Abstract
Surgical interposition of distal ileum into the proximal jejunum is a bariatric procedure that improves the metabolic syndrome. Changes in intestinal and hepatic physiology after ileal interposition (transposition) surgery (IIS) are not well understood. Our aim was to elucidate the adaptation of the interposed ileum, which we hypothesized, would lead to early bile acid reabsorption in the interposed ileum, thus short circuiting enterohepatic bile acid recycling to more proximal bowel segments. Rats with diet-induced obesity were randomized to IIS, with 10 cm of ileum repositioned distal to the duodenum, or sham surgery. A subgroup of sham rats was pair-fed to IIS rats. Physiological parameters were measured until 6 wk postsurgery. IIS rats ate less and lost more weight for the first 2 wk postsurgery. At study completion, body weights were not different, but IIS rats had reversed components of the metabolic syndrome. The interposed ileal segment adapted to a more jejunum-like villi length, mucosal surface area, and GATA4/ILBP mRNA. The interposed segment retained capacity for bile acid reabsorption and anorectic hormone secretion with the presence of ASBT and glucagon-like-peptide-1-positive cells in the villi. IIS rats had reduced primary bile acid levels in the proximal intestinal tract and higher primary bile acid levels in the serum, suggesting an early and efficient reabsorption of primary bile acids. IIS rats also had increased taurine and glycine-conjugated serum bile acids and reduced fecal bile acid loss. There was decreased hepatic Cyp27A1 mRNA with no changes in hepatic FXR, SHP, or NTCP expression. IIS protects against the metabolic syndrome through short-circuiting enterohepatic bile acid recycling. There is early reabsorption of primary bile acids despite selective “jejunization” of the interposed ileal segment. Changes in serum bile acids or bile acid enterohepatic recycling may mediate the metabolic benefits seen after bariatric surgery.
Keywords: biliary enterohepatic recycling, bariatric surgery, ileal transposition, diabetes, hyperlipidemia
restrictive bariatric procedures such as sleeve gastrectomy and adjustable gastric bands, as well as procedures that are both restrictive and potentially malabsorptive such as Roux-en-Y-Gastric Bypass (RYGB), are currently the mainstay of surgical therapy for morbid obesity (8). A “lower intestinal hypothesis” has been postulated to explain the weight loss and multiple metabolic benefits seen with RYGB (4). This hypothesis suggests that enhanced delivery of nutrients to the distal intestine increases secretion of hind-gut anorectic signals, such as glucagon-like-peptide-1 (GLP-1), and peptide YY (PYY) (6). Procedures such as RYGB are complicated, and dissecting the mechanism(s) of these metabolic benefits is difficult on the background of reduced gastric volume and potential malabsorption. Recent reports suggest these same metabolic benefits, such as improved glucose tolerance, can be recapitulated without restricting gastric size or intestinal resection through a procedure termed ileal interposition surgery (IIS), which is sometimes referred to as ileal transposition (3, 5, 7, 25). IIS involves resecting a segment of distal ileum and repositioning it to the proximal jejunum in a properistaltic direction, while maintaining continuity of the gastrointestinal tract and preserving neurovascular connections (Fig. 1A).
Fig. 1.
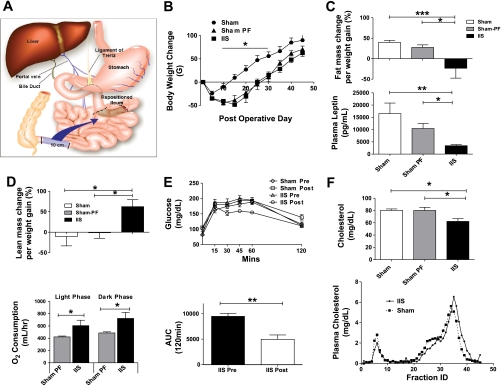
A: illustration depicting ileal interposition surgery. 10 cm of distal small intestine, starting 1 cm proximal to the ileo-cecal junction, is repositioned to the proximal jejunum. This is done by interpositioning the transected segment just beyond the ligament of Treitz. The segment is moved intact with all neurovascular connections and repositioned in a properistaltic direction. B: body weight change post surgery. Rats in the ileal interposition (IIS) surgery group lost more body weight compared with rats in the sham (SH) surgery group. There was a decrease in food intake in the IIS group. For groups IIS, SH, and SH-PF (sham surgery with pair-feeding to an IIS counterpart), n = 6, 7, 8, respectively (*P < 0.05). C: body fat mass change and plasma leptin levels. Magnetic resonance body composition analysis was performed on all rats prior to surgery and then subsequently a second time prior to death during the 5th wk after surgery. The weight gained by the SH and SH-PF groups had an increase in their proportion of fat tissue mass while IIS surgery group less fat mass as a percentage of new body weight gained compared with baseline. Plasma leptin levels were measured at completion of study. For groups IIS, SH, and SH-PF, n = 6, 7, or 8. (*P < 0.05; **P < 0.01; ***P < 0.001). D: body lean mass change and oxygen consumption. The weight gained by the SH and SH-PF groups had no change in their proportion of lean tissue mass, while the IIS surgery group had more lean mass as a percentage of new body weight gained compared with baseline. For groups IT, SH, and SH-PF, n = 6, 7, and 8, respectively. (*P < 0.05). Energy expenditure studies were conducted in the 5th wk after surgery. Oxygen consumption was higher in IIS rats compared with weight-matched SH-PF rats when observed in metabolic cages and given ad libitum access to a high-fat diet for 3 days (*P < 0.05; n = 7 per group). E: glucose tolerance after ileal interposition. Oral glucose tolerance tests were conducted prior to surgery (presurgery) and during the 5th wk after surgery (postsurgery). Rats in the IIS group had an improvement in their glucose tolerance with area under the curve (AUC) for 120 min being significantly less (**P < 0.01). F: plasma cholesterol levels and Fast-performance liquid chromatography (FPLC) fractions after ileal interposition. IIS rats lower plasma cholesterol levels compared with both SH and SH-PF controls. This was also a 25% reduction from presurgery circulating plasma cholesterol levels in the IIS group. For groups IIS, SH, and SH-PF, n = 7, 8, and 8, respectively. (*P < 0.05). Qualitative FPLC fraction analysis of plasma cholesterol lipoproteins heavier HDL particles were observed more in the IIS postsurgery rats (pooled plasma from n = 7 per group).
