Abstract
Chronic alcohol exposure has been shown to increase the gut permeability in the distal intestine, in part, through induction of zinc deficiency. The present study evaluated the molecular mechanisms whereby zinc deficiency mediates alcohol-induced intestinal barrier dysfunction. Examination of zinc finger transcription factors in the gastrointestinal tract of mice revealed a prominent distribution of hepatocyte nuclear factor-4α (HNF-4α). HNF-4α exclusively localizes in the epithelial nuclei and exhibited an increased abundance in mRNA and protein levels in the distal intestine. Chronic alcohol exposure to mice repressed the HNF-4α gene expression in the ileum and reduced the protein level and DNA binding activity of HNF-4α in all of the intestinal segments with the most remarkable changes in the ileum. Chronic alcohol exposure also decreased the mRNA levels of tight junction proteins, particularly in the ileum. Caco-2 cell culture studies were conducted to determine the role of HNF-4α in regulation of the epithelial tight junction and barrier function. Knockdown of HNF-4α in Caco-2 cells decreased the mRNA and protein levels of tight junction proteins in association with disruption of the epithelial barrier. Alcohol treatment inactivated HNF-4α, which was prevented by N-acetyl-cysteine or zinc. The link between zinc and HNF-4α function was confirmed by zinc deprivation, which inhibited HNF-4α DNA binding activity. These results indicate that inactivation of HNF-4α due to oxidative stress and zinc deficiency is likely a novel mechanism contributing to the deleterious effects of alcohol on the tight junctions and the intestinal barrier function.
Keywords: zinc deprivation, oxidative stress, claudin-1, occludin, zonula occludens-1
the intestinal barrier, which prevents the diffusion of macromolecules such as endotoxin from the intestinal lumen in the blood, is composed of the mucosal epithelial cells and the paracellular apical junction complex, in particular the most apical tight junctions (23). Disruption of the intestinal barrier leads to endotoxemia, which, in turn, causes inflammation and hepatitis through stimulating cytokine production (25, 32). Disruption of the intestinal barrier has been well documented in patients with alcoholic liver disease (11, 15, 27). Although acute alcohol abuse has been shown to cause damage of the upper small intestine (22), chronic alcohol consumption had minor impact on the intestinal histology (10, 12), indicating the importance of the paracellular junction molecules in the development of gut leakiness.
Tight junctions have been suggested to play a pivotal role in the regulation of paracellular permeability. Cell culture studies have shown that alcohol, or alcohol metabolites such as acetaldehyde, disrupt the epithelial barrier through disassembling tight junction proteins such as claudins, occludin, and zonula occludens (ZO)-1 (20, 30). Although the precise mechanisms have not been well defined, posttranscriptional modulation of tight junction proteins has been suggested to play an important role in alcohol-, or acetaldehyde-induced, disassembly of tight junction proteins. Alcohol metabolism in the enterocytes was associated with generation of reactive oxygen species (ROS) (2, 4). ROS have been shown to disassemble junction proteins by directly causing protein carbonylation or indirectly inducing tyrosine phosphorylation of tight junction proteins and NF-κB activation (3–6, 31, 34). Inhibition of ROS has been shown to mediate the inhibitory effects of diverse protective agents such as epidermal growth factor and l-cysteine on alcohol-induced disassembly of tight junction proteins (2, 4). These cumulative data suggest that oxidative stress plays a causal role in alcohol-induced epithelial barrier disruption.
Our recent studies demonstrated that the distal, rather than the proximal small intestine is important in the development of alcohol-induced gut leakiness (41). Whereas we found that zinc deficiency due to oxidative stress may interfere with the intestinal barrier function by a direct action on tight junction proteins or by sensitizing to the effects of alcohol, the molecular mechanisms for the effects of zinc deficiency are unclear. It is well known that zinc is the key element in zinc finger structure that is required for DNA binding activity of zinc finger transcription factors (8). Previous studies have shown that oxidative stress can release zinc from zinc finger transcription factors, leading to loss of DNA binding activity (17, 39, 40). Hepatocyte nuclear factor-4α (HNF-4α), a zinc finger transcription factor, is mainly expressed in the liver, intestine, pancreas, and kidney. Previous studies have shown that HNF-4α is essential for enterocyte differentiation (1, 13, 36). Intestinal epithelium-specific knockout of HNF-4α caused an increase in gut permeability in experimental colitis (1), indicating the importance of HNF-4α in regulation of intestinal barrier function. The present study was undertaken to determine if dysfunction of HNF-4α due to zinc deficiency is involved in alcohol-induced intestinal barrier disruption.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice were obtained from Harlan (Indianapolis, IN). All of the mice were treated according to the experimental procedures approved by the Institutional Animal Care and Use Committee. To examine the distribution of zinc finger transcription factors, mice at 4 mo old were anesthetized with Avertin (300 mg/kg), and tissues from each gastrointestinal segment, including stomach, duodenum, jejunum, ileum, cecum, and colon, were taken. For chronic alcohol exposure, mice at 4 mo old were pair-fed a modified Lieber-DeCarli alcohol or isocaloric maltose dextrin control liquid diet for 4 wk with a stepwise feeding procedure as described previously (41). The ethanol content (%, wt/vol) in the diet was 4.8 (34% of total calories) at initiation and gradually increased up to 5.4 (38% of total calories). The amount of food given to the pair-fed mice was that of alcohol-fed mice measured the previous day. The average food intake was 10.4 g·day−1·mouse−1. At the end of the feeding experiment, the mice were anesthetized, and intestinal samples were harvested for analysis. The small intestine segment from the pyloric sphincter to the ligament of Treitz was defined as duodenum. The small intestine segment from the ligament of Treitz to the ileocecal junction was divided into equal length as jejunum and ileum. Protein and RNA samples of the whole intestinal tissues were prepared for analysis.
