0.009 |
| LV Mass Index (mg/m2) | 68.2±19.8 | 90.7±21.8 | 88.0±27.1 | <0.05 |
| Resting Function | ||||
| Heart Rate (bpm) | 70±8 | 71±12 | 68±12 | 0.9 |
| Preload | ||||
| LVEDVI (ml/m2) | 54±8 | 59±12 | 58±19 | 0.6 |
| E/E’ ratio | 12±4 | 12±5 | 20±7*† | 0.003 |
| Contractility | ||||
| PWRI (mm Hg/sec) | 330±80 | 348±59 | 339±69 | 0.8 |
| PRSW (gm/cm2) | 79±19 | 77±19 | 81±40 | 0.9 |
| Ees (mm Hg/ml) | 1.48±0.38 | 1.72±0.38 | 1.79±0.76 | 0.4 |
| Vascular Function | ||||
| Systolic BP (mmHg) | 123±16 | 136±12 | 131±21 | 0.2 |
| Ea (mm Hg/ml) | 1.88±0.40 | 1.97±0.51 | 1.77±0.62 | 0.3 |
| SVRI (dyne*m2/sec*cm-5) | 3430±920 | 3430±750 | 3100±880 | 0.4 |
| Log RHI | 1.33±0.34 | 0.92±0.38* | 0.85±0.42* | 0.009 |
| Endothelial Dysfunction (%) | 0 | 28 | 42* | 0.016 |
| Ventricular arterial coupling | ||||
| Coupling Ratio (Ea/Ees) | 1.32±0.34 | 1.16±0.24 | 1.08±0.35 | 0.2 |
| Ejection Fraction (%) | 58±7 | 58±5 | 60±6 | 0.5 |
| Cardiac Index (L/min*m2) | 2.2±0.5 | 2.4±0.6 | 2.3±0.6 | 0.7 |
Final column reflects overall group ANOVA or χ2.
For between-group comparisons:
p<0.05 vs CON;
p<0.05 vs HTN (ANOVA after Bonferroni)
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionaire; GFR, glomerular filtration rate; BNP, B-type natriuretic peptide; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; LVEDVI, left ventricular end diastolic volume index; PWRI, peak LV power index; PRSW, LV preload recruitable stroke work; Ees, LV end systolic elastance; BP, blood pressure; Ea, arterial elastance; SVRI, systemic vascular resistance index
Resting Cardiovascular Function
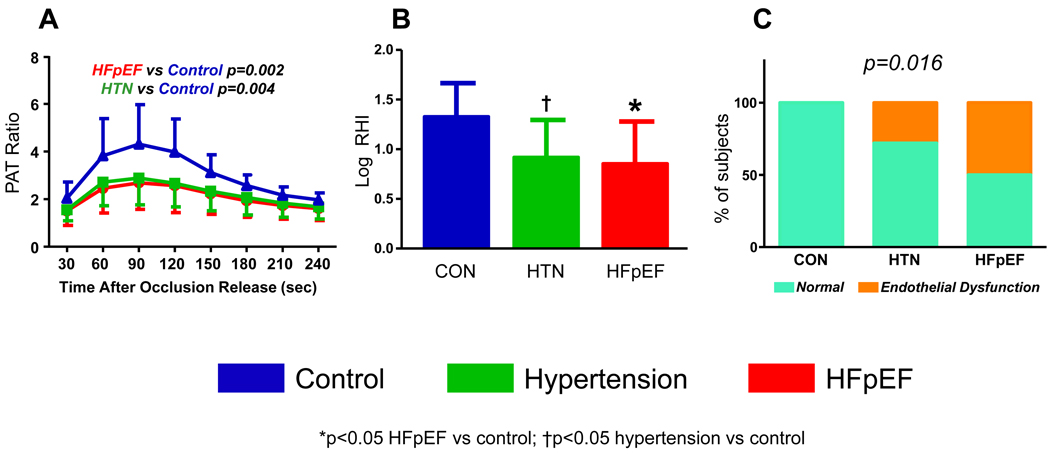
HR, BP, LVEDVI, contractility, ventricular-arterial coupling and cardiac index were similar across groups at rest (Table 1). E/E’ was higher in HFpEF, consistent with diastolic dysfunction. Global vascular function (Ea and SVRI) was not different between groups. However, the hyperemic increase in PAT amplitude after cuff occlusion was blunted in HFpEF and hypertensives compared with controls, consistent with depressed endothelium-dependent vasodilation (Figure 1). Mean RHI were lower in HFpEF and hypertensives compared to controls, but similar in HFpEF and hypertensives (Table 1). The prevalence of endothelial dysfunction was 42% in HFpEF (p<0.05 vs control, p=NS vs hypertension), 28% in hypertensives (p=0.056 vs control) and 0% in controls.