IIS in rats has been reported to produce weight loss, improve glucose tolerance (3, 16, 18, 22, 25), and increase synthesis and release of the “ileal-brake” hormones GLP-1 and PYY (25). In a recent clinical report of 69 adults with type 2 diabetes, the combination of IIS and sleeve gastrectomy resulted in weight loss and greatly improved hemoglobin A1C levels (7). Despite these various clinical and preclinical studies, the mechanisms underlying the reversal of type 2 diabetes in humans and rats seen after IIS and its broader implications for the “lower intestinal hypothesis” remain to be elucidated. Bile acids are critical for lipid absorption and the exclusive site of active bile acid reabsorption lies within that part of the ileum typically used in IIS (1, 11, 22, 23). There is a paucity of data on the adaptive response of the interposed ileal segment and the changes after IIS, if any, in the physiology of ileal bile acid transport and the impact of these adaptive responses on the comorbidities of obesity.
Rats develop obesity and features of the metabolic syndrome when fed a high-fat diet (29), providing an excellent model for determining mechanisms underlying the benefits of bariatric procedures. We performed IIS on high-fat diet-induced obese rats and hypothesized that there would be early and efficient reabsorption of bile acids in the interposed ileal segment. Given that bile acids can act as important signaling molecules in the intestine, brown adipose tissue, and liver, any modulation of their enterohepatic recycling could play a role in explaining the beneficial effects seen after bariatric surgery (17, 26, 28). Therefore, crucial to understanding the powerful effects of IIS to improve components of the metabolic syndrome is delineating the changes in the interposed segment and bile acid transport that accompany changing the ileum's position. The present experiment was conducted to fill these crucial gaps in our knowledge and specifically to understand the functional impact of these changes on the enterohepatic recycling of bile acids.
MATERIALS AND METHODS
Animals.
Adult male Long-Evans rats were ad libitum fed a high-fat, cholesterol-rich diet [41% calories from fat (major source-butter fat); Research Diets, New Brunswick, NJ] for 6 wk, then randomized into groups matched for age and weight to undergo IIS, sham (SH), or sham surgery with pair-feeding to an IIS counterpart (SH-PF). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and Cincinnati Children's Hospital Medical Center.
Surgeries and postoperative care.
Rats anesthetized with isoflurane were placed on a warm platform and for IIS a 10-cm segment of ileum was transected and repositioned just distal to the ligament of Treitz (Fig. 1A) (10). Intestinal biopsies performed at the time of surgery from the segment being interposed were saved for histology and immunohistochemistry (IHC). SH and SH-PF animals received intestinal transections and reanastamoses without change in location. Postoperatively, rats received analgesia for 2 days and had access to a liquid diet (Ensure, Abbott Laboratories, Chicago, IL, USA) for 5 days. They then again consumed a high-fat diet for 6 wk. Body weight and food intake were recorded daily. Each SH-PF rat was paired to an IIS rat and provided food amounts equivalent to the paired IIS rat's previous 24-h intake. Intestinal biopsies were performed at the time of death for histology, quantitative real-time PCR, and IHC.
Body composition analysis.
Echo MRI Whole Body Composition Analyzer (Echo Medical Systems, Houston, TX) (24) was performed on all rats prior to surgery and just prior to death.
Glucose tolerance tests.
Before surgery and just prior to death, rats were fasted overnight (8 h) and then administered a glucose load by oral gavage (1 g of 20% d-glucose). Baseline and test samples were obtained from the tail vein and assessed for blood insulin by a rat insulin ELISA kit (Crystal Chem, Downers Grove, IL) and glucose at 0, 15, 30, 45, 60, and 120 min after glucose administration with a One Touch Glucometer (LifeScan, Milpitas, CA).
Quantitative PCR.
Predesigned, validated gene-specific TaqMan probes were used for real-time PCR analysis. Relative expression was determined by comparison of dT values relative to GAPDH expression using the 2-DDCT method.
Plasma/serum assays.
Plasma leptin assay was performed on the Luminex 100 using the Milliplex MAP Adipokine Panel assay (Linco Research, St. Charles, MO). PYY measurement was performed using Millipore's PYY radioimmunoassay (RIA) kit (Linco Research, St. Charles, MO, USA). Serum bilirubin was measured with bilirubin reagent set (Pointe Scientific, Canton, MI). Active GLP-1 sandwich electrochemiluminescence immunoassay (Meso Scale Discovery, 2400 Imager) was used to detect the GLP-1(7–36) amide and GLP-1(7–37) (Meso Scale Discovery, Gaithersburg, MD). Serum bile acids were assayed using the 3α-hydroxysteroid dehydrogenase method. The reaction was measured at 546 nm using a Hitachi Chemistry 911 analyzer (Hitachi, Tokyo, Japan). Plasma cholesterol and triglycerides were measured by using enzymatic colorimetric assays (Infinity-LSR, Thermo Electron, Waltham, MA).
Lipoprotein separation.