Cell culture.
Caco-2 cells from the American Type Culture Collection (Rockville, MD) were cultured in DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 10 mM HEPES, and 10% FBS, at 37°C in a 5% CO2 environment. Culture medium was changed every 2 days. Caco-2 cells were subcultured after partial digestion with 0.25% trypsin-EDTA, and passages 19–30 were used. Caco-2 cells grown on chamber slides (LabTek, Naperville, IL) were used for immunofluorescence microscopy, whereas Caco-2 cells grown on six-well plates were used for immunoblot analysis. For measurement of epithelial barrier function, Caco-2 cells were cultured on inserts (pore size 0.4 μm; BD Biosciences, San Jose, CA). HNF-4α small-interfering RNA (siRNA) transfection was conducted with human HNF-4α siRNA (Ambion, Austin, TX) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Caco-2 cells were cultured with serum-free medium for 24 h before transfection because Caco-2 cells are known to be difficult for transfection. For alcohol intoxication, ethanol was added to the culture medium at 5% (vol/vol) for 5 h with or without adding N-acetyl-cysteine (NAC) at 2 mM or zinc at 100 μM 30 min before alcohol intoxication. Our previous report has shown that 5% ethanol significantly affected Caco-2 monolayer barrier function, which was prevented by NAC (41). For inducing zinc deficiency, N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) was added to the culture medium at a final concentration of 2, 3, or 4 μM and cultured for 24 h. Zinc sulfate was added at 100 μM zinc together with TPEN to confirm the specificity of the zinc chelating action of TPEN.
Immunoperoxidase procedure.
For localization of zinc finger transcription factors in the gastrointestinal tract, intestinal tissues were fixed in 10% formalin, and paraffin sections were prepared. The antigen retrieval was performed by microwave treatment in 0.01 M citrate buffer (pH 6.0), and the endogenous peroxidase was blocked by incubation with 3% H2O2 in PBS for 10 min. Tissue sections were incubated with polyclonal rabbit anti-HNF-4α, GATA-4, or SP-1 IgG (Santa Cruz Biotechnologies, Santa Cruz, CA) overnight at 4°C. After being rinsed with PBS, the sections were incubated with DAKO EnVision+ Labeled Polymer-horseradish peroxidase (HRP) anti-rabbit IgG (DAKO, Carpinteria, CA) for 30 min. Visualization was conducted using diaminobenzidine as HRP substrate.
Immunofluorescence procedure.
For detection of HNF-4α and tight junction proteins, Caco-2 cells on chamber slides were fixed with methanol at −20°C for 10 min and subjected to the immunofluorescence procedure. Cells on the slides were incubated with rabbit polyclonal antibody against occludin (Zymed Laboratories, San Francisco, CA) or HNF-4α (Santa Cruz Biotechnologies) overnight at 4°C. Slides were then washed in PBS and incubated with Cy3- or Cy2-conjugated donkey anti-rabbit IgG for 30 min.
Immunoblot analysis.
Gastrointestinal tissue extracts and whole cell extracts from Caco-2 cells were prepared. Aliquots containing 40 μg protein for HNF-4α or 30 μg proteins for occludin were loaded on SDS-polyacrylamide gels. After electrophoresis, proteins were transferred to a polyvinylidene fluoride membrane. The membrane was probed with rabbit polyclonal antibody against HNF-4α (Santa Cruz Biotechnologies) or occludin (Zymed) overnight at 4°C. The membrane was then processed with HRP-conjugated donkey anti-rabbit IgG (GE Healthcare, Piscataway, NJ). The protein bands were visualized by an enhanced chemiluminescence detection system (GE Healthcare) and quantified by densitometry analysis.
Real-time RT-PCR assay.
The mRNA levels of HNF-4α and tight junction proteins were assessed by real-time RT-PCR. In brief, the total RNA was isolated and reverse-transcribed with the GenAmp RNA PCR kit (Applied Biosystems, Foster City, CA) and oligo(dT) primers. The forward and reverse primers were designed using Primer Express Software and listed in Table 1. The SYBR green PCR Master Mix (Applied Biosystems) was used for real-time RT-PCR analysis. The relative differences of gene expression among groups were evaluated using cycle time values. The data were normalized to β-actin and expressed as relative changes, setting the values of duodenum as one.
Table 1.