Figure 1. Assessment of Endothelial Function.
[A] Increases in peripheral arterial tonometry (PAT) amplitude with reactive hyperemia are diminished in HFpEF and hypertensives compared with controls, consistent with endothelial dysfunction. [B] Mean reactive hyperemia index (log RHI) is reduced in HFpEF and hypertensives compared with control. [C] Compared with controls, endothelial dysfunction was more prevalent in HFpEF (42% of subjects; p<0.05) and tended to be more common in hypertensives (28% of subjects, p=0.056).
Exercise Performance
Exercise time, peak workload, VO2 at ventilatory threshold, peak VO2 and percent predicted peak VO2 were all impaired in HFpEF compared with controls and hypertensives, while the latter groups were similar (Table 2). Borg effort and dyspnea scores in HFpEF were higher at matched submaximal workload (20W), indicating greater perceived difficulty with exercise. At peak, Borg scores were similar in HFpEF, hypertensives and controls, consistent with maximal subjective effort in all groups. Peak RER tended to be lower in HFpEF, though excluding the subjects who failed to attain a peak RER>1.0 did not affect the differences observed in any parameters (not shown).
Table 2.
Exercise Performance
| Control (n=10) |
Hypertension (n=19) |
HFpEF (n=21) |
p | |
|---|---|---|---|---|
| Exercise Time (seconds) | 831±230 | 801±314 | 497±214*† | 0.0005 |
| Peak Workload (watts) | 96±25 | 91±27 | 55±23*† | <0.0001 |
| Respiratory Exchange Ratio | 1.09±0.07 | 1.09±0.08 | 1.02±0.09 | 0.02 |
| VO2 at VAT (cc/kg/min) | 14.6±2.7 | 13.8±2.6 | 10.4±2.3*† | <0.0001 |
| Peak VO2 (cc/kg/min) | 18.6±3.3 | 18.1±3.5 | 12.7±3.1*† | <0.0001 |
| % Predicted Peak VO2 (%) | 87±22 | 93±24 | 57±18*† | <0.0001 |
| VE/VCO2 slope | 34.0±2.9 | 34.1±4.0 | 35.6±5.0 | 0.7 |
| 20W Borg Effort (6–20) | 8.6±1.6 | 9.2±1.7 | 11.1±2.0*† | 0.003 |
| 20W Borg Dyspnea (0–10) | 0.9±0.7 | 1.0±0.9 | 2.6±1.6*† | 0.0009 |
| Peak Borg Effort (6–20) | 16.4±1.6 | 16.1±1.8 | 15.7±2.2 | 0.7 |
| Peak Borg Dyspnea (0–10) | 5.4±2.2 | 5.1±1.8 | 4.5±2.0 | 0.9 |
Final column reflects overall group ANOVA or χ2.
For between-group comparisons:
p<0.05 vs CON;
p<0.05 vs HTN (ANOVA after Bonferroni)
Abbreviations: VO2, oxygen consumption; VAT, ventilatory anaerobic threshold; VE, minute ventilation; VCO2, carbon dioxide production; W, watts
Reserve Responses at Matched low-level (20 Watts) exercise
Chronotropic reserve
Heart rate increased in HFpEF (+23±6 bpm, p<0.0001), hypertensives (+23±10, p<0.0001), and controls (+26±8, p<0.0001), with no between-group difference (p>0.2).
Preload reserve
LVEDVI increased in HFpEF (+6±9 ml/m2), hypertensives (+5±7 ml/m2), and controls (+11±9 ml/m2) (p<0.0001 for all), with no between-group difference (p>0.2).
