Abstract
Face recognition is a complex cognitive process that requires distinguishable neuronal representations of individual faces. Previous functional magnetic resonance imaging (fMRI) studies using the “fMRI-adaptation” technique have suggested the existence of face-identity representations in face-selective regions, including the fusiform face area (FFA). Here, we present face-identity adaptation findings that are not well explained in terms of face-identity representations. We performed blood-oxygen level–dependent (BOLD) fMRI measurements, while participants viewed familiar faces that were shown repeatedly throughout the experiment. We found decreased activation for repeated faces in face-selective regions, as expected based on previous studies. However, we found similar effects in regions that are not face-selective, including the parahippocampal place area (PPA) and early visual cortex (EVC). These effects were present for exact-image (same view and lighting) as well as different-image (different view and/or lighting) repetition, but more widespread for exact-image repetition. Given the known functional properties of PPA and EVC, it appears unlikely that they contain domain-specific face-identity representations. Alternative interpretations include general attentional effects and carryover of activation from connected regions. These results remind us that fMRI stimulus-change effects can have a range of causes and do not provide conclusive evidence for a neuronal representation of the changed stimulus property.
Keywords: attention, face-identity, fMRI-adaptation, fusiform face area, neuronal representation
Face recognition is an important function of the human visual system. It requires the existence of distinct neuronal representations of individual faces (Puce et al. 1995; Kanwisher et al. 1997; Haxby et al. 2000). These representations have been investigated with the “fMRI-adaptation” method (Grill-Spector and Malach 2001). Several studies using this method (Gauthier et al. 2000; Andrews and Ewbank 2004; Rotshtein et al. 2005) have shown a stronger functional magnetic resonance imaging (fMRI) response to face-identity change than to face-identity repetition in face-selective brain regions, including the fusiform face area (FFA) (Puce et al. 1995; Kanwisher et al. 1997). FFA is defined by its stronger response to faces than to other objects, consistent with a role in detecting the presence of faces (Kanwisher et al. 1997). The fMRI-adaptation results have been interpreted as evidence for the involvement of FFA in representing face identity (see also Grill-Spector et al. 2004). Attempts to directly decode face identity from FFA activity patterns have failed so far, although they succeeded in anterior inferior temporal cortex (aIT) (Kriegeskorte et al. 2007).
Here, we replicate the stronger FFA response to face-identity change than to repetition and examine whether these effects are specific to FFA and other face-selective regions or more widespread. Previous studies might have missed effects outside of face-selective cortex because of spatially restricted analyses or lack of statistical power (but see Pourtois et al. 2005; Ng et al. 2006).
Face-identity repetition effects have been found to be influenced by face familiarity (George et al. 1999; Henson et al. 2002; Eger et al. 2005). The influence of face familiarity has also been investigated by directly comparing activation to familiar with activation to unfamiliar faces, showing modulation of activity by face familiarity in FFA (Henson et al. 2000; Gobbini et al. 2004), aIT (Sergent et al. 1992; Gorno Tempini et al. 1998; Sugiura et al. 2001), consistent with lesion studies (Evans et al. 1995; Marotta et al. 2001), and hippocampus (Leveroni et al. 2000; Bernard et al. 2004; Eger et al. 2005). Familiar-face stimuli used in previous studies were either famous faces or faces of personal acquaintances. Identification of such a face involves both recognition of the perceptual appearance of the face and activation of associated conceptual information such as name and biographical facts (Bruce and Young 1986; Haxby et al. 2000). Previous studies, therefore, did not dissociate the perceptual (looks) and conceptual (biographical information) components of face recognition.
The present study investigates the effects of face-identity repetition and face familiarity on activation in human inferior temporal cortex using a continuous carryover design (Aguirre 2007). Participants were shown faces of different levels of familiarity: “new” (never seen before), “seen” (seen previously, no further information known), and “known” (seen previously, name and biography known). We used images of 4 different perceptually familiar individuals; 2 of them were also biographically familiar. These 4 familiar face identities (2 seen and 2 known) were repeated throughout the experiment to investigate the effects of consecutive face-identity repetition. The subjects’ task was to classify each face image as “new” or “familiar” (either seen or known). The task, thus, diverted attention from differences among the familiar faces. We investigated activity in face-selective as well as non-face–selective regions and also searched for effects outside these regions of interest (ROIs). For optimal stimulus control, we used renderings of textured 3D face models constructed from face photos. Non–face features were masked out, and color histograms equalized to minimize low-level confounds (Fig. 1). View and lighting were varied for each face identity. This allowed us to compare the effects of different-image repetition to those of exact-image repetition.
Figure 1.
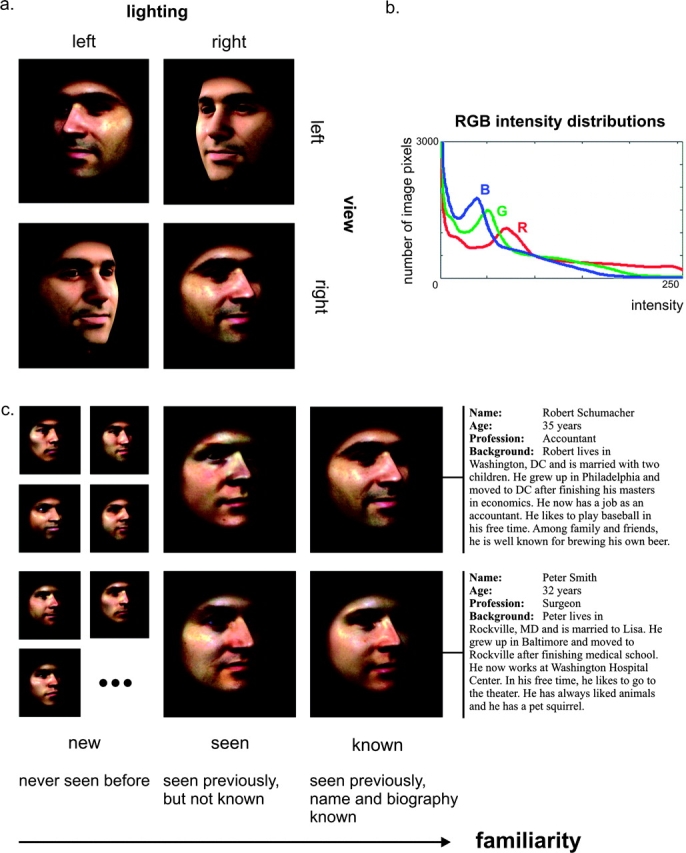
Stimuli. (a) Stimuli were male faces seen from 2 different views and with 2 different lightings. The lower left and upper right faces are counterlit (incongruent view and lighting). (b) Averaged histograms for red, green, and blue (RGB) color channels that were imposed on each image. As a consequence of the color histogram equalization, the images have the same light and spatial-signal energy. (c) Face familiarity was systematically varied, resulting in new, seen, and known faces. The 7 new faces that are shown are a subset of a total of 180 new faces that were used in this study. The 4 familiar faces are the 4 right-most faces.
We expected face-identity repetition effects to be confined to face-selective regions. In addition, we expected perceptual face regions, including the occipital face area (OFA) and FFA, to be equally activated by seen and known faces, and higher-level regions, including aIT and hippocampus, to show stronger activation for known than seen faces.
Materials and Methods
Participants
Eight healthy male volunteers aged between 29 and 40 years (mean age = 34 years) participated in this study. All participants were right-handed and had normal or corrected to normal vision. None of the participants had a history of neurological or psychiatric disorder. Before scanning, the participants received information about the procedure of the experiment and gave their written informed consent for participating. All experiments were conducted in accordance with standards of the Institutional Review Board of the National Institutes of Mental Health, Bethesda, MD.
Stimuli
Stimuli
We took colored photographs of male faces and used FaceGen Modeller 3.1 (Singular Inversions, Vancouver, Canada) to generate 3D face models from these static frontal and profile face photographs. This software uses a morphable face model consisting of a vector space representation of the shape and texture of several 3D face model examples (Blanz and Vetter 1999). Snapshots of the 3D face models were extracted, using combinations of 2 different views (−30 and 30 degrees relative to the sagittal plane) and 2 different lightings (perspective projection, −60 and 60 degrees relative to the sagittal plane, elevation of 30 degrees relative to the center of the head). Subsequently, the snapshots were masked with a soft-faded circular aperture and color histogram equalized, so that the distribution of intensity values in each color channel (red, green, and blue [RGB]) was identical across stimuli (Fig. 1b). This procedure resulted in 24-bit RGB images of 701 × 701 pixels, including the face and part of the neck (Fig. 1a). No hair or ears were shown. All processing steps on the snapshots of the 3D face models were performed using Matlab 7.0 (The MathWorks Inc, Natick, MA). Four male identities were chosen to function as familiar faces (2 seen and 2 known faces). New faces were generated using photographs of males other than the 4 chosen ones and by manipulating 3D face models in FaceGen to create new face identities (Fig. 1c).
Stimulus Presentation
Stimuli were presented using Presentation 9.81 (Neurobehavioral Systems Inc, Albany, CA) and projected onto a translucent screen positioned at the foot of the scanner (at the participants’ feet), using a liquid-crystal display projector. A mirror fixated on the head reception coil enabled participants to see the screen. Face images subtended a vertical visual angle of ∼4 degrees.
Experimental Design and Task (Face Experiment)
Prescanning Training
The participants were familiarized with the stimuli and task 1 or 2 days before scanning. The prescanning training consisted of one 30-min session during which the participants were familiarized with frontal and ±30-degree views of the 4 familiar faces, 2 of which were accompanied by biographical information that needed to be memorized (Fig. 1c). The biographical information was fictive, but realistic, and consisted of name, age, profession, and personal background. The 2 descriptions were matched for information density. In order to ensure that effects were not due to the particular face images used in the different familiarity conditions, half of the participants received the biographical information with one pair of faces and the other half with the other pair. Participants were instructed to closely examine the 4 familiar faces and memorize the biographical information associated with 2 of them. In order to equilibrate visual exposure to seen and known faces, we explicitly asked the subjects to spend an equal amount of time inspecting each of the 4 face images. After a learning period of 10 min, the participants received 18 5-option multiple-choice items and a perceptual face-familiarity test (see Supplementary Material) to check whether their perceptual and conceptual knowledge levels were at a sufficient and stable level (accuracy > 85% per test). Participants that had not reached a sufficient level of familiarity after the first learning period examined the 4 familiar faces again and were then re-tested. Errors made during the tests were reviewed and correct answers were provided. The last 10 min of the prescanning training were used to practice the task to be performed in the scanner (see Task). At the beginning of each subsequent scanning session, participants performed another brief learning session and face-familiarity test to refresh their memories.