Primer sequences for real-time RT-PCR
| Gene | Source | GenBank Accession No. | Sequences (Forward/Reverse 5′-3′) |
|---|---|---|---|
| HNF-4α | Mouse | NM_008261 | GCCTTCTGCGAACTCCTTCTG |
| GGGACGATGTAGTCATTGCCT | |||
| Claudin-1 | Mouse | NM_016674 | GGGGACAACATCGTGACCG |
| AGGAGTCGAAGACTTTGCACT | |||
| Occludin | Mouse | NM_008756 | TTGAAAGTCCACCTCCTTACAGA |
| CCGGATAAAAAGAGTACGCTGG | |||
| ZO-1 | Mouse | NM_009386 | GATCCCTGTAAGTCACCCAGA |
| CTCCCTGCTTGCACTCCTATC | |||
| β-Actin | Mouse | NM_007393 | GGCTGTATTCCCCTCCATCG |
| CCAGTTGGTAACAATGCCATGT | |||
| Claudin-1 | Human | AF134160 | CCAGTCAATGCCAGGTACGAAT |
| TTGGTGTTGGGTAAGAGGTTGTT | |||
| Occludin | Human | NM_002538 | ACAGAGCAAGATCACTATGAGACA |
| TGTTGATCTGAAGTGATAGGTGGA | |||
| ZO-1 | Human | NM_175610 | TGGTGTCCTACCTAATTCAACTCA |
| CGCCAGCTACAAATATTCCAACA | |||
| β-Actin | Human | NM_001101 | CTGGAACGGTGAAGGTGACA |
| AAGGGACTTCCTGTAACAATGCA |
HNF-4α, hepatocyte nuclear factor-4α; ZO-1, zonula occludens-1.
Assessment of HNF-4α function.
Nuclear extracts of intestinal epithelium and Caco-2 cells were prepared using a kit from Active Motif (Carlsbad, CA). The HNF-4α function was assessed by measuring the DNA binding ability with a Trans-AM HNF Family Transcription Factor ELISA Kit from Active Motif. The kit consists of a 96-well plate into which a specific oligonucleotide sequence containing the consensus site of HNF-4α is immobilized. The HNF-4α bound to the consensus site is recognized by a rabbit polyclonal antibody against HNF-4α, followed by incubation with a HRP-conjugated secondary antibody for the colorimetric quantification.
Evaluation of the epithelial monolayer barrier function.
The Caco-2 monolayer barrier function was evaluated by measuring the electrical resistance and paracellular permeability. The transepithelial electrical resistance (TEER) of the filter-grown Caco-2 monolayers was measured with an epithelial voltohmmeter (World Precision Instruments, Sarasota, FL). Electrical resistance was recorded with three consecutive measurements, and the resistance value of the filter alone was subtracted. For determination of paracellular permeability, FITC-dextran (FD-4, FW 4 kDa) was used. FD-4 was added to the apical compartment of Caco-2 cells at a final concentration of 10 mg/ml in DMEM. After 90 min incubation, the basolateral media were collected, and FD-4 penetrated to the basolateral media was measured by a microplate fluorescence reader with an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Statistics.
All data are expressed as means ± SD. The data were analyzed by either ANOVA followed by Newman-Keuls' Multiple-Comparison Test or the Student's t-test. Correlation coefficients were used to determine linear association between intestinal HNF-4α and tight junction protein mRNA variables. In all statistical tests, P values <0.05 were considered as significant.
RESULTS
Distribution of HNF-4α, GATA-4, and SP-1 in the gastrointestinal tract.
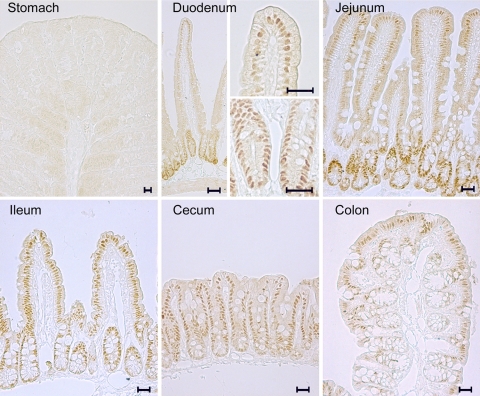
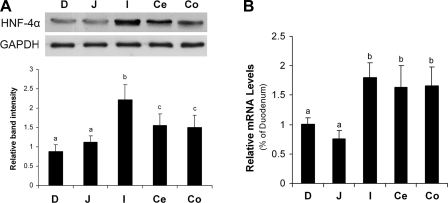
Distribution of zinc finger transcription factors in the gastrointestinal tract was determined by immunohistochemistry. HNF-4α was detected in all of the segments of the gastrointestinal tract except for the stomach (Fig. 1). The distribution of HNF-4α was in the epithelial cells of intestinal mucosa and crypts with exclusive localization in the nuclei. The distribution of HNF-4α gradually increased along the intestine from the proximal to the distal. In contrast, both GATA-4 [Supplemental data 1 (Supplemental data for this article may be found on the American Journal of Physiology: Gastrointestinal and Liver Physiology website.)] and SP-1 (Supplemental data 2) were extensively distributed in the stomach, gradually decreased along the small intestine, and were absent in the cecum and colon. Because our recent studies have shown that chronic alcohol exposure causes zinc deficiency and gut leakiness in the distal intestine (41), HNF-4α was chosen as the target zinc finger transcription factor for further study. Immunoblot analysis demonstrated that the protein levels of HNF-4α were highest in the ileum, followed by the cecum and colon, and lowest in the duodenum and jejunum (Fig. 2A). The mRNA levels of HNF-4α in the distal intestine, including ileum, cecum, and colon, were significantly higher than that in the duodenum and jejunum (Fig. 2B).
Fig. 1.
Distribution of hepatocyte nuclear factor-4α (HNF-4α) in the gastrointestinal tract of mice. HNF-4α was detected by immunohistochemical staining. Exclusive localization of HNF-4α in the nuclei of epithelial cells was found in all of the intestinal segments, but not in the stomach. Scale bars: 20 μM.