Fast-performance liquid chromatography (FPLC) gel filtration was performed on a Pharmacia Smart System equipped with a Superose 6 column. Serum was pooled from each experimental group (n = 7 each), and 200 μl was loaded onto the column. The samples were collected into 45 fractions, and each fraction was analyzed for its cholesterol content using an enzymatic colorimetric assay (Infinity-LSR, Thermo Electron).
Bile acid analysis.
At death, liver, plasma, and intestinal intraluminal contents were collected directly into 90% ethanol from IIS and SH rats. These samples were homogenized, and bile acids were extracted for analysis by GC-MS. An aliquot was taken, and the internal standard nordeoxycholic acid was added. Bile acids were separated into groups on the basis of their state of conjugation, and fractions were then solvolyzed and hydrolyzed, and the methyl ester-trimethylsilyl ether derivatives were prepared for analysis by GC-MS, as previously described (19, 20). Serum bile acids were analyzed by LC-MS, as previously described (9, 13).
Western blot analysis.
Intestinal segments were homogenized, and equal samples loaded on SDS-polyacrylamide gel (Invitrogen, Carlsbad, CA) and probed for ASBT and GAPDH antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) using standard Western blot analysis methods (for further details, see Supplemental materials and methods in the online version of this article).
Energy expenditure studies.
Total energy expenditure and oxygen consumption were monitored using a combined open-circuit indirect calorimetry system (TSE Systems, Midland, MI). The rats were placed in the calorimetry system cages just prior to study completion. (for further details, see Supplemental materials and methods).
Histology and immunohistochemistry.
Standard IHC procedures were used with primary antibodies for anti-synaptophysin (Zymed, CA), anti-ASBT (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-GLP-1 (Bachem, Torrance, CA). The sections were then incubated with goat anti-rabbit Alexa 488, or goat anti-mouse Alexa 488 (Invitrogen, Carlsbad, CA) and viewed with the FITC filter on a standard fluorescence microscope. Positive cells and villi per section were counted by a blinded observer. The width and the height of 10 contiguous villi were measured, and the surface area was estimated using the formula for surface area of a cylinder.
Statistical analyses.
Body weight and food intake over the course of the experiment were analyzed by repeated-measures ANOVA. Cumulative food intake and oxygen consumption were compared with Student's t-test. Plasma and tissue markers, when compared across all three groups at one time point, were analyzed using one-way ANOVA with a post hoc Tukey test.
RESULTS
Body weight, food intake, and metabolic parameters.
Over the first 2 wk postsurgery, IIS rats lost more weight (7.7%) compared with SH rats (4.2%) (P = 0.002; IIS: n = 6, SH: n = 7, SH-PF: n = 8). Postprandial plasma PYY (413.56 ± 0.95.2 pg/ml; P < 0.001) and GLP-1 (13.6 ± 3.1 pg/ml; P = 0.04; IIS: n = 6, SH: n = 7, SH-PF: n = 8) were higher in IIS rats at death (see Supplemental Fig. 2 in the online version of this article), but food intake was lower in IIS rats (155.3 ± 11.0 g) compared with SH rats (204.2 ± 8.6 g) only for the first 2 wk postsurgery. Beyond this initial period, all rats consumed a similar amount (grams/day) of a high-fat diet, gained weight continuously, and at death, there was no significant difference in body weights among the groups (Fig. 1B and Supplemental Fig 1 in the online version of this article).
Over the 6 wk postsurgery, the SH and SH-PF groups had an increase in fat mass (SH: 33.6 ± 7.2 g and SH-PF: 19.0 ± 3.6 g), while the IIS group had a loss of fat mass (−4.1 ± 4.9 g) (see Supplemental Fig. 3 in the online version of this article). This change in fat mass translated to IIS rats having a lower percentage of their body weight gained as fat mass (−23.3 ± 24.2%) compared with SH rats (39.7 ± 4.7%; P < 0.001) or SH-PF rats (27.5 ± 6.3%; P < 0.05) (Fig. 1C; IIS: n = 6, SH: n =7, SH-PF: n = 8). This decreased fat mass in IIS rats was congruent with their lower plasma leptin levels (5,353 ± 1,966 pg/ml) relative to SH (16,512 ± 4,303 pg/ml) and SH-PF (9,569 ± 1,984 pg/ml) rats (Fig. 1C). The IIS group had an increase in lean mass as a percentage of body weight gained (62.4 ± 17.0%) compared with SH rats (−10.3 ± 23.0%; P < 0.05) or SH-PF rats (−1.08 ± 13.6%; P < 0.05) (Fig. 1D; IIS: n = 6, SH: n = 7, SH-PF: n =8). This increase in lean mass was congruent with increased oxygen consumption in IIS rats relative to SH-PF rats in both light (605 ± 88.3 vs. 422.3 ± 14.2 ml/h; P = 0.03) and dark phases of the day (722.6 ± 49.1 vs. 486.3 ± 18.7 ml/h; P = 0.01) (Fig. 1D; n = 7/group).
Glucose tolerance as calculated by the trapezoidal method area under the curve (AUC) was improved for IIS rats compared with before surgery (P < 0.01) (Fig. 1E; IIS: n = 6, SH: n = 7, SH-PF: n =8). There was also a 25% decrease in IIS plasma cholesterol levels, from 79.5 ± 8.6 mg/dl at surgery to 62.3 ± 4.5 mg/dl at study completion (P = 0.009). On FPLC analysis of cholesterol fractions, the IIS rats had lower peaks in both the high-density lipoprotein (HDL) and very low density lipoprotein fractions with a right-shifted curve, indicating an increase in the number of heavier HDL particles (Fig. 1F; Pooled plasma from n = 7 per group). Thus, despite weight gain, the IIS rats had improved metabolic parameters at death.