Contractile reserve
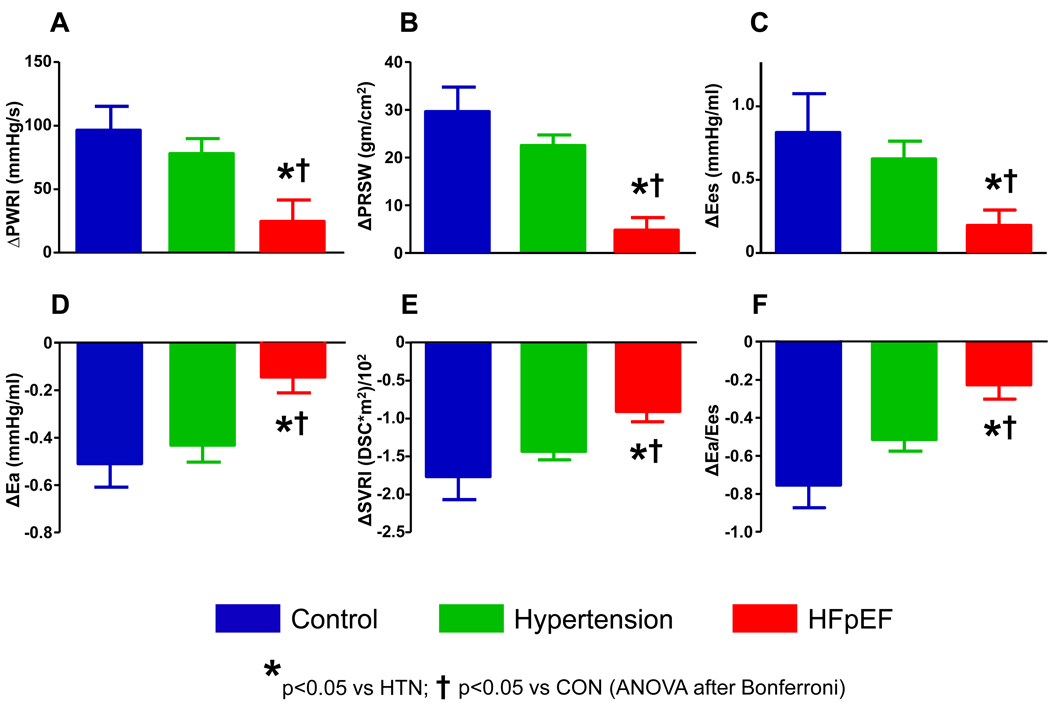
The increase in contractility assessed by Ees, PRSW, and PWRI was 65–85% lower in HFpEF compared with hypertensive and normal controls (Figure 2). LVESVI failed to drop in HFpEF (+2±7 ml/m2) in comparison to hypertensive and healthy controls (−6±5 and −5±5 ml/m2, respectively, both p<0.05 compared to HFpEF).
Figure 2. Contractile, Vascular and Coupling Reserve with low-level exercise (20W).
[A-C] Compared with both controls (blue) and hypertensives (green), contractile reserve was blunted in HFpEF (red) at 20W, evidenced by blunted increases end-systolic elastance (Ees), preload recruitable stroke work (PRSW), and peak power index (PWRI). [D,E] Vasodilation (reduction in arterial elastance, Ea; and systemic vascular resistance index, SVRI) was also impaired in HFpEF. [F] These deficits led to abnormal ventricular-arterial coupling responses (i.e. less reduction in Ea/Ees ratio) in HFpEF compared with controls and hypertensives.
Vascular function and reserve
Vasodilation was attenuated in HFpEF, with less reduction in SVRI and Ea compared with hypertensive and normal controls (Figure 2).
Ventricular vascular coupling reserve
The combination of blunted increases in contractility and impaired vasodilation in HFpEF patients was associated with impaired ventricular-arterial coupling, with less reduction in the Ea/Ees ratio (Figure 2) and less increase EF (+0±8% in HFpEF vs +14±7% in controls and +13±6% in hypertensives, p<0.0001). Augmentation in cardiac index at 20W was lower in HFpEF (+1.1±0.4 L/min*m2) than controls (+2.2±0.9 L/min*m2, p<0.001) and hypertensives (vs +1.8±0.7 L/min*m2, p=0.002).