Face Experiment
A rapid event-related design was used with a stimulus duration of 1 s and interstimulus interval (ISI) of 3 s. A single scanning session consisted of 6 functional runs of 126 trials each (8 min and 24 s per run). These 126 trials consisted of 48 known, 48 seen, and 10 new face trials and 20 baseline trials where no stimulus was shown. The contrasts between the different face types were our main contrasts of interest and were assumed to be smaller than the contrast between faces and baseline. Therefore, we included more face trials than baseline trials. Stimuli were presented in pseudorandom order. Each of 16 different familiar face images (the 4 familiar faces * 2 views * 2 lightings) was repeated 6 times during one run. This resulted in 24 presentations of each face-identity per run (6 repetitions * 2 views * 2 lightings). Thirty percent of these presentations were consecutive face-identity repetitions, that is, they were directly preceded by an image of the same face identity. These consecutive face-identity repetitions were mostly the second same face-identity in a row (first consecutive repetition), but the stimulus sequences also contained instances of more than 2 images of the same face-identity in a row (up to 5 in a row). About half of these consecutive face-identity repetitions were different-image repetitions (different view and/or lighting), the other half were exact-image repetitions (same view and lighting; see Supplementary Table). Each run contained 16 exact-image repetitions, one for each familiar face image. The sequence started and ended with 4 baseline trials. A new pseudorandom sequence was used for each run in the experiment. Participants were scanned 2 or 3 times, resulting in a total of up to 18 runs per participant (with at least 11 good runs per subject, see fMRI Data Preprocessing). Stimuli were presented on a black background while participants fixated a white cross that was displayed close to the bridge of the nose of each face image (see Supplementary Fig. 1).
Task
Participants were instructed to distinguish learned faces from new faces, responding with a right-thumb button-press for a familiar face (either seen or known) and a left-thumb button-press for a new face.
Localization of OFA, FFA, and Parahippocampal Place Area (Functional Localizer Experiment)
Stimuli for the independent functional localizer experiment were gray-scale photographs (252 × 252 pixels) of faces, places and objects masked with a circular aperture. Face identities shown during this experiment were different from the ones used in the face experiment. The stimuli subtended a visual angle of ∼6 × 6 degrees.
Images of the different stimulus categories were presented in 30-s blocks (stimulus duration 700 ms; ISI 300 ms), intermixed with 20-s fixation blocks. Three blocks were presented for each stimulus category, resulting in a total run time of approximately 8 min. Stimuli were presented on a black background, centered with respect to a white cross that participants fixated on during the run. Participants performed a one-back repetition detection task on the images, responding with a left-thumb button-press for each consecutive repetition (3–5 repetitions per block).
Magnetic Resonance Imaging
Functional Measurements
Blood-oxygen level–dependent (BOLD) fMRI was performed using a 3-Tesla General Electric VH/3 MRI scanner, equipped with a custom-built 16-channel MRI digital receiver (Bodurka et al. 2004). A receive-only whole-brain 16-element surface-coil array (NOVA Medical Inc, Wilmington, MA) was used to achieve high spatial resolution for functional studies with good sensitivities to small BOLD signal changes (Bodurka et al. 2007). Twenty 2-mm axial slices (no gap) were acquired, covering the occipital and temporal lobes including the anterior pole, using single-shot full k-space gradient-recalled echo planar imaging (EPI). EPI parameters were as follows: interleaved slice order, EPI matrix size = 128 × 96 pixels, voxel volume = 1.95 × 1.95 × 2 mm3, echo time (TE) = 42 ms, repetition time (TR) = 2 s. Each functional run consisted of 252 volumes (8 min and 24 s per run). The total amount of data acquired for the face experiment was equivalent to 12 h, 25 min, and 4 s of scanning (8 subjects, 11 runs per subject).
Anatomical Measurements
Functional scans were superimposed on high-resolution T1-weighted whole-brain anatomical scans (voxel volume = 0.98 × 0.98 ×1.2 mm3), acquired with a fast spoiled gradient echo recalled (FSPGR) sequence.
Statistical Analysis
Behavioral Data
Reaction time data were analyzed using SPSS 15.0 (SPSS Inc, Chicago, IL). Reaction times of incorrect responses and reaction times that were more than 3 standard deviations from the mean were discarded. We performed a random-effects analysis of variance (ANOVA) for repeated measures including factors for face-identity repetition and familiarity. Face-identity repetition effects were tested up to the second consecutive repetition and compared for seen and known faces (reaction times for new faces could not be included because these faces were not repeated throughout the experiment). Reaction times for the third, fourth, and fifth consecutive repetition were not included in this analysis because there were only few trials for these conditions (see Supplementary Table). Reaction times for the first consecutive repetition were split into a different-image and an exact-image repetition condition. An additional random-effects ANOVA for repeated measures was used to test for effects of familiarity (including new faces), view, and lighting. Insignificant interaction terms were stepwise removed from the models. Post-hoc paired t-tests were performed to investigate significant main effects in more detail.
fMRI Data Preprocessing
MRI data preprocessing and analysis were performed using BrainVoyager QX 1.8 (Brain Innovation, Maastricht, the Netherlands). The first 4 data volumes of each scan were discarded to allow the fMRI signal to reach a steady state. Runs with excessive head-motion or imaging artifacts were excluded from analysis, leaving at least 11 runs per subject. To ensure that each subject-specific data set contributed equally to the results, we used exactly 11 runs per subject for analysis. For the subjects that had more than 11 runs, we randomly chose 11 runs from the whole set. Preprocessing steps performed on the functional data volumes were as follows: slice scan time correction, motion correction (first nondiscarded volume of run as reference volume), temporal high-pass filtering with a filter of 3 cycles per run for the face experiment (corresponding to a cut-off frequency of 0.006 Hz) and 2 cycles per run for the functional localizer experiment (corresponding to a cut-off frequency of 0.004 Hz), and spatial smoothing by convolution with a Gaussian kernel of 6-mm full width at half maximum (FWHM) for the face experiment and 4 mm FWHM for the functional localizer experiment. Spatial smoothing was performed to increase sensitivity to extended activations (activation blobs) and improve intersubject correspondence for group analysis (mapping). In the functional localizer approach, intersubject correspondency is determined by means of individual ROI definitions; therefore a slightly smaller smoothing kernel (4 mm FWHM) was chosen, allowing for a more precise definition of the shape of the region in each subject. Functional data were manually aligned to same-session high-resolution structural whole-brain scans and transformed into Talairach stereotactic space. If no same-session structural scan was available (30% of sessions), functional data were manually aligned to a structural scan from a different session for that subject. Visual comparison of activation loci before and after alignment indicated good alignment quality. All time courses were converted to percent signal change.
Multiple Linear Regression
We performed a fixed-effects group analysis by multiple linear regression of the time course at each voxel. Cognitive predictors were created using the Boynton hemodynamic impulse response function (Boynton et al. 1996), assuming an instantaneous rectangular neuronal response to the 1-s stimulus presentations. In order to keep the number of predictors reasonable (especially given the amount of data to be analyzed simultaneously), we constructed 4 slightly different models (Supplementary Fig. 1). These models investigated the effects of face-identity repetition, face familiarity, face-identity, and view and lighting. The first 2 models will be described below. A description of the other 2 models and associated results can be found in the Supplementary Methods and Results.
The first model was used to test for effects of face-identity repetition. The first consecutive repetition of a specific face identity (one of the 4 familiar faces) was named rep1, the second consecutive repetition of that same face identity was named rep2, and so forth, up to rep5. As described before, these repetitions could be either different-image repetitions (different view and/or lighting) or exact-image repetitions (same view and lighting). The rep1 trials were most frequent and contained a relatively large proportion (0.62) of exact-image repetitions (see Supplementary Table). We therefore split rep1 into 2 predictors to separately investigate the effects of different-image and exact-image face-identity repetition. Any familiar face stimulus that was not a consecutive face-identity repetition was named rep0 (identity change). New faces were never repeated throughout the experiment and were therefore modeled by a separate predictor. The face-identity repetition model consisted of subject-specific predictors for rep0, rep1_different, rep1_exact, rep2, rep3, rep4, rep5 and new faces, and confound-mean predictors for each subject and run (Supplementary Fig. 1a). Contrasts of interest were the following: 1) rep0 versus rep1_different, 2) rep0 versus rep1_exact, 3) rep1_different versus rep1_exact, 4) rep1_different versus rep2, and 5) rep1_exact versus rep2. The first contrast compared face-identity change with first consecutive different-image face-identity repetition, the second compared face-identity change with first consecutive exact-image face-identity repetition, the third compared different-image repetition with exact-image repetition, the fourth compared first consecutive different-image face-identity repetition with second consecutive face-identity repetition, and the fifth compared first consecutive exact-image face-identity repetition with second consecutive face-identity repetition. We did not test contrasts comparing brain responses to more than 2 consecutive repetitions because there were only few trials for rep3, rep4, and rep5 (see Supplementary Table). In addition, we investigated face-identity repetition effects separately for seen and known faces and tested for an interaction between face-identity repetition and familiarity.
The second model was used to test directly for face familiarity effects and consisted of subject-specific predictors for new faces, seen faces and known faces, and confound-mean predictors for each subject and run (Supplementary Fig. 1b). The following 2 contrasts were performed using t-tests: 1) new versus seen faces and 2) seen versus known faces. The first contrast searched for effects of face novelty and perceptual face familiarity, whereas the second isolated effects of added conceptual familiarity.
Results were corrected for serial autocorrelation in the temporal domain. Maps were thresholded to control the average false-discovery rate (FDR) to be <0.05. For both models, one contrast map was a priori chosen as reference and thresholded at FDR < 0.05. The other contrast maps were then thresholded using the t-value associated with that FDR. This created consistent thresholding across maps independent of differences across maps in the number of activated voxels. The reference contrast maps were those maps that focused on our main questions. For face-identity repetition, face-identity change versus first consecutive different-image face-identity repetition (rep0 vs. rep1_different) was chosen as reference contrast map. For face familiarity, seen versus known faces was chosen as reference contrast map.