Adaptation of the interposed ileal segment.
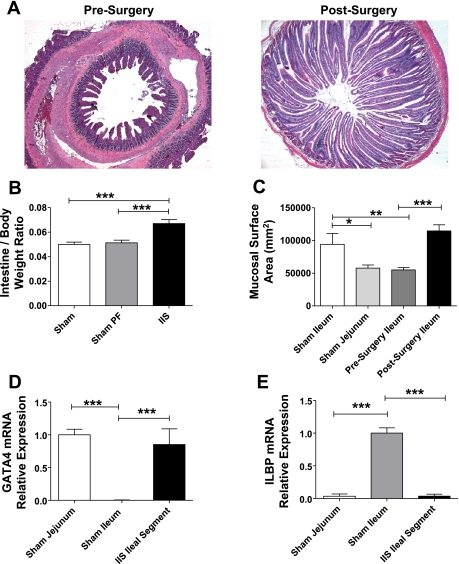
At study completion, there was a significant increase in length and number of villi in the interposed ileal segment compared with the native ileum at surgery (Fig. 2A). The overall intestinal weight at death was increased in the IIS group (36.81 ± 1.8 g) compared with that of the SH group (27.85 ± 1.7 g) and SH-PF groups (29.32 ± 1.1 g; P = 0.0018). The ratio of intestine weight to body weight was increased in the IIS rats compared with SH and SH-PF rats (P < 0.001) (Fig. 2B; IIS: n = 6, SH: n = 7, SH-PF: n = 8). Further, the calculated surface area of the interposed ileal segment was comparable to that of normal jejunum (Fig. 2C; IIS: n = 6, SH: n = 7, SH-PF: n = 8). At the mRNA level, the proximal intestinal marker, GATA4 was increased in the interposed ileal segment compared with the baseline of normal ileum (Fig. 2D; IIS: n = 7, SH: n = 8). A reduction in mRNA of the ileal specific marker, ileal lipid binding protein, was seen in the interposed ileal segment compared with normal ileum (Fig. 2E; IIS: n = 7, SH: n = 8). Together, these features demonstrate that the interposed ileal segment underwent postsurgical adaptation to a more jejunal-like histological and gene expression phenotype.
Fig. 2.
A: histological adaptation of the interposed segment. Photomicrographs (×10 magnification) of hematoxylin-and-eosin-stained sections of the same segment of ileum first at the time of surgery and then at the time of death 6 wk later. There is marked increase in the length and number of villi in the IIS rats. B: intestine-to-body weight ratios. Intestine weight normalized for body weight of the rat in IIS rats postsurgery was increased in IIS rats. For groups IIS, SH, and SH-PF, n = 6, 7, and 8, respectively (***P < 0.001). C: surface area of jejunum and ileum at surgery and study completion. Surface area of intestinal segments was estimated by using a formula of a cylinder and the ileal segment adapted to its new proximal environment to increase its surface area within 6 wk to jejunal levels. For groups IIS, SH, and SH-PF, n = 6, 7, and 8, respectively (*P < 0.05; **P < 0.01; ***P < 0.001). D: adaptation of epithelial jejunal specific gene GATA4 mRNA levels of proximal intestinal marker GATA4 were measured by RT-PCR and expressed in relative expression units to GAPDH (***P < 0.001). For groups IIS and SH, n =7 and 8, respectively. E: adaptation of epithelial ileal specific gene ILBP. mRNA levels of distal intestinal marker ILBP were measured by RT-PCR and expressed in relative expression units to GAPDH. (***P < 0.001). For groups IIS and SH, n = 7 and 8, respectively.
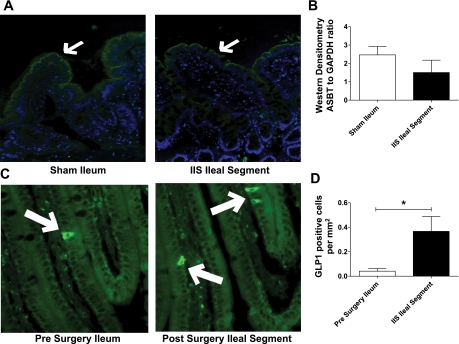
Additional characteristic features of the ileum included changes in the active bile salt reabsorption protein apical sodium bile acid transporter (ASBT) and enteroendocrine cells that secrete GLP-1. ASBT protein expression was demonstrated by IHC on both native ileum and the interposed ileal segment at death (Fig. 3A). This was further confirmed by quantitative densitometry of Western blots for ASBT showing that the interposed ileal segment had comparable protein levels to that of native ileum (Fig. 3B; IIS: n = 7, SH: n = 8). Ileal segment and native ileum that stained positive for GLP-1-containing cells (Fig. 3C), and there were more cells per surface area staining positive for GLP-1 in the interposed ileal segment compared with the native ileum (P < 0.05; Fig. 3D; IIS: n = 6, SH: n = 7, SH-PF: n = 8). These ASBT/GLP data, together with the histological and ILBP/GATA4 changes, suggest that the interposed ileal segment underwent a selective jejunization postsurgery.
Fig. 3.
A: protein estimation for apical sodium bile acid transporter (ASBT) in ileal segment by immunohistochemistry (IHC). SH ileum and IIS ileal segment protein extracts probed for ASBT by IHC. Representative photomicrograph sections are shown with white arrows at ASBT fluorescence. B: protein estimation for ASBT in ileal segment by Western blot analysis. Quantification of Western blots was done by densitometry ratios of ASBT to GAPDH (for groups IIS and SH, n = 7 and 8, respectively). C: glucagon-like-peptide-1 (GLP-1) staining in ileum and ileal segment at study completion. Photomicrographs (×20 magnification) of histological sections stained for enteroendocrine cell marker GLP1 (positive cells stain green by fluorescent IHC). More positive stained cells can be seen in the transposed segment after surgery. D: quantification of GLP-1-positive cells per surface area. Positive cells quantified by single unbiased observer. For group IT, SH, and SH-PF, n = 6, 7, and 8, respectively (*P < 0.05).