Fig. 2.
Protein and mRNA levels of HNF-4α in the gastrointestinal tract of mice. A: immunoblot analysis of HNF-4α protein levels. The immunoblot bands were quantified by densitometry analysis, and the HNF-4α-to-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratios were calculated. B: real-time RT-PCR assay of HNF-4α mRNA levels. The gene expressions, normalized to β-actin, were expressed as relative changes, setting the values of duodenum as one. Results are means ± SD (n = 3 experiments in A, n = 4 in B). Significant differences (P < 0.05) between intestinal segments were determined by ANOVA and are indicated by different letters. D, duodenum; J, jejunum; I, ileum; Ce, cecum; Co, colon.
Effects of chronic alcohol exposure on intestinal HNF-4α and tight junction proteins.
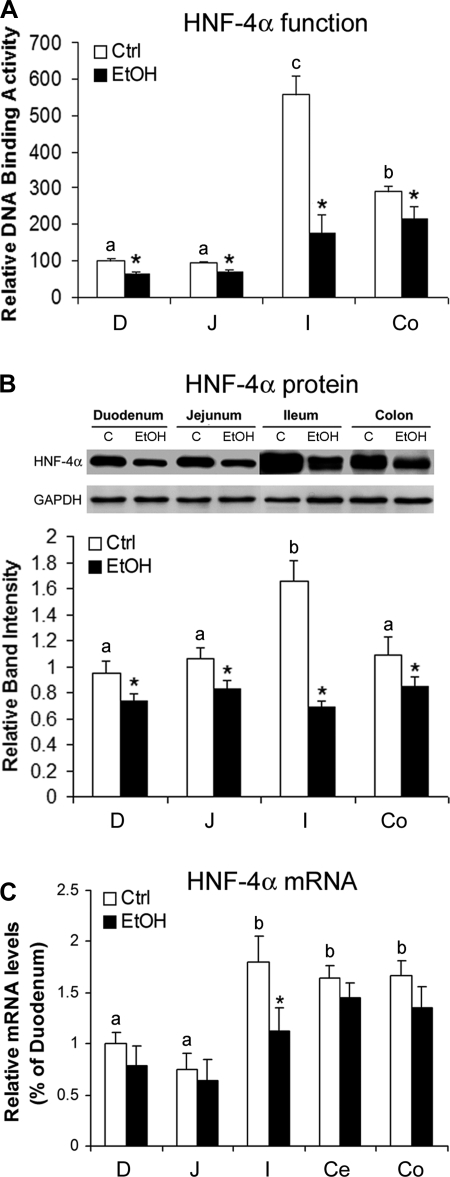
To determine if chronic alcohol exposure affects intestinal HNF-4α function and if the effects are at the transcriptional or posttranscriptional level, DNA binding activity and protein and mRNA levels of HNF-4α were determined. As shown in Fig. 3A, chronic alcohol exposure significantly inhibited HNF-4α function in all of the intestinal segments, and the most prominent effect was in the ileum, a 70% decrease in DNA binding activity compared with 20–30% decreases in the duodenum, jejunum, and colon. In accordance, the immunoblot analysis showed that chronic alcohol exposure significantly decreased the HNF-4α protein level in all of the intestinal segments with the greatest reduction in the ileum (Fig. 3B). However, chronic alcohol exposure only caused a significant decrease in the mRNA level of HNF-4α in the ileum (Fig. 3C).
Fig. 3.
Effects of chronic alcohol exposure on intestinal HNF-4α in mice. A: HNF-4α function was assessed by measuring DNA binding activity with a Trans-AM HNF Family Transcription Factor ELISA Kit. EtOH, ethanol; Ctrl, control. B: HNF-4α protein levels were measured by immunoblot analysis. The immunoblot bands were quantified by densitometry analysis. C: HNF-4α mRNA levels were measured by real-time RT-PCR. The gene expressions, normalized to β-actin, were expressed as relative changes, setting the values of duodenum as one. Results are means ± SD (n = 6 in A; n = 3 in B; n = 4 in C). Significant differences (P < 0.05) among intestinal segments were determined by ANOVA and are indicated by different letters. *Significant differences (P < 0.05) between alcohol-fed and pair-fed mice for each intestinal segment determined by t-test.
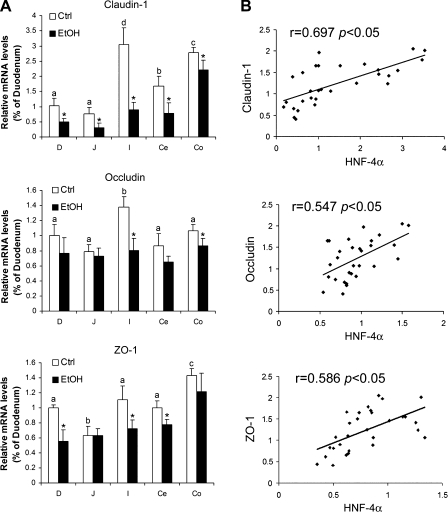
To determine the possible link between HNF-4α and tight junctions, the mRNA levels of tight junction proteins were measured. As shown in Fig. 4A, chronic alcohol exposure caused a significant decrease in claudin-1 mRNA in all of the intestinal segments, occludin mRNA in the ileum and colon, and ZO-1 mRNA in the duodenum, ileum, and cecum. The ileum exhibited the largest decreases of all the genes examined. Highly positive correlations were found between the intestinal HNF-4α mRNA and the intestinal mRNAs of claudin-1 (r = 0.697), occludin (r = 0.547), and ZO-1 (r = 0.586) (Fig. 4B).