Reserve Responses at Peak Exercise
Chronotropic reserve
Peak heart rate was reduced in HFpEF compared to both controls and hypertensives (Table 3). HRR was lower in HFpEF (56±17%) compared with hypertensives (79±20%, p<0.001) and controls (93±17%, p<0.0001), even after adjusting for chronic beta-blocker use. Among HFpEF subjects with peak RER>1.0, the prevalence of chronotropic incompetence was 57%.
Table 3.
Cardiovascular Reserve Responses at Peak Exercise
| Control (n=10) |
Hypertension (n=19) |
HFpEF (n=21) |
p | |
|---|---|---|---|---|
| Chronotropic Reserve | ||||
| Δ Heart Rate | +82±21 | +65±15 | +47±17*† | <0.0001 |
| Preload Reserve | ||||
| Δ LV EDVI (ml/m2) | +13±15 | +7±10 | +5±9 | 0.2 |
| Contractile Reserve | ||||
| Δ PWRI (mmHg/sec) | +471±179 | +391±119 | +139±103*† | <0.0001 |
| Δ PRSW (gm/cm2) | +112±53 | +93±54 | +27±23*† | <0.0001 |
| Δ Ees (mmHg/ml) | +2.87±1.52 | +2.18±0.94 | +0.88±0.82*† | 0.0002 |
| Δ LV ESVI (ml/m2) | −5±7 | −7±6 | +1±9† | 0.004 |
| Vascular Reserve | ||||
| Δ Ea (mmHg/ml) | +0.19±0.62 | −0.07±0.42 | +0.22±0.37 | 0.16 |
| Δ SVRI (dyne*m2/s*cm−5) | −2070±730 | −1890±610 | −1110±530*† | 0.0006 |
| Δ Digital PAT Amplitude | +2.52±0.99 | +2.33±1.38 | +1.46±0.77* | 0.027 |
| Coupling Reserve | ||||
| Δ Coupling Ratio (Ea/Ees) | −0.93±0.22 | −0.67±0.24 | −0.21±0.39*† | <0.0001 |
| Δ Ejection Fraction (%) | +16±8 | +16±7 | +3±7 | <0.0001 |
| Δ CI (L/min*m2) | +5.1±2.2 | +4.1±1.1 | +2.2±1.1*† | <0.0001 |
Final column reflects overall group ANOVA or χ2.
For between-group comparisons:
p<0.05 vs CON;
p<0.05 vs HTN (ANOVA after Bonferroni)
Abbreviations: Δ, peak change; EDVI, end diastolic volume index; PWRI, peak power index; PRSW, preload recruitable stroke work; Ees, end systolic elastance; Ea, arterial elastance; SVRI, systemic vascular resistance index; PAT, peripheral arterial tonometry; CI, cardiac index
Preload reserve
EDVI tended to increase more in controls but this was not significant (p=0.2).
Contractile reserve
Increases in contractility at peak exercise were ~65% lower in HFpEF compared to hypertensives and controls for each load-independent measure (p<0.001). Peak exercise reduction in ESVI was impaired in HFpEF.
Vascular reserve
Exercise reduction in SVRI and augmentation in peripheral blood flow (PAT amplitude) were both blunted in HFpEF compared to hypertensives and controls although the changes in Ea were similar across groups at peak.
Ventricular vascular coupling reserve
Contractile and vascular reserve impairments produced abnormal dynamic ventricular-arterial coupling responses at peak exercise in HFpEF, with less reduction in the Ea/Ees ratio and less increase in EF and cardiac index. Reflecting the potent differences in contractile reserve function, systolic BP increased less in HFpEF (34±25 mmHg) than in hypertensive (56±23 mmHg, p<0.05) or healthy (76±28 mmHg, p<0.05) controls.
Impact of Coronary Disease
No subject displayed ischemic ECG or wall motion changes during exercise. After adjusting for history of coronary disease, all differences in endothelial function and ventricular-vascular reserve remained significant (not shown). Subgroup analysis restricted to only subjects without history of coronary disease showed similar impairments in low-level and peak contractile reserve in HFpEF, with the exception of the increase in Ees at 20W which was no longer significant (not shown). Among subjects without coronary disease, the prevalence of endothelial dysfunction was 0% in controls, 31% in HFpEF and 31% in hypertensives (each p=0.02 compared with control). In this subgroup, log RHI tended to be lower in HFpEF compared with control (0.96±0.42 vs 1.33±0.34), though this difference was no longer significant (p=0.09 after Bonferroni).