Fecal and small intestinal content bile acid composition.
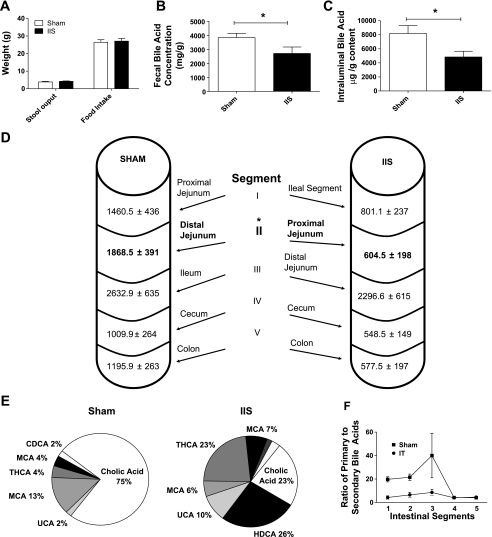
In the week prior to study completion, daily food intake, and stool output (Fig. 4, A and B; IIS: n = 6, SH-PF: n = 8) were not different between IIS and SH groups though IIS rats had lower fecal total bile acid concentrations (2,703 ± 457 μg/g) compared with SH rats (3,832 ± 305 μg/g; P = 0.04) (Fig. 4B; IIS: n = 7, SH: n = 7). At study completion, the total quantity of intraluminal chymal bile acids was also found to be lower in IIS rats (4,828 ± 815 μg) compared with SH rats (8,168 ± 1,126 μg; P = 0.04) (Fig. 4C; IIS: n = 6, SH: n = 7).
Fig. 4.
A: stool output and food intake prior to study completion. In the week prior to study completion, stool output and food intake data were collected for 48 h. Average food intake and stool output per rat are plotted. For groups IIS and SH, n = 6 and 8, respectively. B: fecal bile acid concentration. Fecal bile acid concentration was measured by LC-MS technology in collected pellets described in A. There was reduced fecal bile acid concentration in the IIS group of rats. For groups IIS and SH, n = 7 and 7, respectively. (*P < 0.05). C: total intraluminal bile acid content. Bile acid content in intraluminal content was quantified using GC-MS and found to be reduced overall in the IIS rats postsurgery. D: segmental intraluminal bile acid content. Intraluminal chymal contents were further divided into five segments as detailed in the diagram and were lower in the segment just following the IIS interposed ileal segment (segment II) compared with corresponding SH segment (highlighted in bold). For groups IIS and SH, n = 6 and 7, respectively (P < 0.05). E: compositional analysis of intraluminal content. Pie chart of bile acid composition analysis for Segment I in SH and IIS rats. Percentage for cholic acid (CA), ursocholic acid (UCA), β murocholic acid (β MCA), tetrahydroxy hyocholic acid (THCA), and muricholic acid (MCA) are shown. For groups IIS and SH, n = 6 and 7, respectively (*P < 0.05). F: primary-to-secondary bile acid ratio. Primary bile acid [cholic acid (CA), chenodeoxycholic acid (CDCA), and β-muricholic acid (β MCA)] to secondary bile acid ratio was calculated for all five intraluminal intestinal segments. There was reduced ratio seen in IIS rats for Segments I and II with similar ratios seen in segments IV and V. For groups IIS and SH, n = 6 and 7, respectively.
The distribution of bile acids quantitatively and qualitatively differed along the length among the groups when the analysis of the intraluminal content of subsections of intestine was performed (numbered I-V caudal to distal) (Fig. 4D; IIS: n = 6, SH: n = 7). The quantity of bile acids in segment II in IIS rats (proximal jejunum: segment just distal to the interpositioned ileal segment) was lower (604.5 ± 198 μg) compared with its complementary segment (distal jejunum) in SH rats (1,868.5 ± 391 μg; P < 0.01) (Fig. 4D).
Compositional analysis of intestinal bile acids in IIS rats revealed a lower percentage of primary bile acids [cholic acid (CA), chenodeoxycholic acid (CDCA), and β-muricholic acid (β-MCA)] in segments I–III (79.33%, 83.91%, and 88.99%, respectively) compared with SH rats (94.67%, 94.85%, 96.35%) (P = 0.007; Fig. 4E; IIS: n = 6, SH: n =7). The largest contribution to the intraluminal bile acids was from CA in both IIS and SH rats, and it was significantly lower for IIS rats in both segment I (CA: IIS, 239.3 ± 82.9 μg/g; SH, 1,041.3 ± 309.5 μg/g; P = 0.04) and segment II (CA: IIS, 248.9 ± 113.9 μg/g; SH, 1,312 ± 285.5 μg/g; P = 0.008). The primary-to-secondary bile acid ratios for intestinal segments IV or V (cecum and colon) were similar among IIS and SH rats. Together, these observations produce a reversal of the normal small intestinal ratio of primary to secondary bile acids such that intestinal segments I–III in IIS rats (interposed ileal segment, proximal jejunum, and distal jejunum) had a lower ratio of primary to secondary bile acids compared with SH intestinal segments I–III (proximal jejunum, distal jejunum, and native ileum) (Fig. 4F; IIS: n = 6, SH: n = 7). This significantly altered quantity and composition of intestinal bile acids in IIS rats suggest an early and efficient reabsorption of primary bile acids by the interposed ileal segment of IIS rats.