Fig. 4.
Effects of chronic alcohol exposure on expression of tight junction proteins in mice. A: mRNA levels of tight junction proteins. The mRNA levels of claudin-1, occludin, and ZO-1 were measured by real-time RT-PCR. The gene expressions, normalized to β-actin, were expressed as relative changes, setting the values of duodenum as one. Results are means ± SD (n = 4). Significant differences (P < 0.05) among intestinal segments were determined by ANOVA and are indicated by different letters. *Significant differences (P < 0.05) between alcohol-fed and pair-fed mice for each intestinal segment determined by t-test. B: correlations between mRNA levels of HNF-4α and tight junction proteins. Correlation coefficients were used to determine linear association between intestinal HNF-4α and tight junction protein mRNA variables.
Effects of HNF-4α siRNA transfection on tight junction proteins and epithelial barrier function in Caco-2 enterocytes.
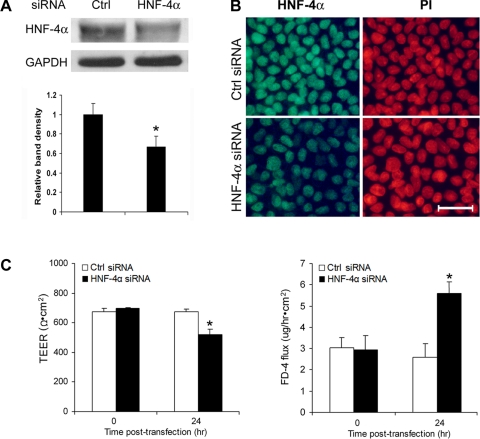
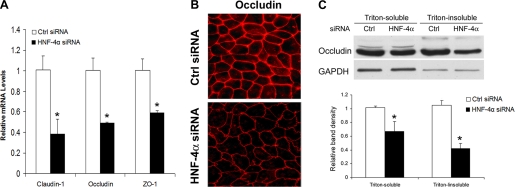
HNF-4α siRNA transfection was performed in differentiated Caco-2 cells after 21 days of culture. Immunoblot and immunocytochemistry showed that siRNA transfection for 24 h reduced HNF-4α protein levels (Fig. 5A) and nuclear distribution in the Caco-2 cells (Fig. 5B). Epithelial barrier function analysis showed that HNF-4α siRNA transfection for 24 h caused epithelial barrier dysfunction, as indicated by significant decrease in the TEER value and increase in paracellular permeability to FD-4 (Fig. 5C). Real-time RT-PCR showed that knockdown of HNF-4α resulted in downregulation of claudin-1, occludin, and ZO-1 expression (Fig. 6A). Reduction of tight junction proteins was further demonstrated by immunocytochemical staining and immunoblot analysis of occludin. Immunocytochemical staining demonstrated not only a reduced intensity of occludin at the tight junction but also a zipper-like distribution, a pattern commonly found in undifferentiated Caco-2 cells (Fig. 6B). Immunoblot analysis showed a reduction of the occludin protein levels in both Triton-soluble and Triton-insoluble fractions (Fig. 6C).
Fig. 5.
Effects of HNF-4α small-interfering RNA (siRNA) transfection on the epithelial barrier of Caco-2 cells. Caco-2 cells were transfected with human HNF-4α siRNA or control siRNA on day 21 for 24 h. A: immunoblot analysis of HNF-4α protein levels. The immunoblot bands were quantified by densitometry analysis. B: immunocytochemical localization of HNF-4α. The nuclei were counterstained by propidium iodide (PI). C: epithelial barrier function. The epithelial barrier function of insert-cultured Caco-2 cells was assessed by measuring the transepithelial electrical resistance (TEER) with an epithelial voltohm-meter and the paracellular FD-4 penetration from the apical to the basolateral compartments. Results are means ± SD (n = 3 in A; n = 8 in C). *Significant differences (P < 0.05) between control siRNA and HNF-4α siRNA detected by t-test (A). *Significant differences (P < 0.05) between time points determined by ANOVA (C).
Fig. 6.
Effects of HNF-4α siRNA transfection on tight junction proteins. Caco-2 cells were transfected with HNF-4α siRNA for 24 h. A: real-time RT-PCR assay of gene expression of tight junction proteins. The mRNA levels, normalized to β-actin, were expressed as relative changes, setting the values of controls as one. B: immunocytochemical staining of occludin. C: immunoblot analysis of occludin protein levels. The immunoblot bands were quantified by densitometry analysis. Results are means ± SD (n = 4 in A; n = 3 in C). *Significant differences (P < 0.05) between control siRNA and HNF-4α siRNA detected by t-test (A and C).
Mechanistic link of oxidative stress and zinc deprivation in alcohol-induced HNF-4α inactivation.