ROI Definition
Six ROIs were defined in each hemisphere, based on 1) the block localizer experiment (OFA, FFA, and parahippocampal place area [PPA]), 2) the contrast faces > baseline performed on even runs of the face experiment (hippocampus and aIT), and 3) anatomical landmarks (early visual cortex [EVC]). OFA and FFA were defined for each subject by the contrast faces > objects and places, and PPA was defined by places > objects and faces. Maps were thresholded using FDR < 0.01 and a cluster threshold of 200 voxels. Hippocampus and aIT were each defined at 2 different sizes using fixed-effect group results for faces > baseline in the face experiment. Half of the data from the face experiment was used to define hippocampus and aIT, the other half was used to test for effects of interest (see ROI Analysis). The large-sized regions were defined first, using a threshold that resulted in a contiguous set of voxels well separated from other nearby clusters of activation (uncorrected P values were 8.0 × 10−15 for hippocampus and 1.6 × 10−4 for aIT). Then, in order to define the small-sized regions, the threshold was increased until the above regions were reduced by half. We only report ROI results for the large-sized hippocampus and aIT because results for the small-sized regions were qualitatively similar. In each subject, we used high-resolution anatomical data to manually define the calcarine sulcus. EVC was defined by centering ellipsoids (radii were 12 × 5 × 5 mm) on 11 consecutive points along the calcarine sulcus. The resulting ROI included V1 and likely portions of V2 and V3.
ROI Analysis
Data from each ROI were averaged across voxels to obtain an average time course per subject. These time courses were concatenated and used for a fixed-effects group analysis. The above models for face-identity repetition and familiarity were fit to the ROI average time course using multiple linear regression. Results were corrected for serial autocorrelation in the temporal domain. Contrasts of interest identical to the ones used for mapping (see Multiple Linear Regression) were computed.
To investigate whether face-identity repetition effect sizes differed across regions, we performed paired t-tests on subject-specific contrast values (random-effects analysis) for all possible pairs of ROIs that showed significant face-identity repetition effects. For each region, subject-specific contrast values were computed by subtracting subject-specific beta-values for one condition (e.g., rep1_different) from subject-specific beta-values for the other condition (e.g., rep0). We performed region comparisons for the following 2 contrasts: face-identity change versus first consecutive different-image face-identity repetition (rep0 vs. rep1_different) and face-identity change versus first consecutive exact-image repetition (rep0 vs. rep1_exact).
Results
Behavioral Results
Subjects performed a binary classification task, in which they responded with a button press to indicate whether the presented face was “new” or “familiar.” (The familiar faces could be either “seen” or “known,” but the task did not require distinguishing between them.) Accuracy across subjects was 95% or higher in all conditions. In order to investigate the effects of face-identity repetition on reaction time and the influence of face familiarity on these effects, we performed a 2-way ANOVA for repeated measures. Reaction times for new faces were not included in this analysis. The analysis showed a significant main effect of face-identity repetition (F(3) = 24.537, P < 0.01). Paired t-tests showed that reaction times for face-identity change trials (rep0) were higher than for first consecutive different-image face-identity repetition trials (rep1_different) (t(7) = 6.355, P < 0.01), which in turn were higher than for first consecutive exact-image face-identity repetition trials (rep1_exact) (t(7) = 4.732, P < 0.01) (Fig. 2). Reaction times for second consecutive face-identity repetition trials (rep2) were higher than for first consecutive exact-image face-identity repetition trials (rep1_exact) (t(7) = 3.758, P < 0.01, Fig. 2). Note that second consecutive face-identity repetition trials were mainly different-image repetitions. Face-identity repetition effects were not significantly different for seen and known faces. A separate paired t-test showed that reaction times for new faces were significantly higher than those for identity-change trials (t(7) = 4.905, P < 0.01, Fig. 2). We performed an additional 3-way ANOVA for repeated measures to investigate the influence of familiarity (including new faces), view, and lighting on reaction time. This analysis yielded a significant main effect of familiarity (F(1.148, Greenhouse-Geisser corrected for nonsphericity) = 23.110, P < 0.01), attributable to significantly higher reaction times for new than seen, and new than known faces (P < 0.01 for both contrasts). There was no significant difference in reaction times for seen as compared with known faces. This suggests that the seen and known faces were equally perceptually familiar, consistent with the purpose of our familiarity manipulation. We also found a significant interaction effect between view and lighting (F(1) = 9.398, P < 0.05), due to higher reaction times for faces with incongruent as compared with congruent view and lighting.
Figure 2.
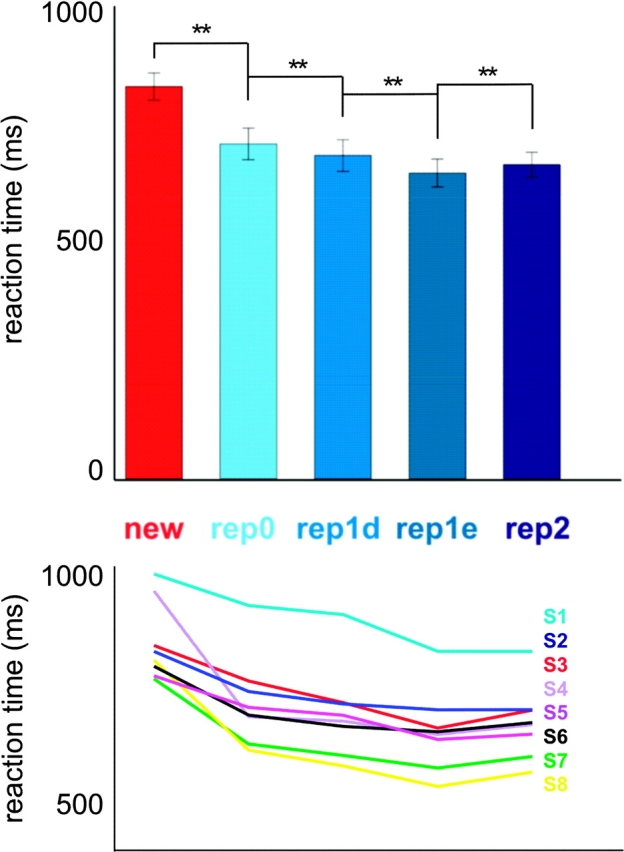
Reaction times for face-identity change (rep0) were higher than for face-identity repetition. The upper panel shows mean reaction times across subjects and associated error bars (random-effects standard error of the mean) for new faces, face-identity change trials, and face-identity consecutive repetition trials. Values for the rep3, rep4, and rep5 predictors are not shown because these were based on only few trials. Four relevant contrasts (new vs. rep0, rep0 vs. rep1_different (rep1d), rep1_different vs. rep1_exact (rep1e), and rep1_exact vs. rep2) were tested for significance using paired t-tests. Significant contrasts are shown and denoted with **(P < 0.01). The lower panel shows the reaction times of each individual subject for the conditions shown in the upper panel, in order to give a more detailed picture of the between-subject variation.
fMRI Results
Face-Identity Change Versus Consecutive Face-Identity Repetition: Identity Change Elicited More Activation Than Repetition Across Early Visual and Posterior Inferior Temporal Cortex
To investigate the effects of face-identity repetition, we contrasted face-identity change trials with face-identity repetition trials. In order to distinguish the effects of different-image face-identity repetition from the effects of exact-image repetition, we separately contrasted face-identity change (rep0) with different-image repetition (rep1_different) and with exact-image repetition (rep1_exact). Both contrasts showed a larger response for identity-change trials than identity-repetition trials across early visual and posterior inferior temporal cortex (Fig. 3) (thresholded using FDR < 0.05 for the contrast rep0 vs. rep1_different, corresponding to a t-value of |3.01|). This face-identity change effect was less widespread and more left-lateralized for the different-image contrast (Fig. 3a) than for the exact-image contrast (Fig. 3b). The regions that were activated more strongly for face-identity change than different-image face-identity repetition (Fig. 3a) overlapped with anterior parts of EVC, posterior parts of OFA (little overlap), inferior medial parts of FFA, and inferior parts of PPA. A small additional cluster in anterior inferior temporal cortex (Talairach coordinates: 19, 2, −18) also responded more strongly to change than different-image repetition (Fig. 3a). Almost complete overlap was found between ROIs (EVC, OFA, FFA, PPA) and regions showing stronger responses to face-identity change than exact-image repetition. Several small clusters in inferior frontal regions also responded more strongly to change than exact-image repetition (Fig. 3b). Three small clusters (<100 voxels) in left amygdala (−20, −8, −12), left middle temporal gyrus (−55, −34, −8), and left anterior middle temporal gyrus (−37, 16, −22) showed a smaller response to face-identity change trials than to exact-image repetition trials (Fig. 3b).
Figure 3.