Serum and hepatic bile acid metabolism.
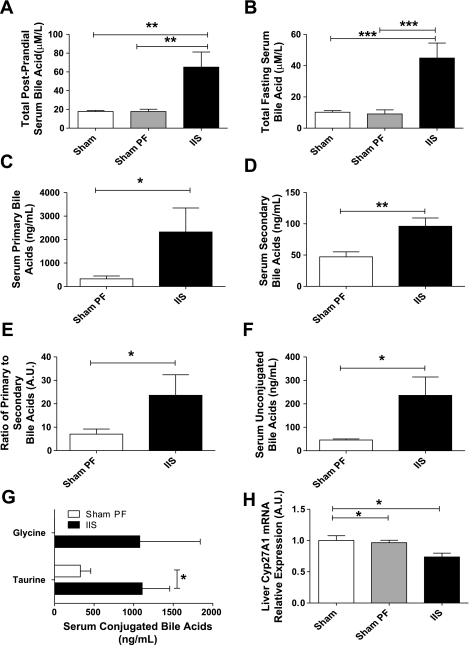
Postprandial (45 min after a fixed liquid meal) serum total bile acid levels were higher in the IIS group (65.2 ± 16.1 μM/l) compared with SH (17.8 ± 1.2 μM/l) and SH-PF rats (17.7 ± 2.5 μM/l) (P = 0.0008; Fig. 5A; IIS: n = 6, SH: n = 7, SH-PF: n = 8). Similarly, IIS rats had higher fasting serum total bile acid levels compared with SH and SH-PF (P = 0.0003; Fig. 5B; IIS: n = 6, SH-PF: n = 8). IIS rats had higher levels of both primary (IIS: 2,324 ± 1,025 ng/ml; SH: 323.5 ± 126.2 ng/ml; P = 0.04; Fig. 5C; IIS: n = 6, SH-PF: n = 8) and secondary bile acids (IIS: 96.00 ± 13.27 ng/ml; SH: 47.14 ± 8.1 ng/ml; P = 0.007) compared with SH-PF rats (Fig. 5D; IIS: n = 6, SH-PF: n = 8). Of note, the relative levels of primary bile acids were much higher in magnitude compared with secondary bile acids in either group. The ratio of primary to secondary serum bile acids in serum was also higher in IIS compared with SH-PF rats (IIS: 23.61 ± 8.7; SH-PF: 7.050 ± 2.2; P = 0.05; Fig. 5E; IIS: n = 6, SH-PF: n = 8). Further, IIS rats had increased levels of unconjugated bile acids (IIS: 236.0 ± 78.4 ng/ml; SH-PF: 45.7 ± 4.8 ng/ml; P = 0.02; Fig. 5F; IIS: n = 6, SH-PF: n = 8). There was also an increase in taurine-conjugated bile acids in the IIS rats (IIS: 1,108 ± 347.8 ng/ml; SH-PF: 324.9 ± 131.8 ng/ml; P = 0.04) with a novel appearance of glycine-conjugated serum bile acids that were absent in SH rats (IIS: 792.9 ± 558.3 ng/ml; SH-PF: none detected; Fig. 5G; IIS: n = 6, SH-PF: n = 8).
Fig. 5.
A: serum postprandial bile acid level. Serum was collected after an overnight fast and 45 min after a fixed meal for postprandial total serum bile acid levels. These were significantly higher in rats that were in the IIS group compared with SH or SH-PF groups. For groups IIS, SH, and SH-PF; n = 6, 7, and 8, respectively (**P < 0.01). B: serum fasting bile acid level. Fasting serum bile acid levels were significantly higher in rats that were in the IIS group compared with SH or SH-PF groups. For groups IIS and SH-PF, n = 6 and 8, respectively (**P < 0.01). C: serum primary bile acid level. Postprandial serum primary bile acid levels by LC-MS were significantly higher in rats that were in the IIS group compared with SH-PF group. For groups IIS and SH-PF, n =6 and 8, respectively (*P < 0.05). D: serum secondary bile acid level. Postprandial serum secondary bile acid levels by LC-MS were significantly higher in rats that were in the IIS group compared with SH-PF group. For groups IIS and SH-PF, n = 6 and 8, respectively. (**P < 0.01). E: serum primary to secondary bile acid ratio. Primary bile acid CA, CDCA, and β MCA to secondary bile acid ratio by LC-MS was reduced in IIS rats. For groups IIS and SH-PF, n = 6 and 8, respectively (*P < 0.05). F: serum unconjugated bile acid level. Postprandial serum unconjugated bile acid levels by LC-MS were significantly higher in rats that were in the IIS group compared with SH-PF group. For IIS and SH-PF groups, n = 6 and 8, respectively. (*P < 0.05). G: serum conjugated bile acids level. Postprandial serum-conjugated bile acid (taurine and glycine conjugated) levels by LC-MS were significantly higher in rats that were in the IIS group compared with SH-PF group. There were no glycine-conjugated bile acids measured in the SH-PF group. For groups IIS and SH-PF, n = 6 and 8, respectively (*P < 0.05). H: liver mRNA levels of bile acid production regulating enzymes. The mRNA expression of Cyp7A1 was not different between groups but Cyp27A1 that regulates the alternate pathway of bile acid production was suppressed in the IIS rats compared with the SH and SH-PF rats. For groups IIS, SH, and SH-PF, n = 7, 8, and 8, respectively. (*P < 0.05).