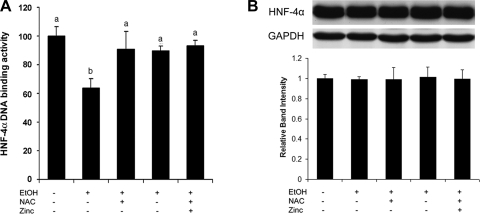
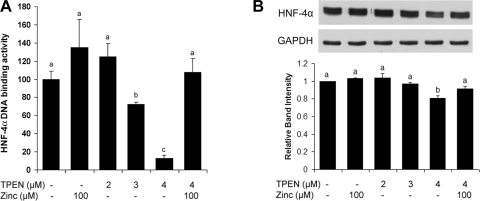
To determine if alcohol exposure causes HNF-4α inactivation via oxidative stress-induced zinc release from HNF-4α, Caco-2 cells were treated with alcohol with or without NAC or zinc supplementation. Alcohol treatment significantly inhibited the DNA binding activity of HNF-4α (Fig. 7A), whereas the protein level of HNF-4α was not affected by alcohol (Fig. 7B). Either NAC or zinc supplementation attenuated alcohol-induced HNF-4α inactivation, but NAC and zinc did not have a synergistic effect. NAC or zinc alone did not affect HNF-4α DNA binding and HNF-4α expression. To define the link between zinc deprivation and HNF-4α inactivation, zinc deprivation in Caco-2 cells was achieved by TPEN treatment at 2, 3, and 4 μM. As shown in Fig. 8A, the DNA binding activity of HNF-4α was markedly inhibited by TPEN at both 3 and 4 μM. However, the HNF-4α protein level was significantly reduced by TPEN only at 4 μM (Fig. 8B). The effects of TPEN on HNF-4α DNA binding activity and protein level were normalized by zinc pretreatment.
Fig. 7.
Effects of alcohol on HNF-4α protein and function in Caco-2 cells. Caco-2 cells were exposed to 5% alcohol for 24 h, and NAC and zinc were supplemented to determine the role of oxidative stress and zinc deprivation. A: HNF-4α function was assessed by measuring HNF-4α DNA binding activity with a Trans-AM HNF Family Transcription Factor ELISA kit. B: immunoblot assay of HNF-4α protein levels. The immunoblot bands were quantified by densitometry analysis. Results are means ± SD (n = 6 in A; n = 3 in B). Significant differences (P < 0.05) between time points were determined by ANOVA and are indicated by different letters in A.
Fig. 8.
Effects of zinc deprivation on HNF-4α protein and function in Caco-2 cells. Caco-2 cells were treated on day 21 with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) at 2, 3, and 4 μM or 4 μM TPEN plus 100 μM zinc for 24 h. A: HNF-4α function was assessed by measuring HNF-4α DNA binding activity with a Trans-AM HNF Family Transcription Factor ELISA kit. B: immunoblot assay of HNF-4α protein levels. The immunoblot bands were quantified by densitometry analysis. Results are means ± SD (n = 6 in A; n = 3 in B). Significant differences (P < 0.05) between time points were determined by ANOVA and are indicated by different letters.
DISCUSSION
The results obtained demonstrated that HNF-4α predominantly distributes in the distal part of the intestine. Chronic alcohol exposure significantly inhibited the mRNA and protein levels and DNA binding activity of HNF-4α, particularly in the ileum, which is correlated well with our previous observations (41) on alcohol-induced ROS accumulation and zinc deprivation in the ileum. Caco-2 cell culture showed that HNF-4α critically regulates expression of tight junction proteins, and inactivation of HNF-4α due to oxidative stress and/or zinc deprivation accounts for alcohol-induced epithelial barrier dysfunction. These data provide evidence for the first time that HNF-4α critically regulates tight junction proteins, and inactivation of HNF-4α is likely an important mechanism underlying alcohol-induced epithelial barrier dysfunction.
HNF-4α belongs to the nuclear hormone receptor superfamily and is distributed in the liver, intestine, pancreas, and kidney (33, 35). As a liver-enriched transcription factor, HNF-4α has been shown to bind to the reporters of >1,200 genes involved in most aspects of hepatocyte function (26). Liver-specific conditional knockout of HNF-4α in adult mice led to repression of a multitude of genes encoding metabolic proteins/enzymes as well as cell junction and adhesion proteins (28). Although HNF-4α has been shown to regulate a number of genes in the intestine, most previous studies on intestinal HNF-4α were focused on its role in enterocyte differentiation. A bioinformatic study demonstrated that genes that are upregulated during fetal to adult and crypt to villus differentiation have an overrepresentation of potential HNF-4α-binding sites in their promoters (36). An embryonic colon-HNF-4α-null mouse model showed developmental and functional defects, including failure of crypt formation, perturbed goblet cell maturation, and disrupted expression of a number of genes involved in colon function (13). The present study demonstrated that HNF-4α predominantly distributes in the distal intestine and regulates tight junction proteins at the transcriptional level. Therefore, HNF-4α is likely a critical regulator in both intestinal development and physiological functions, including intestinal barrier function.