Global Reserve Dysfunction and Exercise Intolerance in HFpEF
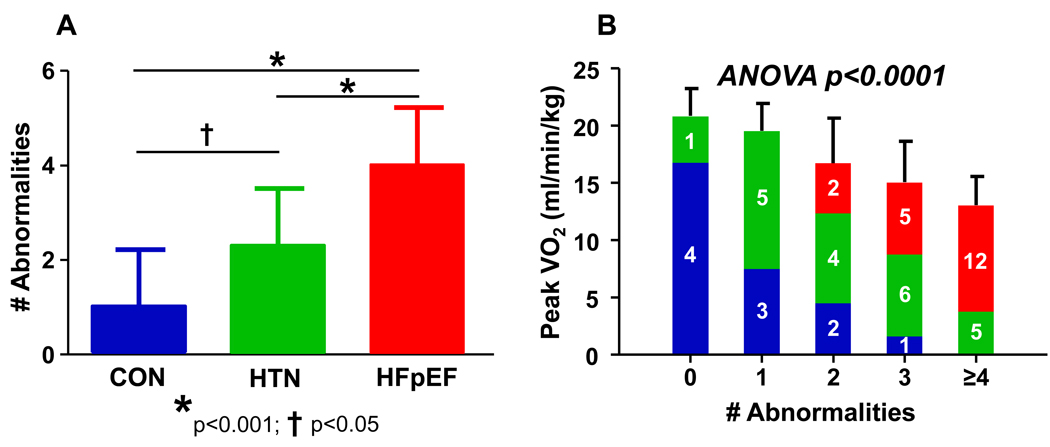
Several indices of cardiovascular reserve function including chronotropic (ΔHR), contractile (ΔPWRI), vascular (ΔSVRI, ΔPAT), endothelial (log RHI) and ventricular-arterial (ΔEa/Ees) coupling responses were each significantly associated with peak VO2 (Table 4). The number of individual reserve abnormalities (defined as <25th percentile values observed in the healthy controls) were tabulated for each subject. HFpEF patients had the greatest number of abnormalities (Figure 3A), and the presence of more reserve abnormalities was associated with progressively more depressed exercise capacity (Figure 3B). Of note, several indices of cardiovascular reserve function also correlated with subjective dyspnea and fatigue at matched low-level workload (Table 4).
Table 4.
Relationships between Reserve and Peak exercise capacity or Symptoms at matched low level workload (20W)
| Pearson r | p | |
|---|---|---|
| Peak VO2 | ||
| Chronotropic reserve (ΔHR) | 0.70 | <0.0001 |
| Contractile reserve (ΔPWRI) | 0.73 | <0.0001 |
| Endothelial function (Log RHI) | 0.43 | 0.003 |
| Vascular reserve (ΔSVRI) | −0.40 | 0.009 |
| Vascular reserve (Δ Log PAT) | 0.52 | 0.0007 |
| Coupling reserve (ΔEa/Ees) | −0.51 | 0.0006 |
| 20W Borg Fatigue | ||
| Chronotropic reserve (ΔHR) | −0.37 | 0.007 |
| Contractile reserve (ΔPWRI) | −0.44 | 0.004 |
| Endothelial function (Log RHI) | −0.52 | 0.0002 |
| Vascular reserve (ΔSVRI) | 0.37 | 0.02 |
| Vascular reserve (Δ Log PAT) | −0.47 | 0.003 |
| Coupling reserve (ΔEa/Ees) | 0.52 | 0.0006 |
| 20W Borg Dyspnea | ||
| Chronotropic reserve (ΔHR) | −0.48 | 0.0005 |
| Contractile reserve (ΔPWRI) | −0.61 | <0.0001 |
| Endothelial function (Log RHI) | −0.39 | 0.007 |
| Vascular reserve (ΔSVRI) | 0.37 | 0.02 |
| Vascular reserve (Δ Log PAT) | −0.32 | 0.052 |
| Coupling reserve (ΔEa/Ees) | 0.49 | 0.001 |
Figure 3. Global Reserve Dysfunction.