In the liver, Cyp27A1 mRNA level was lower in IIS rats compared with SH and SH-PF rats postsurgery (Fig. 5H; P < 0.05; IIS: n = 6, SH-PF: n = 8), while no difference was detected in mRNA expression of FXR, SHP, Cyp7A1, or Cyp8B1 (see Supplemental Fig. 4 in the online version of this article). mRNA expression of the major transporters regulating hepatocyte uptake and export of bile acids (sodium taurocholate cotransporting polypeptide, NTCP, and bile salt export pump, BSEP) were also not different (see Supplemental Fig. 4 in the online version of this article). Thus, IIS rats had significantly higher levels of circulating serum bile acids, with their composition analysis demonstrating an increase in conjugation activity compared with SH rats. These data, together, suggest an early and efficient reabsorption of primary bile acids by the interposed ileal segment of IIS rats, leading to suppression of the alternative bile acid production pathway in the liver.
DISCUSSION
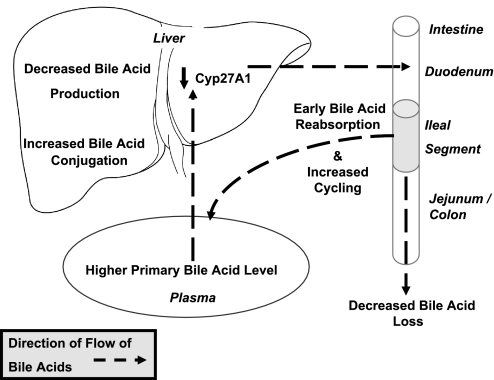
Our experiment focused on the mechanistic role of ileal adaptation and bile acid enterohepatic recycling in IIS's potent ability to improve multiple aspects of the metabolic syndrome. Rats after IIS had a short-term reduction in food intake with commensurate loss in body weight immediately after surgery. IIS rats then regained weight but gained more lean mass and less fat mass than controls. We also saw weight gain-independent improvements in glucose tolerance and “selective” adaptation of the ileal segment. The repositioned segment increased its surface area but retained its bile acid absorptive capacity, while increasing the density of enteroendocrine cells. To summarize our results, IIS short-circuits the normal enterohepatic recycling of bile acids, with weight and energy-intake-independent improvement in body composition, plasma cholesterol, and glucose tolerance (Fig. 6).
Fig. 6.
Schematic of bile acid and cholesterol physiology in IIS rats.
Most bariatric procedures significantly alter intestinal anatomy and, therefore, alter the enterohepatic recycling of bile acids. Patients postbariatric surgery have higher serum bile acid concentrations, and these elevated bile acid levels correlate inversely with glucose and triglyceride levels and directly with GLP-1 levels (14, 17). Bile acids are also signaling molecules for metabolic targets, including the G protein-coupled receptor TGR5 (2) and for intestinal GLP-1 secretion (26). How bariatric surgery results in elevated bile acid levels and what role the altered enterohepatic recycling plays in the observed metabolic improvements is, however, not well understood. The distal ileum has the highest expression of the bile acid transporter ASBT and is the usual site for the greatest proportion of active bile acid reabsorption (21). The interposition of a segment of ileum to a more proximal location, in animals or humans, produces improvements in insulin resistance and weight loss (7, 10, 15, 18, 27). There have been no previous reports on the adaptive response of the interposed segment nor on the influence this has on bile acid homeostasis. The present experiment was conducted to better define these gaps in our knowledge and specifically to understand the adaptive response of the interposed ileal segment and its impact on bile acid enterohepatic recycling.
The induction of GLP-1 by increased serum bile acids has been shown to improve liver and pancreatic function and enhance glucose tolerance in obese mice (26). In addition, given the recent data that serum bile acids are higher in humans with prior gastric bypass (17), our findings of elevations in serum bile acids, GLP-1 and increased oxygen consumption in IIS rats merit closer analysis. While we did not observe any change in brown adipose tissue TGR5 mRNA levels, we did find elevated serum bile acid levels and signs consistent with increased energy expenditure. Our energy expenditure data are also consistent with the increased lean tissue mass observed in IIS rats. Locomotor activity levels were similar in SH-PF and IIS rats, suggesting that the increased oxygen consumption rates after IIS were not due to increased activity-induced thermogenesis, raising the possibility that bile acids are acting as signaling molecules through altered levels of ileal hormones (GLP-1, PYY) with effects on energy partitioning.
Investigating the status of bile acids within the intestinal tract, we found a decrease in intraluminal bile acid concentration distal to the interposed ileal segment, specifically of the primary bile acids. Further, we found an increased circulating level of total and primary bile acids in serum along with an increase in extent of taurine and glycine bile acid conjugation by the liver. Interestingly, the intraluminal bile acids quantity in the distal segments of the IIS rats was higher again after the decrease seen after the interposed segment. We speculate that the interposed segment may have altered motility, thus explaining this finding. Together, these data in IIS rats suggest an efficient proximal reabsorption of primary bile acids by the interposed ileal segment and consequently shorter and more frequent bile acid recycling (Fig. 6). Endogenous bile acid production is regulated closely by bile acids, which act as ligands for farnesoid X receptor (FXR), and influence the regulation of bile acid synthesis in the classical (Cyp7A1) and acidic pathways (Cyp27A1) (11). We found mRNA of Cyp27A1 was downregulated in IIS rat livers, suggesting a decrease in bile acid production through the acidic pathway. Selective suppression of Cyp27A1, as has been reported in the hamster liver, leads to decreased production of chenodeoxycholic acid (12).
GATA factors together are now understood to play important roles in determining regional identity in intestinal programming. Our data regarding suppression of GATA4 in the interposed ileal segment (Fig. 3D) suggest that changes in GATA factors may be part of a process in play in our experiment. Since the “jejunization” in our experiment was not complete, we speculate that there may be other (non-GATA) factors that might be important in determining jejunal identity. In summary, our bile acid and selective jejunization data together indicate a short-circuiting of the enterohepatic bile acid recycling by the interposed ileal segment, resulting in elevated levels of bile acids in the plasma and a decrease in hepatic bile acid production through the acidic pathway.