Dysfunction of hepatic HNF-4α has been documented in disease conditions such as cirrhosis (7), hepatocellular carcinoma (19), and alcoholic liver disease (14). In a tumor progression mouse model, the highly invasive fast-growing dedifferentiated variant (fgHCC) exhibited loss of characteristic hepatocyte markers in association with loss of HNF-4α expression (19). Forced reexpression of HNF-4α in cultured fgHCC cells not only reversed the progressive phenotype but also induced fgHCC cells to reestablish an epithelium in association with induction of ZO-1 protein. These data indicate that loss of HNF-4α expression is an important determinant of disease progression. Although the status of HNF-4α in intestinal diseases remains unclear, a recent study showed that knockout of HNF-4α in the intestinal epithelial cells in mice increases the susceptibility to experimental inflammatory bowl disease (1). Interestingly, the intestinal permeability was significantly higher in intestinal epithelial HNF-4α null mice in acute colitis. Thus HNF-4α may have an impact on initiation and progression of intestinal disease through regulating gut permeability. The present study showed that chronic alcohol exposure significantly reduced the mRNA level of HNF-4α in the ileum. However, significant decreases in the protein level and DNA binding activity of HNF-4α were found in all of the intestinal segments, with the most remarkable change in the ileum. The findings suggest that chronic alcohol exposure could affect the intestinal HNF-4α at both transcriptional and posttranscriptional levels, in particular the latter. Our recent studies showed that chronic alcohol exposure increases gut permeability in the ileum, but not in the duodenum and jejunum (41). The most predominant loss of HNF-4α mRNA, protein, and DNA binding activity in the ileum after chronic alcohol exposure is correlated well with the decreased expression of tight junction proteins and the increased gut permeability.
Tight junctions, which are located at the most apical and seal the intercellular space, are organized by interactions among a variety of proteins, including transmembrane proteins such as occludin and claudins, intracellular plaque proteins such as ZOs, and cytoskeleton proteins such as actin and myosin filaments (23). Loss of intestinal tight junction proteins such as ZO-1, occludin, and claudin-1 has been reported to be associated with gut leakiness in patients with inflammatory bowel disease (18), alcoholic liver disease (37), celiac disease (24, 29), and experimental colitis (16). However, the mechanisms underlying the direct cause for disassembling the proteins from the tight junctions have been poorly understood. Modifications of tight junction proteins at the posttranslational level have been suggested to mediate oxidative stress-induced disassembly of tight junction proteins. Previous studies showed that the interactions between tight junction proteins are dependent on Ser/Thr phosphorylation, which is determined by a balance between protein kinase C and protein phosphatases (32, 30, 31). On the other hand, phosphorylation at tyrosine residues has been suggested to account for ROS-induced dissociation of occludin and ZO-1 complex. A recent study demonstrated a micro-RNA-dependent mechanism. An increase in siRNA-212 expression was associated with reduction of ZO-1 protein in the colon biopsy samples from alcoholic patients (37). Caco-2 cell culture studies further demonstrated that siRNA-212 expression decreases the ZO-1 protein. The present study showed that tight junction proteins can also be downregulated at the transcription level due to dysfunction of HNF-4α after chronic alcohol exposure. Cell culture studies further demonstrated that knockdown of HNF-4α leads to repression of tight junction proteins, indicating an important role of HNF-4α in regulation of tight junction integrity and barrier function.
Zinc is an essential cofactor required for the structure and function of a large number of zinc proteins. Prooxidants such as superoxide and aldehydes have been shown to release zinc, leading to inactivation of zinc proteins (17). HNF-4α is a zinc finger transcription factor, and zinc is required for maintaining the DNA binding activity of the zinc finger motif (33, 16). The present study showed that alcohol exposure to Caco-2 cells inactivates HNF-4α without affecting its protein level, indicating that loss of zinc from the zinc finger of HNF-4α due to oxidative stress is likely a direct cause of HNF-4α inactivation. Chronic ethanol exposure has been shown to induce oxidative stress as indicated by alterations of redox status and free malondialdehyde in the intestine of rats, although differential regulation was found from proximal to distal intestinal segments (38). Our previous studies have shown that chronic alcohol exposure induced ROS accumulation and zinc deprivation in the ileum of mice (41). Although a lower zinc intake has been reported in alcoholics (9), the present study suggests that zinc release under alcohol-induced oxidative stress may lead to cellular zinc deficiency and dysfunction of zinc proteins even under adequate dietary zinc conditions. Furthermore, zinc treatment alone showed a stimulating effect on HNF-4α DNA binding in Caco-2 cells, suggesting an increase in zinc coordination to HNF-4α under zinc-supplemented conditions. Thus zinc supplementation alone may stimulate intestinal HNF-4α function, leading to enhancement of the tight junctions.
In conclusion, HNF-4α prominently distributes in the lower intestine segments of mice and exclusively localizes in the nuclei of the mucosal and crypt epithelial cells. Chronic alcohol exposure inactivates HNF-4α at both transcriptional and posttranscriptional levels, in particular, in the ileum. HNF-4α plays an important role in maintenance of epithelial barrier function through regulating tight junction proteins. Inactivation of HNF-4α due to oxidative stress and zinc deficiency is likely a novel mechanism contributing to the deleterious effects of alcohol on the tight junctions and the intestinal barrier function.
GRANTS
C. J. McClain and Y. J. Kang are Distinguished University Scholars of the University of Louisville. W. Zhong (D.V.M.) is an awardee of the State Scholarship Fund of China Scholarship Council. This research was supported in part by the National Institutes of Health grants (C. J. McClain, Y. J. Kang, and Z. Zhou) and the Veterans Administration (C. J. McClain).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Xinguo Sun for technical assistance and Marion McClain for review of this manuscript.