[A] HFpEF subjects displayed a greater number of abnormalities in cardiovascular reserve function than hypertensives and normal controls, and hypertensives had more abnormalities than healthy controls. [B] The presence of a greater number of reserve abnormalities was associated with more severely depressed exercise capacity. Numbered colored bars indicate total number of subjects in each grouping for controls (blue), hypertensives (green) and HFpEF subjects (red). P value reflects 1-way ANOVA testing relationship of number of abnormalities versus peak VO2.
DISCUSSION
This study found evidence for global impairment in cardiovascular reserve function in HFpEF compared with normal and hypertensive controls, including limitations in chronotropic, contractile, endothelial and vascular reserve, resulting in markedly impaired ventricular-arterial coupling responses to exercise. Depressed reserve responses correlated with reduced exercise capacity and greater subjective symptoms at low-level workload, and the accumulation of more individual abnormalities was associated with progressively greater impairment in exercise capacity. These data confirm and extend upon a growing body of evidence demonstrating that the pathophysiology of HFpEF is complex and characterized by global impairment in multiple domains of cardiovascular reserve function.
Contractile Reserve
Patients with HFpEF have a “normal” ejection fraction, but EF is a rather poor measure of contractility because of its sensitivity to load and chamber remodeling(17,18). To accurately assess contractility, preload and afterload must both be accounted for(19). Using load-independent measures, we observed that contractile reserve responses with exercise were blunted in HFpEF at peak exercise. However, it is difficult to discern whether differences in peak contractility alone are meaningful, because HFpEF subjects reach lower peak workloads. In other words, are observed deficits in contractile reserve in HFpEF a mechanism or consequence of exercise limitation?
The current study resolves this question by demonstrating that at matched, low-level workload (20W), contractile reserve is impaired in HFpEF. In an earlier study, we found inotropic reserve impairments in HFpEF compared with hypertensives at peak, but not low-level exercise(5). However, the hypertensive control group in the latter study had more severe limitation (peak VO2 70% predicted) and more abnormal ventricular remodeling (~90% with LV hypertrophy). The current findings are consistent with recent reports from other groups showing attenuated increases in EF with exercise(20,21), and reduced tissue-Doppler systolic shortening velocities and strain(22).
The mechanisms limiting contractile reserve in HFpEF remain speculative. While one prior study reported that resting contractility in HFpEF is similar to normals(19), a recent population-based study found that chamber and myocardial contractility are subtly but significantly impaired in HFpEF(18). We speculate that these “mild” impairments in resting contractility become more limiting during the stress imposed by exercise. Abnormalities in calcium handling may contribute, as Liu et al. demonstrated a blunted force-frequency relationship in human HFpEF(23). Finally, both systolic and diastolic reserve may be affected by abnormalities in energy substrate bioavailability, as have recently been demonstrated in HFpEF(21,24).
Endothelial Function and Vasodilator reserve
Investigators first noted endothelial dysfunction in patients with HF and reduced EF (HFrEF) in the early 1990’s(25,26), and recent work has suggested that this may contribute to symptoms of breathlessness and fatigue by enhancing abnormal skeletal muscle signaling during exercise(27). However, few studies have examined endothelial function in HFpEF.
Hundley and colleagues measured exercise capacity and flow mediated arterial dilation (FMAD) in the femoral artery by MRI in 9 subjects with HFpEF, comparing them to 11 normal controls and 10 HFrEF subjects(28). Exercise capacity was reduced in both HFrEF and HFpEF, but FMAD was impaired only in HFrEF. However, flow-mediated vasodilation in large conduit arteries (e.g. femoral) may differ from that observed in the microvasculature (as in the current study). We now show for the first time that endothelial function is impaired in HFpEF compared with apparently healthy controls, assessed at the microvasculature. Part of this deficit may be related to atherosclerosis, though RHI remained lower in HFpEF after adjusting for coronary disease, and mean RHI values were similar in HFpEF patients with or without coronary disease. Hypertensives also displayed endothelial dysfunction, but had preserved exercise capacity, possibly related to preservation of other components of reserve function. Endothelial dysfunction correlated with reduced exercise capacity and greater symptoms, suggesting a role in contributing to objective and subjective exertional intolerance in HFpEF.