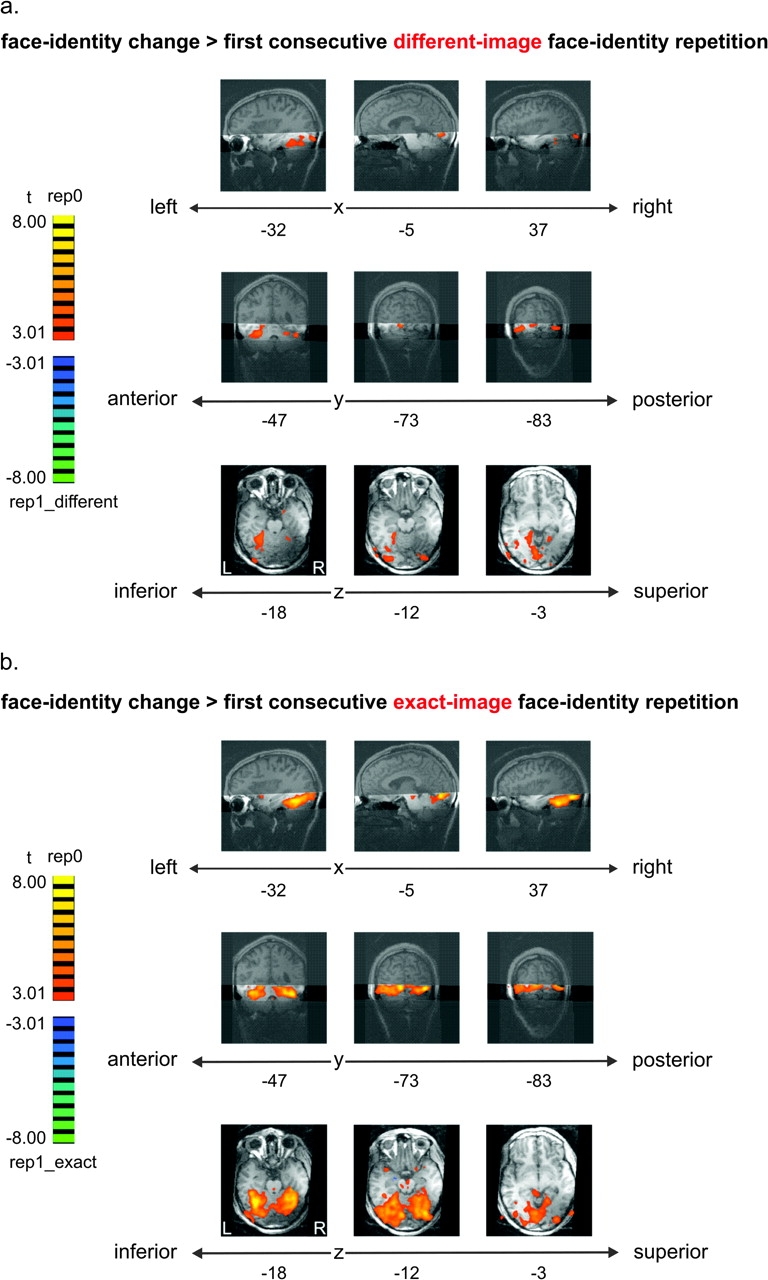
Face-identity change (rep0) elicited more activation than face-identity repetition across early visual and posterior inferior temporal cortex (including regions that are not face-selective). Effects were more widespread for exact-image than different-image repetition. (a) Face-identity change (rep0) versus first consecutive different-image face-identity repetition (rep1_different) (FDR < 0.05). (b) Face-identity change (rep0) versus first consecutive exact-image face-identity repetition (rep1_exact). In both panels, fixed-effects group results are displayed on single-subject high-resolution anatomical slices. The position of the measured slab is indicated by transparent masks overlaid on sagittal and coronal slices. Slices along different points on the x-, y-, and z-axes show stronger activation for rep0 than rep1 (orange/yellow) in EVC as well as in inferior temporal regions, overlapping with OFA, FFA, and PPA. More activation for rep1 than rep0 is shown in blue/green. The most superior slice along the z-axis shows activation based on only three-quarters of the data (data with very low slab position were removed).
Contrasting activation to the first consecutive face-identity repetition (either rep1_different or rep1_exact) with activation to the second consecutive face-identity repetition (rep2) yielded several clusters in early visual and posterior inferior temporal cortex that showed less activation to the second than to the first consecutive face-identity repetition (map thresholds identical to the threshold for the contrast rep0 vs. rep1_different: t = |3.01|). Clusters found in the different-image contrast overlapped with clusters found in the exact-image contrast. Several additional clusters were found in the different-image contrast as compared with the exact-image contrast. These additional clusters were located in right EVC and right posterior inferior temporal cortex.
ROI results were consistent with the mapping results and indicated decreased responses with face-identity repetition in EVC, OFA, FFA, and PPA (Fig. 4). All these regions showed a decreased response to exact-image repetition as compared with face-identity change. Bilateral face-selective regions (OFA, FFA) and left EVC and PPA also showed a decreased response to different-image face-identity repetition as compared with face-identity change. A smaller response to second consecutive face-identity repetition than first consecutive face-identity repetition was found in right EVC for the exact-image repetition contrast (rep1_exact > rep2) (Fig. 4) and in bilateral EVC and right OFA, FFA, and PPA for the different-image contrast (rep1_different > rep2) (significance not shown).
Figure 4.
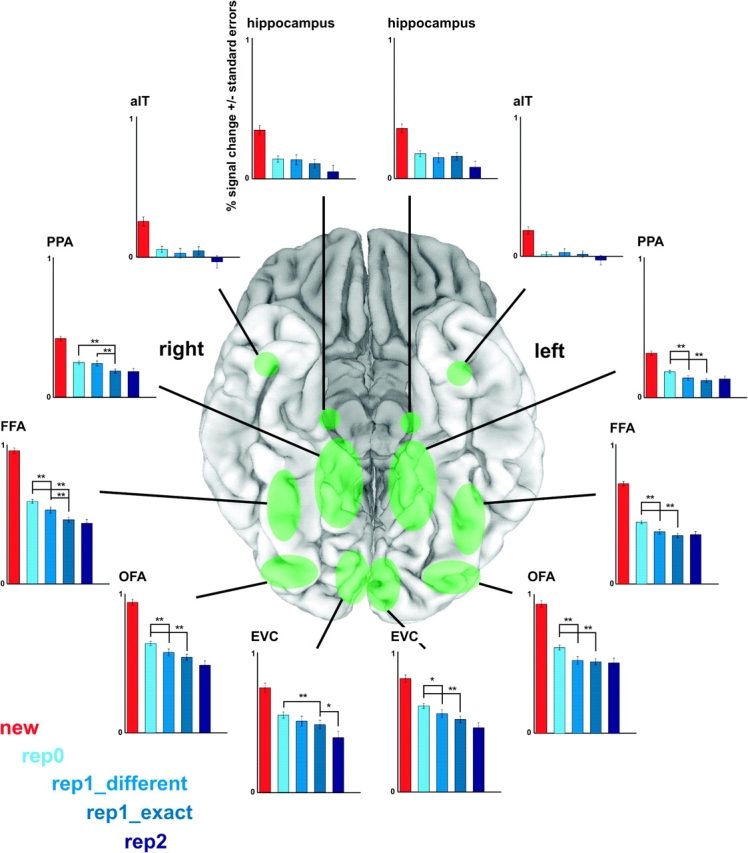
ROI analysis for face-identity repetition effects. Face-selective regions (OFA, FFA) as well regions that are not face-selective (EVC, PPA) showed more activation for face-identity change (rep0) than repetition. These regions (including left EVC and PPA) showed these effects for both exact-image and different-image repetition. Approximate ROI locations are shown (in green) on a ventral view of the cortex (shown here: MNI template colin27). Graphs show beta-values and associated standard errors for the new (red), rep0 (i.e. identity change; light blue), rep1_different (blue), rep1_exact (blue gray), and rep2 (dark blue) predictors, averaged across subjects. Values for the rep3, rep4, and rep5 predictors are not shown because these were based on only few trials. Six relevant contrasts (new vs. rep0, rep0 vs. rep1_different, rep0 vs. rep1_exact, rep1_different vs. rep1_exact, rep1_different vs. rep2, and rep1_exact vs. rep2) were tested for significance. The new versus rep0 contrast was significant for all tested regions (P < 0.01) (not shown). The rep1_different versus rep2 contrast was significant for bilateral EVC and right OFA, FFA, and PPA (P < 0.05) (not shown). For the other 4 tested contrasts, significant contrasts are shown and denoted with **(P < 0.01) or *(P < 0.05). ROIs were defined using independent data. See Table 1 for abbreviations and ROI details (including ROI-defining contrasts).
Our EVC ROI did not include cortex that represents the central visual field (foveal confluence of retinotopic areas V1/2/3), which is where we presented our stimuli. In order to investigate the effects of face-identity repetition in this region, we created 2 additional ROIs located at the left and right foveal confluence (FOV). These ROIs were centered at Talairach coordinates −29, −78, −11 and 25, −80, −9 (spherical ROIs with a volume of 1437 mm3 each, center Talairach coordinates taken from Dougherty et al. 2003). These ROIs as well showed a decreased response to face-identity repetition (both exact-image and different-image repetition) as compared with face-identity change (Supplementary Fig. 2). Hippocampus and aIT did not show significant face-identity repetition effects (Fig. 4).
Different-Image Face-Identity Repetition Elicited More Activation Than Exact-Image Repetition in Right Posterior Inferior Temporal Cortex
Contrasting activation to different-image face-identity repetition (rep1_different) with activation to exact-image repetition (rep1_exact) resulted in several clusters in posterior inferior temporal cortex that showed more activation for different-image than exact-image repetition (map threshold identical to the threshold for the contrast rep0 vs. rep1_different: t = |3.01|, not shown). These clusters were mainly right-lateralized. Parts of these clusters overlapped with FFA and PPA. Exact-image repetition elicited more activation than different-image repetition in right amygdala (15, −4, −17) and left parahippocampal gyrus (−16, −26, −27) (map threshold: t = |3.01|).
ROI results were consistent with the mapping results. Right FFA and PPA showed more activation to different-image face-identity repetition than exact-image repetition; activation to these 2 conditions was statistically indistinguishable in other regions (Fig. 4).
Differences in Strength of Face-Identity Change Effects Across Regions
We reported face-identity change effects in EVC, OFA, FFA, and PPA. Previous studies have consistently reported face-identity change effects in face-selective regions (OFA, FFA), but not non–face-selective regions (EVC, PPA) (see Discussion). Could this discrepancy be explained by differences in the strength of the effect across regions? Related to that, could our widespread findings be explained by increased sensitivity due to the large amount of data we analyzed? In order to investigate these questions, we performed the following 2 analyses: 1) comparison of effect sizes across regions and 2) analysis of a subset of our data (6 runs per subject instead of 11; we analyzed odd runs only).
(1) Effect-size comparison across regions. We performed effect-size comparisons for face-identity change versus first consecutive different-image face-identity repetition (rep 0 vs. rep1_different, “different-image contrast”) and for face-identity change versus first consecutive exact-image repetition (rep0 vs. rep1_exact, “exact-image contrast”). Different-image effect sizes were significantly smaller in right PPA than bilateral FFA (P < 0.05 for both comparisons) and left PPA (P < 0.01). Exact-image effect sizes were significantly larger in right FFA than in bilateral PPA, right EVC and right OFA (P < 0.01 for all comparisons). In addition, exact-image effect sizes were significantly smaller in bilateral PPA than in right OFA (P < 0.05 for both comparisons). These findings suggest that there are some differences in effect size across regions. In particular, face-identity change effects in (right) PPA seem to be smaller than face-identity change effects in face-selective regions. In addition, the strongest face-identity change effects are found in (right) FFA.