In conclusion, our data provide novel insights into mechanisms behind the metabolic improvements seen after bariatric surgeries. In our experiment, IIS rats alone had metabolic improvements and not their pair-fed sham-operated controls. The unique difference between IIS and pair-fed shams was that the IIS rats had a short-circuited enterohepatic bile acid recycling with increased levels of serum taurine and glycine conjugated and primary bile acids. These data highlight the potential mechanistic role for circulating bile acids in the metabolic benefits seen after bariatric surgery. Further studies along this path may help uncover therapeutic targets that could reproduce these metabolic benefits seen after bariatric surgical procedures in a much more scalable and noninvasive manner.
GRANTS
This work was supported by the National Institutes of Health NICHD K12 HD028827 (to R. Kohli), National Institute of Diabetes and Digestive and Kidney Diseases 1K08DK084310 (to R. Kohli), Children's Digestive Health and Nutrition Foundation-George Ferry Young Investigator Award (to R. Kohli) and Ethicon Endosurgery, Johnson and Johnson, Cincinnati, Ohio (to R. Kohli and R. J. Seeley). This work also received support from the Cincinnati Digestive Health Center-Public Health Service Grant P30 DK078392.
DISCLOSURES
Randy J. Seeley is a consultant for Amylin Pharmaceuticals, Eli Lilly, and Johnson & Johnson, Zafgen, and Merck. Rohit Kohli and Matthias H. Tschoep are consultants for Johnson & Johnson.
Supplementary Material
REFERENCES
- 1.Baker RD, Searle GW. Bile salt absorption at various levels of rat small intenstine. Proc Soc Exp Biol Med 105: 521–523, 1960 [DOI] [PubMed] [Google Scholar]
- 2.Baxter JD, Webb P. Metabolism: bile acids heat things up. Nature 439: 402–403, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Boza C, Gagner M, Devaud N, Escalona A, Munoz R, Gandarillas M. Laparoscopic sleeve gastrectomy with ileal transposition (SGIT): A new surgical procedure as effective as gastric bypass for weight control in a porcine model. Surg Endosc 22: 1029–1034, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, Baskin DG, Havel PJ. Ileal Interposition Surgery Improves Glucose and Lipid Metabolism and Delays Diabetes Onset in the UCD-T2DM Rat. Gastroenterology 138: 2437–2446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings DE, Overduin J, Foster-Schubert KE, Carlson MJ. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis 3: 109–115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Paula AL, Macedo AL, Prudente AS, Queiroz L, Schraibman V, Pinus J. Laparoscopic sleeve gastrectomy with ileal interposition (“neuroendocrine brake”)–pilot study of a new operation. Surg Obes Relat Dis 2: 464–467, 2006 [DOI] [PubMed] [Google Scholar]
- 8.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med 356: 2176–2183, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Hagio M, Matsumoto M, Fukushima M, Hara H, Ishizuka S. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J Lipid Res 50: 173–180, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Koopmans HS, Sclafani A. Control of body weight by lower gut signals. Int J Obes 5: 491–495, 1981 [PubMed] [Google Scholar]
- 11.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126: 322–342, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki Y, Bouscarel B, Ikegami T, Honda A, Doy M, Ceryak S, Fukushima S, Yoshida S, Shoda J, Tanaka N. Selective inhibition of CYP27A1 and of chenodeoxycholic acid synthesis in cholestatic hamster liver. Biochim Biophys Acta 1588: 139–148, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Melgarejo T, Williams DA, O'Connell NC, Setchell KD. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig Dis Sci 45: 407–414, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 58: 1400–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Ohneda A, Tsuchiya T, Naito H, Sasaki I, Ohneda M, Kawamura T. Increased plasma glucagon-like immunoreactivity in dogs with ileojejunal transposition. Tohoku J Exp Med 162: 95–108, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C, Donini A. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 15: 1258–1264, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 17: 1671–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabench Pereferrer F, Hernandez Gonzalez M, Blanco Blasco S, Sanchez Marin A, Morandeira Rivas A, Del Castillo Dejardin D. [The effects of ileal transposition, gastrojejunal bypass and vertical gastroplasty on the regulation of ingestion in an experimental obesity model associated with diabetes mellitus type 2]. Cir Esp 85: 222–228, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Setchell KD, Dumaswala R, Colombo C, Ronchi M. Hepatic bile acid metabolism during early development revealed from the analysis of human fetal gallbladder bile. J Biol Chem 263: 16637–16644, 1988 [PubMed] [Google Scholar]
- 20.Setchell KD, Lawson AM, Tanida N, Sjovall J. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J Lipid Res 24: 1085–1100, 1983 [PubMed] [Google Scholar]
- 21.Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr 32: 407–417, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Strader AD. Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav 88: 277–282, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Strader AD, Clausen TR, Goodin SZ, Wendt D. Ileal interposition improves glucose tolerance in low-dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg 19: 96–104, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Strader AD, Shi H, Ogawa R, Seeley RJ, Reizes O. The effects of the melanocortin agonist (MT-II) on subcutaneous and visceral adipose tissue in rodents. J Pharmacol Exp Ther 322: 1153–1161, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288: E447–E453, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thulesen J, Hartmann B, Kissow H, Jeppesen PB, Orskov C, Holst JJ, Poulsen SS. Intestinal growth adaptation and glucagon-like peptide 2 in rats with ileal–jejunal transposition or small bowel resection. Dig Dis Sci 46: 379–388, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.