During normal exercise, arterial resistance decreases in order to accommodate large increases in flow with minimal increment in pressure(6). Prior studies have demonstrated using derived indices of arterial load, such as SVRI and Ea, that exercise vasodilation is blunted in HFpEF(5,20,22). The current findings confirm these studies using the same derived vascular measures, and importantly extending upon them by demonstrating for the first time that directly-measured peripheral vasodilation (change in digital PAT amplitude with exercise) is also depressed in HFpEF.
Ventricular-Arterial Interaction with Exercise
Abnormal vasorelaxation, combined with blunted contractile reserve, led to abnormal ventricular-arterial coupling in HFpEF. In the pressure-volume plane, contractility is expressed by end-systolic elastance (Ees), defined by the slope and intercept of the end systolic pressure-volume relationship, while afterload is defined by effective arterial elastance (Ea), a lumped parameter incorporating both mean and pulsatile vascular load(6). Ventricular-arterial interaction is described by the coupling ratio (Ea/Ees). Under normal circumstances, Ea/Ees drops with exercise, because the increase in Ees exceeds the change in Ea, leading to an increase in EF(6). The normal exercise drop in Ea/Ees becomes impaired with aging(29), and Phan et al. recently found that the drop in the ratio of end systolic volume to stroke volume (which is related to Ea/Ees) was impaired in HFpEF compared with hypertensives at 50% maximal effort(21). The current findings confirm and extend upon the latter, showing that abnormal ventricular-arterial coupling is present both at matched, objective low-level workload and throughout exercise in HFpEF compared to hypertensives and normal controls.
Chronotropic Reserve
The current data confirm previously reported impairment in peak chronotropic reserve and its relationship to exercise limitation(5,21). Heart rate reserve was lower in the HFpEF patients, and over half met criteria for chronotropic incompetence(9). In contrast to an earlier study(5) and to contractile and vascular reserves in this study, heart rate responses were not blunted at submaximal workload in HFpEF, making it difficult to discern whether chronotropic incompetence contributed to exercise limitation in HFpEF or was simply related to the lower peak workload achieved.
Preload Reserve
While diastolic dysfunction was present at rest, exercise changes in diastolic compliance and relaxation were not assessed in this study. Kitzman et al. found that EDVI failed to increase with exercise in HFpEF patients compared to controls(30), whereas in the current study and in an earlier report(5), EDVI increased by 5–10% in HFpEF during exercise. However, nearly half of the patients in the Kitzman study had either infiltrative or hypertropic cardiomyopathy, diseases known to produce the most extreme forms of diastolic dysfunction. These patients were excluded from the latter analyses, and this may explain the apparent discrepancies in preload reserve. We observed a trend toward greater EDVI reserve in healthy controls at peak exercise compared with HFpEF and hypertensives, and the absence of a significant difference may be related to the small sample size in the healthy controls. Finally, changes in filling pressures with exercise, which are known to be abnormal in HFpEF(30,31), were not assessed in this study, and therefore the current results should not be interpreted as minimizing the importance of diastolic reserve in HFpEF(30).
Clinical Implications
Because diastolic dysfunction is readily detectable in most HFpEF patients and plausibly explains many symptoms, it has traditionally been conceptualized as the sole or predominant mechanism. This pathophysiologic model is similar to other disorders where a single lesion (e.g. cortisol excess) produces a wide variety of clinical sequelae (bone loss, hypertension, glucose intolerance). Our data shows that rather than being a disease of diastolic dysfunction alone, HFpEF is characterized by a number of abnormalities in endothelial and ventricular-vascular reserve function which contribute in a coordinated fashion in patients with HFpEF. We speculate and the epidemiology studies suggest that HFpEF is not due to one systemic disease, but rather, in the majority of cases, represents a culmination of a number of different disease processes associated with aging, hypertension, and diabetes. Understanding the pleiotropic nature of reserve limitation of HFpEF may allow for more focussed and tailored therapies in individual patients, and future research will hopefully identify the specific mechanistic processes that produce global reserve dysfunction in HFpEF.