(2) Face-identity change analysis on subset of data. We repeated the face-identity change analysis on a subset of our data to investigate the robustness of our effects. We used 6 runs per subject (odd runs only), which corresponds to about half of the data. As for the effect-size comparisons, we investigated the different-image contrast (rep0 vs. rep1_different) and the exact-image contrast (rep0 vs. rep1_exact). Contrast maps were thresholded using the FDR method to account for multiple testing (FDR < 0.05 for the contrast rep0 vs. rep1_different, corresponding to a t-value of |3.86|). As for the full data set, face-identity change elicited more activity in early visual and posterior inferior temporal cortex. Nevertheless, the spatial extent of effects was noticeably smaller, especially for the different-image contrast (Supplementary Fig. 3). This reduction in spatial extent of the effects was less, but still noticeable, when the threshold was set to the threshold used for the full data set maps: t = |3.01| (not shown). Reducing the amount of data affected different-image repetition effects in both face-selective and non–face-selective regions (see Supplementary Fig. 4, ROI analysis). Different-image repetition effects for OFA, FFA, and PPA showed a reduction in significance or even disappeared (left PPA), while they appeared or increased in significance for EVC. Exact-image repetition effects were of similar significance as for the full data set.
Effect-size comparisons between regions on the reduced data set showed that effect sizes in PPA were overall smaller than in OFA and FFA. In particular, different-image effect sizes were significantly smaller in right PPA than bilateral EVC, bilateral FFA, left OFA and left PPA (P < 0.01 for the comparison with right FFA, P < 0.05 for all other comparisons). Exact-image effect sizes were significantly smaller in bilateral PPA than bilateral OFA and right FFA (P < 0.01 for the comparisons with left OFA and right FFA, P < 0.05 for the comparison with right OFA). These effect-size comparison results are consistent with the results of the full data set. In addition, they suggest that effect sizes in EVC are similar to effect sizes in face-selective regions.
Face-Identity Repetition Effects Were Similar for Seen and Known Faces
To test whether face familiarity would influence face-identity repetition effects, we compared face-identity repetition effects for seen and known faces. This comparison was made for each of the 5 contrasts that were investigated for main effects of face-identity repetition. These contrasts were: 1) rep0 versus rep1_different, 2) rep0 versus rep1_exact, 3) rep1_different versus rep1_exact, 4) rep1_different versus rep2, and 5) rep1_exact versus rep2. Mapping did not show significant differences in face-identity repetition effects between seen and known faces, except for the contrast between face-identity change (rep0) and first consecutive exact-image face-identity repetition (rep1_exact). Three small regions, located in posterior occipital cortex and cerebellum, showed the following interaction effect: for seen faces, activation for face-identity change was stronger than for exact-image repetition (rep0 > rep1_exact), whereas for known faces, the opposite pattern of response was found (rep0 < rep1_exact). One of these small clusters was located within our right EVC ROI. Maps were thresholded at a t-value of |3.65|, corresponding to FDR < 0.05 for the interaction contrast that compared the difference between rep0 and rep1_different for seen with that for known faces. ROI analysis indicated that right EVC, right aIT, and right hippocampus showed differential face-identity repetition effects for seen as compared with known faces for either one (EVC, FFA) or 2 (hippocampus) of the above contrasts comparing consecutive identity repetitions (P < 0.05 for each contrast). There was no clear pattern to these results: in some cases, seen faces showed a decrease in activation with repetition, whereas known faces showed the opposite trend, and vice versa in others.
New Versus Seen: New Faces Elicited More Activation Than Seen Faces Across Early Visual and Inferior Temporal Cortex
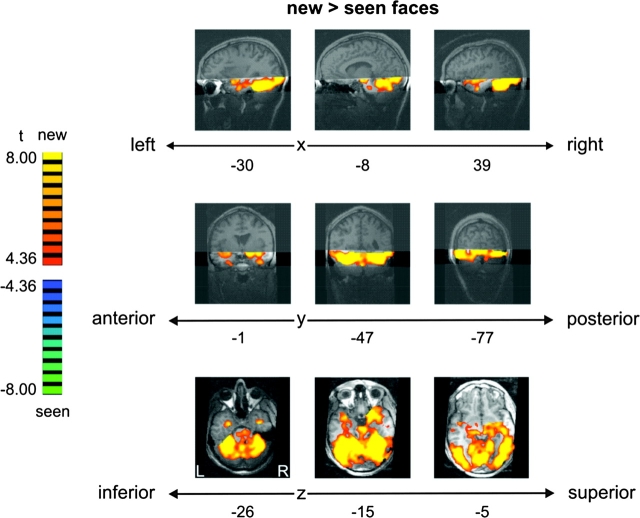
To investigate the influence of face novelty and perceptual face familiarity on face-related activation, we compared activation to new and seen faces. New faces (i.e., faces never seen before) elicited a larger response than seen faces across a large portion of occipital and inferior temporal cortex, including EVC, OFA, FFA, PPA, hippocampus, and aIT (thresholded at FDR < 0.05 for the contrast seen vs. known, corresponding to a t-value of |4.36|) (Fig. 5). There were no regions that showed a larger response to seen than new faces. Consistent with these findings, all ROIs (see Table 1) showed an increased response to new as compared with seen faces (Fig. 6).
Figure 5.
New faces elicited more activation than seen faces across early visual and inferior temporal cortex (t = |4.36|, associated with FDR < 0.05 for seen-known contrast, not shown). Fixed-effects group results are displayed on single-subject high-resolution anatomical slices. Position of the measured slab is indicated by transparent masks overlaid on sagittal and coronal slices. Slices along different points on the x-, y- and z-axes show stronger activation for new than seen faces (orange/yellow) in EVC as well as in (anterior) inferior temporal regions, including OFA, FFA, PPA, hippocampus, and aIT. There were no regions showing more activation for seen than new faces. Note that the most superior slice along the z-axis shows activation based on only three-quarters of the data (data with very low slab position were removed).
Table 1.
ROI details
| ROI | Definition | Hemisphere | Mean Talairach (x, y, z) | Mean size (mm3) |
| EVC | Calcarine | Left | −5, −87, −6 | 1817 |
| Sulcus | Right | 5, −87, −6 | 1817 | |
| OFA | Faces > places and objects | Left | −43, −75, −12 | 1315 |
| Right | 42, −72, −12 | 1835 | ||
| FFA | Faces > places and objects | Left | −37, −46, −18 | 1858 |
| Right | 38, −43, −18 | 2034 | ||
| PPA | Places > faces and objects | Left | −24, −43, −10 | 3169 |
| Right | 23, −42, −10 | 3552 | ||
| Hippocampus | Faces > baseline | Left | −21, −22, −9 | 131 |
| Right | 23, −20, −9 | 142 | ||
| aIT | Faces > baseline | Left | −26, −6, −27 | 382 |
| Right | 35, −3, −25 | 427 |
Mean Talairach coordinates denote the center of gravity of the ROIs. EVC was defined using anatomical landmarks; OFA, FFA, and PPA were defined using independent data from a separate block localizer experiment; and hippocampus and aIT were defined using independent data from the face experiment (even runs).
Figure 6.
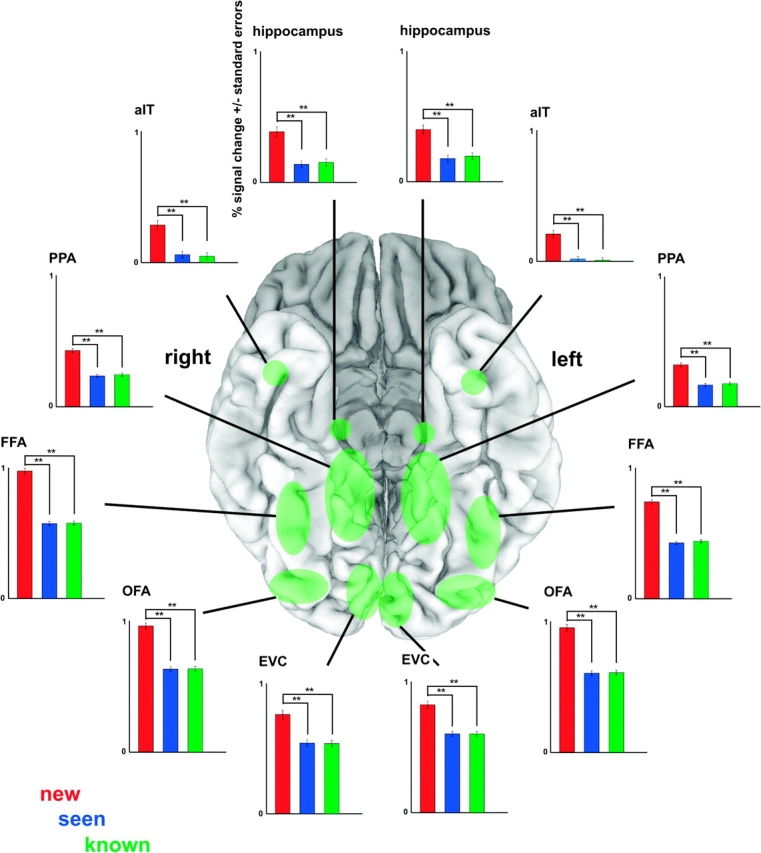
ROI analysis for face-familiarity effects. All ROIs showed more activation to new than seen and known faces. Seen and known faces elicited equal activation. Approximate ROI locations are shown (in green) on a ventral view of the cortex (shown here: MNI template colin27). Graphs show percent signal change and associated standard errors for the new (red), seen (blue), and known (green) predictors, averaged across subjects. All possible contrasts (new vs. seen, new vs. known, and seen vs. known) were tested for significance. Significant contrasts are shown and denoted with **(P < 0.01). ROIs were defined using independent data. See Table 1 for abbreviations and ROI details (including ROI-defining contrasts).