REFERENCES
- 1.Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ, Inoue Y. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis 14: 908–920, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction, and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther 291: 1075–1085, 1999 [PubMed] [Google Scholar]
- 3.Banan A, Farhadi A, Fields JZ, Mutlu E, Zhang L, Keshavarzian A. Evidence that nuclear factor-kappa B activation is critical in oxidant-induced disruption of the microtubule cytoskeleton, and barrier integrity and that its inactivation is essential in epidermal growth factor-mediated protection of the monolayers of intestinal epithelia. J Pharmacol Exp Ther 306: 13–28, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther 294: 997–1008, 2000 [PubMed] [Google Scholar]
- 5.Banan A, Zhang LJ, Farhadi A, Fields JZ, Shaikh M, Keshavarzian A. PKCβ1 isoform activation is required for EGF-induced NF-κB inactivation, and IκBα stabilization and protection of F-actin assembly and barrier function in enterocyte monolayers. Am J Physiol Cell Physiol 286: C723–C738, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Inhibition of oxidant-induced nuclear factor-kappaB activation, and inhibitory-kappaB alpha degradation and instability of F-actin cytoskeletal dynamics and barrier function by epidermal growth factor: key role of phospholipase-gamma isoform. J Pharmacol Exp Ther 309: 356–368, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Berasain C, Herrero JI, García-Trevijano ER, Avila MA, Esteban JI, Mato JM, Prieto J. Expression of Wilms' tumor suppressor in the liver with cirrhosis: relation to hepatocyte nuclear factor 4 and hepatocellular function. Hepatology 38: 148–157, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271: 1081–1085, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Bergheim I, Parlesak A, Dierks C, Bode JC, Bode C. Nutritional deficiencies in German middle-class male alcohol consumers: relation to dietary intake and severity of liver disease. Eur J Clin Nutr 57: 431–438, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bhonchal S, Nain CK, Prasad KK, Nada R, Sharma AK, Sinha SK, Singh K. Functional, and morphological alterations in small intestine mucosa of chronic alcoholics. J Gastroenterol Hepatol 23: e43–e48, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1: 179–182, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Bode JC, Knüppel H, Schwerk W, Lorenz-Meyer H, Dürr HK. Quantitative histomorphometric study of the jejunal mucosa in chronic alcoholics. Digestion 23: 265–270, 1982 [DOI] [PubMed] [Google Scholar]
- 13.Garrison WD, Battle MA, Yang C, Kaestner KH, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha is essential for embryonic development of the mouse colon. Gastroenterology 130: 1207–1220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang X, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-a in mice subjected to a long-term ethanol administration. Am J Pathol 172: 916–925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Kroencke KD, Klotz LO. Zinc fingers as biological redox switches? Antioxid Redox Signal 11: 1015–1027, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kröncke KD. Zinc finger proteins as molecular targets for nitric oxide-mediated gene regulation. Antioxid Redox Signal 3: 565–575, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 159: 2001–2009, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarevich NL, Cheremnova OA, Varga EV, Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI, Engelhardt NV, Duncan SA. Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology 39: 1038–1047, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 276: G965–G974, 1999 [DOI] [PubMed] [Google Scholar]
- 21.McClain CJ, Marsano L, Burk RF, Bacon B. Trace metals in liver disease. Semin Liver Dis 1: 321–339, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Millan MS, Morris GP, Beck IT, Henson JT. Villous damage induced by suction biopsy and by acute ethanol intake in normal human small intestine. Dig Dis Sci 25: 513–525, 1980 [DOI] [PubMed] [Google Scholar]
- 23.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology, and pathophysiology of tight junctions. I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 279: G250–G254, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Montalto M, Cuoco L, Ricci R, Maggiano N, Vecchio FM, Gasbarrini G. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated and treated celiac disease. Digestion 65: 227–233, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 228: 882–890, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas, and liver gene expression by HNF transcription factors. Science 303: 1378–1381, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules, and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742–747, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS, Duncan SA. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet 34: 292–296, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Pizzuti D, Bortolami M, Mazzon E, Buda A, Guariso G, D'Odorico A, Chiarelli S, D'Incà R, De Lazzari F, Martines D. Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig Liver Dis 36: 337–341, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50: 638–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 447: 171–183, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Rao RK. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci 13: 7210–7226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part I. the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol Rev 54: 129–158, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278: 49239–49245, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Sladek FM, Zhong WM, Lai E, Darnell JE., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev 4: 2353–2365, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Stegmann A, Hansen M, Wang Y, Larsen JB, Lund LR, Ritié L, Nicholson JK, Quistorff B, Simon-Assmann P, Troelsen JT, Olsen J. Metabolome, transcriptome, and bioinformatic cis-element analyses point to HNF-4 as a central regulator of gene expression during enterocyte differentiation. Physiol Genomics 27: 141–155, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res 32: 355–364, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Tian J, Brown LA, Jones DP, Levin MS, Wang L, Rubin DC, Ziegler TR. Intestinal redox status of major intracellular thiols in a rat model of chronic alcohol consumption. J Parenter Enteral Nutr 33: 662–668, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid Redox Signal 3: 535–548, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Wilcox DE, Schenk AD, Feldman BM, Xu Y. Oxidation of zinc-binding cysteine residues in transcription factor proteins. Antioxid Redox Signal 3: 549–564, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. Role of zinc deficiency in alcohol-induced intestinal barrier disruption. Am J Physiol Gastrointest Liver Physiol 298: G625–G633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.