Limitations
This is a cross sectional study and cannot assess causality. Pressure and flow were not directly measured, but rather estimated from non-invasive surrogates. While these derived parameters have been validated in prior studies against invasive hemodynamic measurements(10–13), there is inherently greater variability compared with the gold standard measures. Because of image foreshortening during exercise, EDVI was determined from SV and EF rather than 2D imaging alone. This assumes that mitral regurgitation was not significant, which was not measured directly.
Conclusions
Heart failure is often conceptualized as being caused by isolated, discrete disease mechanisms, such as diastolic or systolic dysfunction. However, HFpEF is a disease of the elderly, and with aging, patients acquire multiple comorbidities and processes which integrate in complex ways to produce symptoms and exercise intolerance. The current results, taken in concert with other recent studies, suggest that in most cases, HFpEF is not simply the result of a single impairment in one component of cardiovascular function, but rather a culmination of global limitations of cardiovascular reserve function—chronotropy, inotropy, lusitropy and vasodilatation, all resulting in impaired ventricular arterial coupling, depressed cardiac output response, and subjective and objective exercise intolerance. Recognition that reserve dysfunction in HFpEF is a global process affecting many cardiovascular responses to stress will aid in the design and testing of future therapeutic strategies for HFpEF.
Acknowledgments
BAB was supported the Mayo Clinic CTSA, the NIH (UL RR024150) and the Marie Ingalls Career Development Award in Cardiovascular Research.
Abbreviations
- HFpEF
Heart Failure with preserved Ejection Fraction
- PAT
Peripheral arterial tonometry
- RH
Reactive hyperemia
- PWRI
Peak LV power index
- Ees
LV end systolic elastance
- PRSW
LV preload recruitable stroke work
- Ea
Effective arterial elastance
- SVRI
Systemic vascular resistance index
- VO2
Oxygen consumption
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- 1.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. Jama. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS. Heart failure with a normal ejection fraction (HFNEF): embracing complexity. J Card Fail. 2009;15:561–564. doi: 10.1016/j.cardfail.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitzman DW, Groban L. Exercise intolerance. Heart Fail Clin. 2008;4:99–115. doi: 10.1016/j.hfc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg GA. Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–153. [PubMed] [Google Scholar]
- 8.Lam CS, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114:2070–2082. doi: 10.1161/CIRCULATIONAHA.105.561944. [DOI] [PubMed] [Google Scholar]
- 10.Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 11.Sharir T, Feldman MD, Haber H, et al. Ventricular systolic assessment in patients with dilated cardiomyopathy by preload-adjusted maximal power. Validation and noninvasive application. Circulation. 1994;89:2045–2053. doi: 10.1161/01.cir.89.5.2045. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284:H744–H750. doi: 10.1152/ajpheart.00455.2002. [DOI] [PubMed] [Google Scholar]
- 14.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. doi: 10.1016/j.tcm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Rozanski A, Qureshi E, Bauman M, Reed G, Pillar G, Diamond GA. Peripheral arterial responses to treadmill exercise among healthy subjects and atherosclerotic patients. Circulation. 2001;103:2084–2089. doi: 10.1161/01.cir.103.16.2084. [DOI] [PubMed] [Google Scholar]
- 17.Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- 18.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111:2306–2312. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 20.Ennezat PV, Lefetz Y, Marechaux S, et al. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Phan TT, Abozguia K, Nallur Shivu G, et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Liu CP, Ting CT, Lawrence W, Maughan WL, Chang MS, Kass DA. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy. Systolic versus diastolic determinants. Circulation. 1993;88:1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- 24.Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 26.Katz SD, Krum H, Khan T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J Am Coll Cardiol. 1996;28:585–590. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]
- 27.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 28.Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 29.Najjar SS, Schulman SP, Gerstenblith G, et al. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 30.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 31.Borlaug BA, Sorajja P, Nishimura RA, Lam CS, Redfield MM. Do Exercise Hemodynamics enhance Diagnosis of Heart Failure with preserved Ejection Fraction? Circulation. 2009 doi: 10.1161/CIRCHEARTFAILURE.109.930701. (abs) [DOI] [PMC free article] [PubMed] [Google Scholar]