Seen Versus Known: Known and Unknown Familiar Faces Elicited Equal Activation
To localize activation associated with conceptual face information, we contrasted activation to seen (i.e., unknown familiar faces) with activation to known faces. Mapping did not yield regions activated significantly differently by seen than known faces (thresholded at FDR < 0.05 for the contrast seen vs. known, corresponding to a t-value of |4.36|). The more powerful ROI analysis also did not reveal activation differences between seen and known faces (Fig. 6).
Discussion
We investigated the effects of face-identity repetition and face familiarity on activation in human inferior temporal cortex. We observed a decreased BOLD response to repeated faces in OFA and FFA, as expected based on previous literature. However, these effects were not confined to face-selective regions: other regions in occipital and inferior temporal cortex, including EVC and PPA, displayed a similar effect. These face-identity repetition effects in face-selective and non–face-selective regions were present for both different-image and exact-image face-identity repetition but were clearly reduced in spatial extent for different-image repetition. Previous studies have interpreted face-identity repetition effects in face-selective regions as an indication of the existence of specialized face-identity representations. Following this logic, our results could be taken as evidence for the presence of face-identity representations outside of face-selective regions. However, this interpretation is not plausible for EVC and PPA given their known response properties. Alternative interpretations include residual attentional effects (despite our task control) and carryover of activation from face-identity regions to other visual regions. These alternative explanations, which are discussed below, could also apply to the identity-change effects in face-selective regions, including FFA.
Face-identity repetition effects were similar for seen and known faces. A direct comparison of activation to seen and known faces did not yield significant results. The infrequent new faces (never seen before), which were excluded from the identity-repetition analysis, elicited a stronger response across a large portion of occipital and inferior temporal cortex than the 4 familiar faces (see Supplementary Discussion for a more detailed discussion on our face-familiarity findings).
Face-Identity Repetition Effects in Inferior Temporal Cortex
Consistent with previous studies, we found a greater response to face-identity change than repetition in face-selective regions (Gauthier et al. 2000; Andrews and Ewbank 2004; Winston et al. 2004; Pourtois et al. 2005, but see Epstein et al. 1999).
Interestingly, we observed similar face-identity repetition effects in non–face-selective regions in inferior temporal cortex (including PPA). Face-identity repetition effects outside of face-selective regions have been reported in several previous studies (Sugiura et al. 2001; Pourtois et al. 2005; Ng et al. 2006; Dricot et al., 2008a, 2008b). Widespread repetition-related response decreases have also been found using stimuli other than faces (Epstein et al. 2003) and using designs that blocked stimuli by category (Avidan et al. 2002; Chao et al. 2002). Other studies did not find face-identity repetition effects in non–face-selective inferior temporal cortex (Gauthier et al. 2000; Henson et al. 2002; Andrews and Ewbank 2004; Rotshtein et al. 2005; Henson and Mouchlianitis 2007; Summerfield et al. 2008) or did not investigate activity in these regions (Winston et al. 2004; Eger et al. 2005; Loffler et al. 2005). Possible explanations of differences between studies in spatial extent of repetition effects will be discussed below.
Face identity need not be represented in face-selective regions. In line with this thought, face-identity repetition effects in inferior temporal regions that are not face-selective have often been interpreted as evidence for the existence of neuronal face-identity representations in these regions (e.g., Avidan et al. 2002; Dricot et al. 2008a, 2008b). Pattern-information analysis as well has suggested the existence of face-identity representations in a region that did not show a clear face-selective response (i.e., right anterior temporal cortex; Kriegeskorte et al. 2007). PPA could theoretically contain small subsets of “face-identity neurons” that give rise to face-identity change effects (as suggested by Avidan et al. 2002). However, to our knowledge, there is currently no direct evidence for this possibility; it has merely been used as an interpretation of fMRI-adaptation findings. Based on its functional response properties, PPA is an unlikely candidate for representing face-identity. PPA responds strongly to scenes of the local visual environment and only weakly to faces (Epstein et al. 1998). Consistent with this, a large proportion of parahippocampal neurons in macaque prefers eccentric stimulus positions, and only a small proportion responds to complex object images (Sato and Nakamura 2003). Furthermore, the most prominent consequence of parahippocampal lesions is loss of the ability to navigate through spatial environments (Aguirre and D'Esposito 1999). These response properties suggest that PPA is involved in processing spatial environments, not face-identity recognition.
Face-Identity Repetition Effects in EVC
We found face-identity repetition effects even in EVC (including V1 and possibly portions of V2/3). Such effects have not previously been discussed to our knowledge. It appears unlikely that these effects reflect a domain-specific face-identity representation in EVC. Face identity is a high-level stimulus feature, and EVC is known to be sensitive to low-level stimulus properties. Activity in V1, V2, and V3 is modulated by varying low-level stimulus properties, including orientation, spatial frequency, and direction of motion (e.g., Hubel and Wiesel 1968; Levitt et al. 1994; Gegenfurtner et al. 1997). Sensitivity to low-level stimulus properties could underlie exact-image face-identity repetition effects. However, the different-image face-identity repetition effects we found are hard to explain in terms of sensitivity to low-level stimulus properties: identity change was not confounded with low-level feature change because view and lighting changes (associated with large low-level feature changes) occurred on identity-change as well as on different-image identity-repetition trials. Nevertheless, considerable proportions of neurons in V2 and V3 have been shown to also respond to more complex stimulus features, including combinations of orientations and (moving) gratings (Gegenfurtner et al. 1997; Hegdé and Van Essen 2000; Anzai et al. 2007). However, it appears unlikely that these response characteristics produce sensitivity to face features with invariance across view or lighting changes.
Our study is not the first to report fMRI-adaptation effects that are inconsistent with known functional properties of early visual areas (in particular V1). Boynton and Finney (2003) failed to find orientation-sensitive fMRI-adaptation effects in V1 (but see Tootell et al. 1998) despite neurophysiological evidence for sensitivity to orientation and spatial frequency in V1 (Hubel and Wiesel 1968; Movshon and Lennie 1979; Mueller et al. 1999), possibly attributable to the short adaptation duration that was used (Fang et al. 2005). Similar discrepancies between short-term fMRI-adaptation effects and known sensitivity to orientation were found for area V2 (Boynton and Finney 2003; Fang et al. 2005). Another discrepancy can be found in Chao et al. (2002), who reported long-term repetition effects in EVC for complex object stimuli (animals and tools). It would not be in line with known response properties of EVC to interpret these findings in terms of invariant object representations. A more likely explanation of these findings would be that Chao et al. (2002) used exact-image repetitions: object changes were associated with low-level feature changes, whereas object repetitions were not. These reports, as well as the face-identity repetition effects in EVC that we report here, indicate that fMRI-adaptation results might 1) not accurately reflect neuronal sensitivity profiles in early visual areas and 2) reflect sensitivity to stimulus properties other than the stimulus property of interest (particularly when exact-image repetitions are used).
Alternative Explanations for Stimulus-Change Responses
The fMRI-adaptation paradigm is based on the logic that stimulus-change fMRI effects in a specific brain region can be interpreted to indicate that the region contains neurons that are sensitive to the changed stimulus property (for a review, see Grill-Spector et al. 2006). Sensitivity to a stimulus property is taken to indicate that the brain region represents that stimulus property (e.g., face identity). However, the findings from EVC and PPA suggest that some caution is needed when interpreting fMRI stimulus-change effects in any brain region in terms of neuronal sensitivity for the changed stimulus property. Additional support for this assertion can be found in 2 studies that directly investigated the relationship between neuronal selectivity as measured by classical electrophysiological methods and neuronal adaptation measured using an adaptation paradigm in higher-order visual cortex (Tolias et al. 2005; Sawamura et al. 2006; see also Krekelberg et al. 2006). Results from these studies indicated that selectivity inferred from adaptation does not consistently match directly measured neuronal selectivity. These considerations render an interpretation of fMRI-adaptation findings in terms of local neuronal sensitivity for the changed stimulus property no more likely than other possible interpretations. We will discuss 3 alternative explanations for stimulus-change responses.
Automatic Attention
At the cognitive level of description, a plausible alternative interpretation of our effects is that a change in face identity detected by the subject results in an attentional response. Such a response could activate a wider network within the visual system. Our task drew attention away from differences among the 4 familiar faces. However, the attentional response to face changes could be automatic and task-independent. Under natural conditions, a new face implies the presence of a new person to be recognized, and recognition will typically be followed by more general memory access, and a host of other processes required for appropriate behavior. Attention has been shown to enhance responses to preferred stimuli in object-selective cortex (Wojciulik et al. 1998; O'Craven et al. 1999; Murray and Wojciulik 2004) as well as early visual regions (Liu et al. 2005). In addition, attention has been shown to modulate repetition effects (Eger et al. 2004; Murray and Wojciulik 2004; Henson and Mouchlianitis 2007), as indicated by decreased or abolished repetition effects for ignored as compared with attended stimuli. Task manipulations that affect the amount of attention allocated to a stimulus can also influence the strength of repetition effects (e.g., see Henson et al. 2002). Together, these results suggest that attention plays an important role in repetition-related brain responses. If a face-identity change triggered attention automatically, it could give rise to increases in activity that surpass the location where face identities are distinguished. Although the strong response to the infrequent “new” faces can be accounted for as an oddball effect, our face-identity change findings among the 4 familiar faces cannot be explained as an oddball effect because identity-change trials were much more frequent than identity-repetition trials (70% and 30% of all presented familiar faces, respectively). An automatic attentional response to face changes (even when they occur on most trials) is a more plausible explanation.
Carryover of Activation
At the neuronal level of description, a possible cause of face-identity change responses outside of face-identity regions is activation carryover: the region distinguishing the identities and therefore exhibiting release from adaptation might activate connected regions. An example of carryover in the visual system can be found in Tolias et al. (2005). They showed by cell recording that neurons in macaque area V4, which are not generally selective for direction of visual motion, nevertheless respond to “changes” of the direction of motion. They interpret this finding in terms of activation carryover from area MT/V5, whose neurons are strongly selective for direction of motion. A V4 cell pooling outputs from MT/V5 cells across all directions would not be sensitive to direction per se but it would reflect a release from adaptation occurring in MT/V5 after a direction change. Carryover of activation, thus, need not imply carryover of neuronal tuning properties. In other words, carryover could be unspecific: activation could be passed on without relaying stimulus information. Alternatively, stimulus information (e.g., face-identity information) could truly be passed on from one region to connected regions (specific carryover). In either case, face-identity change could activate regions not primarily involved in representing face identity. Carryover may explain our face-identity change effects in early visual regions. Feedback, which can be seen as a form of carryover, from higher-order visual regions involved in face-identity representation could have activated EVC (see also Williams et al. 2008). Such spreading (or carryover) of activation could be functionally interpreted as an attentional effect, but carryover could also occur in the absence of an attentional effect. Carryover could, for example, activate a specialized network (e.g., the face network) to initiate a more comprehensive cognitive process (e.g., recognition, memory access, and response selection).
Neuronal Sensitivity to Stimulus Changes
Another way in which changing a specific stimulus property could elicit effects that might not reflect neuronal sensitivity to this property is if these effects instead reflect processing of the change itself. For example, an abrupt change of stimulus position can elicit an apparent motion percept, activating the motion-sensitive human middle temporal region (hMT/V5+) (e.g., Muckli et al. 2005). By the logic of fMRI adaptation, a position-change effect in hMT/V5+ could be interpreted as indicating that the region represents the spatial location of static visual objects. However, this would not be consistent with what is known about hMT/V5+. Instead hMT/V5+ responds to visual motion, that is, the change of spatial location.
A change-detection explanation is most compelling when the change in question occurs under natural conditions. This is not the case for our study, because faces do not naturally morph from one identity to another. However, change detection is central to visual perception. The involvement of a more general change-detection mechanism cannot be ruled out. Note that neuronal adaptation provides one possible mechanism for change detection, but other mechanisms, including the Reichardt motion detector (Reichardt 1969), can serve this purpose as well.
The above face-identity change explanations could account for neuroimaging findings associated with the behavioral face-inversion effect (Yovel and Kanwisher 2005; Mazard et al. 2006): face-identity change might not be detected with equal sensitivity for upside–down faces, and therefore fail to engage general attentional or carryover mechanisms, resulting in comparable activation for face-identity change and repetition. Similar reasoning would explain the absence of face-identity change effects for upright faces in patients with acquired prosopagnosia or developmental prosopamnesia (Schiltz et al. 2006; Williams et al. 2007): if face-identity repetition is perceptually indistinguishable from face-identity change, then change and repetition will elicit equal activation. Interestingly, Avidan et al. (2005) reported intact face-identity change effects in congenital prosopagnosic patients. This finding seems inconsistent with the above face-identity change interpretations; however, it can be explained by an effect of stimulus change (face-identity change was associated with physical stimulus change whereas face-identity repetition was not).
Why Did Several Previous Studies Fail to Report Widespread Face-Identity Change Effects?
Several previous studies reported widespread face-identity change effects consistent with our present results (Sugiura et al. 2001; Pourtois et al. 2005; Ng et al. 2006). Other studies, however, have found effects restricted to face-selective regions. This discrepancy suggests that features of the experimental design might influence the strength and spatial extent of repetition effects. We consider 6 different features in turn.
Different-Image Repetition Versus Exact-Image Repetition
Most studies that reported face-identity change effects outside of face-selective regions included exact-image repetitions (e.g., Sugiura et al. 2001; Pourtois et al. 2005; Ng et al. 2006), but so did most studies that reported face-identity change effects confined to face-selective regions (e.g., Gauthier et al. 2000; Henson et al. 2002; Andrews and Ewbank 2004). In our study, we could directly compare the effects of different-image face-identity repetition to the effects of exact-image face-identity repetition. Face-identity change effects involving exact-image repetition were clearly more widespread than face-identity change effects involving different-image repetition (consistent with Pourtois et al. 2005). Non–face-selective regions in the right hemisphere did not respond more strongly to face-identity changes than to different-image face-identity repetitions, but did respond more strongly to face-identity changes than to exact-image face-identity repetitions (see Vuilleumier et al. 2002, for a similar laterality effect). A likely explanation for these findings is that face-identity change trials are confounded with stimulus change for the exact-image comparison, but not for the different-image comparison. This could elicit adaptation effects in any region with sensitivity to any of the changed stimulus properties. Alternatively, this could result in a larger attentional response to face-identity change for the exact-image than the different-image comparison. Nevertheless, face-identity change effects involving different-image repetition were still quite widespread, that is, these effects were found in face-selective regions as well as outside face-selective regions (e.g., left EVC and PPA). These results suggest that the use of exact-image repetitions contributes to widespread face-identity change effects but cannot by itself explain the existence of face-identity change effects outside of face-selective regions.
Temporal Lag Between Presentations
Another factor that has been shown to influence repetition effects is the temporal lag between the first and second presentation of a stimulus. Immediate repetition (i.e., no intervening stimuli) and delayed repetition (i.e., other stimuli intervening) are associated with qualitatively different behavioral and neuronal effects (Bentin and Moscovitch 1988; Bentin and Peled 1990; Epstein et al. 2008). The effects of immediate repetition reported in Epstein et al. (2008) seem to be more widespread than those of delayed repetition, especially in posterior visual cortex. This finding seems consistent with our data and interpretation: the attentional response to change might be stronger in immediate-repetition designs than in delayed-repetition designs. Repetition-lag by itself cannot account for the differences in spatial extent of repetition effects between studies: Andrews and Ewbank (2004) used immediate repetition and found effects confined to face-selective regions; Pourtois et al. (2005) used delayed repetition and found more widespread repetition effects (their Fig. 2).
Repetition Frequency Versus Change Frequency
A third factor that has been shown to modulate repetition effects is frequency of repetition. Repetition frequency influences the subject's expectations and can affect the strength of repetition effects (Summerfield et al. 2008): effects are reduced when repetitions occur with relatively low probability. This modulation of effect strength is consistent with an attentional interpretation: infrequent changes are “oddballs” and will trigger a larger attentional response. The spatial extent of repetition effects did not seem to be influenced by probability of repetition (Summerfield et al. 2008). Note that changes were frequent in our study (70% of the trials were face-identity change trials), thus the oddball explanation cannot account for our findings.
Stimulus Variety
All previous studies reporting widespread repetition effects used stimuli from one category only (Sugiura et al. 2001; Epstein et al. 2003; Pourtois et al. 2005; Ng et al. 2006) or blocked stimuli by category (Avidan et al. 2002; Chao et al. 2002). However, other studies with limited stimulus variety did not report widespread effects (Henson et al. 2002; Andrews and Ewbank 2004).
Number of Distinct Stimuli
Our study used a relatively small stimulus set (16 different familiar face images: 4 identities, 2 views, 2 lightings), which could have led to automatic stimulus–response binding (Dobbins et al. 2004). This could possibly have resulted in repetition effects in regions that are not primarily involved in representing face-identity. However, it is important to note that both immediate face-identity change and repetition trials can be considered delayed repetitions of the 16 specific face images. In our study, automatic stimulus–response binding would therefore apply equally to repetitions and changes.
Statistical Power
Our study had a larger amount of data than most previous studies. This might have provided us with increased power to detect widespread face-identity change effects. In order to test this possibility, we repeated our face-identity change analysis on only half of our data. This control analysis showed an overall reduction in the spatial extent of our face-identity change effects, especially for face-identity change effects involving different-image repetition. Consistent with this, ROI analysis indicated that reducing the amount of data did not significantly affect exact-image repetition effects but did affect different-image repetition effects in both face-selective and non–face-selective regions. The strongest different-image effect reduction was seen in left PPA: the effect disappeared, resulting in an absence of the different-image effect in bilateral PPA. In contrast to the other regions, different-image repetition effects in EVC became stronger. These differences between regions suggested by our control analysis are consistent with results of effect-size comparisons between regions. These comparisons showed that face-identity change effects overall were smaller in PPA (but not EVC) than in face-selective regions. This was true for the full as well as the reduced data set and for face-identity change effects involving different-image as well as exact-image repetition. In conclusion, our different-image effects reported for left PPA might indeed be due to increased statistical power. This does not mean that there are no face-identity change effects in PPA; it does indicate that face-identity change effects in PPA are weaker than in face-selective regions and EVC.
Our findings indicate several possible causes of widespread face-identity change effects. In the literature, similar widespread effects have sometimes, but not always, been reported. No single design feature has consistently been associated with widespread face-identity change effects. Combinations of design features might explain the discrepancies between studies in spatial extent of stimulus-change effects. For example, frequent, immediate, exact-image repetitions of stimuli from one object category could be associated with attentional effects and elicit more widespread repetition effects. Some of these features apply to our design. However, it is important to note that our study is not special in this regard: most fMRI-adaptation studies use designs that include several of these features. Finally, even if widespread effects can be avoided by means of particular repetition designs, this would not prove that repetition-related effects indicate neuronal tuning.
Implications for the Interpretation of FFA Results
Our findings question the interpretation of face-identity change effects as conclusive evidence for the presence of neurons tuned to face identity. The alternative explanations are likely to hold for the non–face-selective regions EVC and PPA. They might also hold for FFA. Direct evidence for face-identity tuning could be provided by fMRI or cell recording of responses to single face-image presentations. In the macaque, single-unit recordings from the middle face patch, a possible homologue of the human FFA, suggested that the face-category effect is dominant, but that the region does carry some amount of face-identity information in its population response as well (Tsao et al. 2006). Using high-resolution fMRI and pattern-information analysis, we have previously attempted and failed to detect face-identity information in the FFA or its vicinity, although we did detect such information in right aIT (Kriegeskorte et al. 2007). Current evidence strongly suggests that the FFA serves a key role in face recognition. Consistent with such a role, the strongest face-identity change effects in our study were found in right FFA. However, the evidence that its role consists in distinguishing individual faces is not conclusive.
Conclusion
We reported widespread effects of face-identity change despite well-controlled stimuli. Effects were found in face-selective and non–face-selective regions in inferior temporal cortex and in EVC. These effects were found for exact-image face-identity repetition as well as for different-image face-identity repetition, although exact-image repetition was associated with more widespread effects than different-image repetition. Face-identity-change effects found in previous fMRI-adaptation studies have commonly been interpreted to indicate the existence of face-identity representations. However, alternative interpretations, including general attentional and activation carryover effects, are plausible as well and better account for our widespread effects. These alternative interpretations might also contribute to face-identity change effects in face-selective regions, including FFA whose fMRI activity patterns do not strongly distinguish individual faces (Kriegeskorte et al. 2007).
More generally, fMRI stimulus-change effects are widely interpreted in terms of neuronal sensitivity. This interpretation promises to reveal information represented in fine-grained patterns of activity even within a single voxel. However, our findings add to the evidence (Tolias et al. 2005; Sawamura et al. 2006) that stimulus-change effects do not provide conclusive evidence for neuronal tuning to the changed stimulus property.
Funding
Intramural Research Program of the National Institute of Mental Health at the National Institutes of Health.
Supplementary Material
Supplementary Methods and Results, Supplementary Discussion, Supplementary Figures, Supplementary Table, and Face-familiarity Test Material can be found at: http://www.cercor.oxfordjournals.org/.
Supplementary Material
Acknowledgments
We thank John Crawford for helping take the photographs for our stimuli, Ziad Saad for the cortical surface reconstruction, and Alex Martin for his comments on a draft of the paper. Conflict of Interest: None declared.
References
- Aguirre GK. Continuous carry-over designs for fMRI. Neuroimage. 2007;35:1480–1494. doi: 10.1016/j.neuroimage.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, D'Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122:1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. Neuroimage. 2004;23:905–913. doi: 10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Anzai A, Peng X, Van Essen DC. Neurons in monkey visual area V2 encode combinations of orientations. Nat Neurosci. 2007;10:1313–1321. doi: 10.1038/nn1975. [DOI] [PubMed] [Google Scholar]
- Avidan G, Hasson U, Hendler T, Zohary E, Malach R. Analysis of the neuronal selectivity underlying low fMRI signals. Curr Biol. 2002;12:964–972. doi: 10.1016/s0960-9822(02)00872-2. [DOI] [PubMed] [Google Scholar]
- Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-processing in congenital prosopagnosia: 2. Functional neuroimaging findings. J Cogn Neurosci. 2005;17:1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Bentin S, Moscovitch M. The time course of repetition effects for words and unfamiliar faces. J Exp Psychol Gen. 1988;117:148–160. doi: 10.1037//0096-3445.117.2.148. [DOI] [PubMed] [Google Scholar]
- Bentin S, Peled BS. The contribution of task-related factors to ERP repetition effects at short and long lags. Mem Cognit. 1990;18:359–366. doi: 10.3758/bf03197125. [DOI] [PubMed] [Google Scholar]
- Bernard FA, Bullmore ET, Graham KS, Thompson SA, Hodges JR, Fletcher PC. The hippocampal region is involved in successful recognition of remote and recent famous faces. Neuroimage. 2004;22:1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Blanz V, Vetter T. A morphable model for the synthesis of 3D faces. Proceedings of the 26th annual conference on Computer graphics and interactive techniques. 1999:187–194. [Google Scholar]
- Bodurka J, Ledden P, van Gelderen P, Chu R, de Zwart JA, Duyn J. Scalable multichannel MRI data acquisition system. Magn Reson Med. 2004;51:165–171. doi: 10.1002/mrm.10693. [DOI] [PubMed] [Google Scholar]
- Bodurka J, Ye F, Petridou N, Murphy KM, Bandettini P. Mapping the MRI voxel volume in which thermal noise matches physiological noise—implications for fMRI. Neuroimage. 2007;34:542–549. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Finney EM. Orientation-specific adaptation in visual cortex. J Neurosci. 2003;23:8781–8787. doi: 10.1523/JNEUROSCI.23-25-08781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Chao LL, Weisberg J, Martin A. Experience-dependent modulation of category-related cortical activity. Cereb Cortex. 2002;12:545–551. doi: 10.1093/cercor/12.5.545. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- Dricot L, Sorger B, Schiltz C, Goebel R, Rossion B. The roles of “face” and “non-face” areas during individual face perception: evidence by fMRI adaptation in a brain-damaged prosopagnosic patient. Neuroimage. 2008a;40:318–332. doi: 10.1016/j.neuroimage.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Dricot L, Sorger B, Schiltz C, Goebel R, Rossion B. Evidence for individual face discrimination in non-face selective areas of the visual cortex in acquired prosopagnosia. Behav Neurol. 2008b;19:75–79. doi: 10.1155/2008/561476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E, Henson RNA, Driver J, Dolan RJ. BOLD repetition decreases in object-responsive ventral visual areas depend on spatial attention. J Neurophysiol. 2004;92:1241–1247. doi: 10.1152/jn.00206.2004. [DOI] [PubMed] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. Neuroimage. 2005;26:128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Epstein R, Graham KS, Downing PE. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37:865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM. Two kinds of fMRI repetition suppression? Evidence for dissociable neural mechanisms. J Neurophysiol. 2008;99:2877–2886. doi: 10.1152/jn.90376.2008. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy. Brain. 1995;118:1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- Fang F, Murray SO, Kersten D, He S. Orientation-tuned fMRI adaptation in human visual cortex. J Neurophysiol. 2005;94:4188–4195. doi: 10.1152/jn.00378.2005. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JD, Anderson AW. The fusiform ‘face area’ is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC, Levitt JB. Functional properties of neurons in macaque area V3. J Neurophysiol. 1997;77:1906–1923. doi: 10.1152/jn.1997.77.4.1906. [DOI] [PubMed] [Google Scholar]
- George N, Dolan RJ, Fink GR, Baylis GC, Russell C, Driver J. Contrast polarity and face recognition in the human fusiform gyrus. Nat Neurosci. 1999;2:574–580. doi: 10.1038/9230. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. Neuroimage. 2004;22:1628–1635. doi: 10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMRI-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;75:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gorno Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RSJ. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hegdé J, Van Essen DC. Selectivity for complex shapes in primate visual area V2. J Neurosci. 2000;20:RC61. doi: 10.1523/JNEUROSCI.20-05-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Mouchlianitis E. Effect of spatial attention on stimulus-specific haemodynamic repetition effects. Neuroimage. 2007;35:1317–1329. doi: 10.1016/j.neuroimage.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Gorno-Tempini ML, Dolan RJ. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb Cortex. 2002;12:178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJA. Adaptation: from single cells to BOLD signal. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20:878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Kiper DC, Movshon JA. Receptive fields and functional architecture of macaque V2. J Neurophysiol. 1994;71:2517–2542. doi: 10.1152/jn.1994.71.6.2517. [DOI] [PubMed] [Google Scholar]
- Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron. 2005;45:469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler G, Gordon GE, Wilkinson F, Goren D, Wilson HR. Configural masking of faces: evidence for high-level interactions in face-perception. Vis Res. 2005;45:2287–2297. doi: 10.1016/j.visres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Marotta JJ, Genovese CR, Behrmann M. A functional MRI study of face recognition in patients with prosopagnosia. Neuroreport. 2001;12:1581–1587. doi: 10.1097/00001756-200106130-00014. [DOI] [PubMed] [Google Scholar]
- Mazard A, Schiltz C, Rossion B. Recovery from adaptation to facial identity is larger for upright than inverted faces in the human occipito-temporal cortex. Neuropsychologia. 2006;44:912–922. doi: 10.1016/j.neuropsychologia.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Mueller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285:1405–1408. doi: 10.1126/science.285.5432.1405. [DOI] [PubMed] [Google Scholar]
- Muckli L, Kohler A, Kriegeskorte N, Singer W. Primary visual cortex activity along the apparent-motion trace reflects illusory perception. PLoS Biol. 2005;3:e265. doi: 10.1371/journal.pbio.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SO, Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nat Neurosci. 2004;7:70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979;278:850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- Ng M, Ciaramitaro VM, Anstis S, Boynton GM, Fine I. Selectivity for the configural cues that identify gender, ethnicity, and identity of faces in human cortex. Proc Natl Acad Sci USA. 2006;103:19552–19557. doi: 10.1073/pnas.0605358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Craven KM, Downing PE, Kanwisher N. fMRI evidence for objects as the units of attentional selection. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Portraits or people? Distinct representations of face identity in the human visual cortex. J Cogn Neurosci. 2005;17:1043–1057. doi: 10.1162/0898929054475181. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Reichardt W. Movement perception in insects. In: Reichardt W, editor. Processing of optical data by organisms and by machines. New York: Academic Press; 1969. [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;81:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Sato N, Nakamura K. Visual response properties of neurons in the parahippocampal cortex of monkeys. J Neurophysiol. 2003;90:876–886. doi: 10.1152/jn.01089.2002. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the fMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Sorger B, Caldara R, Ahmed F, Mayer E, Goebel R, Rossion B. Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cereb Cortex. 2006;16:574–586. doi: 10.1093/cercor/bhj005. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, Sato N, Nakamura A, Kato T, Hatano K, Schormann T, Zilles K, Sato K, et al. Activation reduction in anterior temporal cortices during repeated recognition of faces of personal acquaintances. Neuroimage. 2001;13:877–890. doi: 10.1006/nimg.2001.0747. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci. 2008;11:1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias AS, Keliris GA, Smirknakis SM, Logothetis NK. Neurons in macaque area V4 acquire directional tuning after adaptation to motion stimuli. Nat Neurosci. 2005;8:591–593. doi: 10.1038/nn1446. [DOI] [PubMed] [Google Scholar]
- Tootell RBH, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci USA. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat Neurosci. 2002;5:491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Williams MA, Baker CI, Op de Beeck HP, Shim WM, Dang S, Triantafyllou C, Kanwisher N. Feedback of visual object information to foveal retinotopic cortex. Nat Neurosci. 2008;11:1439–1445. doi: 10.1038/nn.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Berberovic N, Mattingley JB. Abnormal fMRI adaptation to unfamiliar faces in a case of developmental prosopamnesia. Curr Biol. 2007;17:1259–1264. doi: 10.1016/j.cub.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Winston JS, Henson RNA, Fine-Goulden MR, Dolan RJ. fMRI-Adaptation reveals dissociable neural representations of identity and expression in face perception. J Neurophysiol. 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in human fusiform gyrus: fMRI study. J Neurophysiol. 1998;79:1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. The neural basis of the behavioral face-inversion effect. Curr Biol. 2005;15:2256–2262. doi: 10.1016/j.cub.2005.10.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.