Abstract
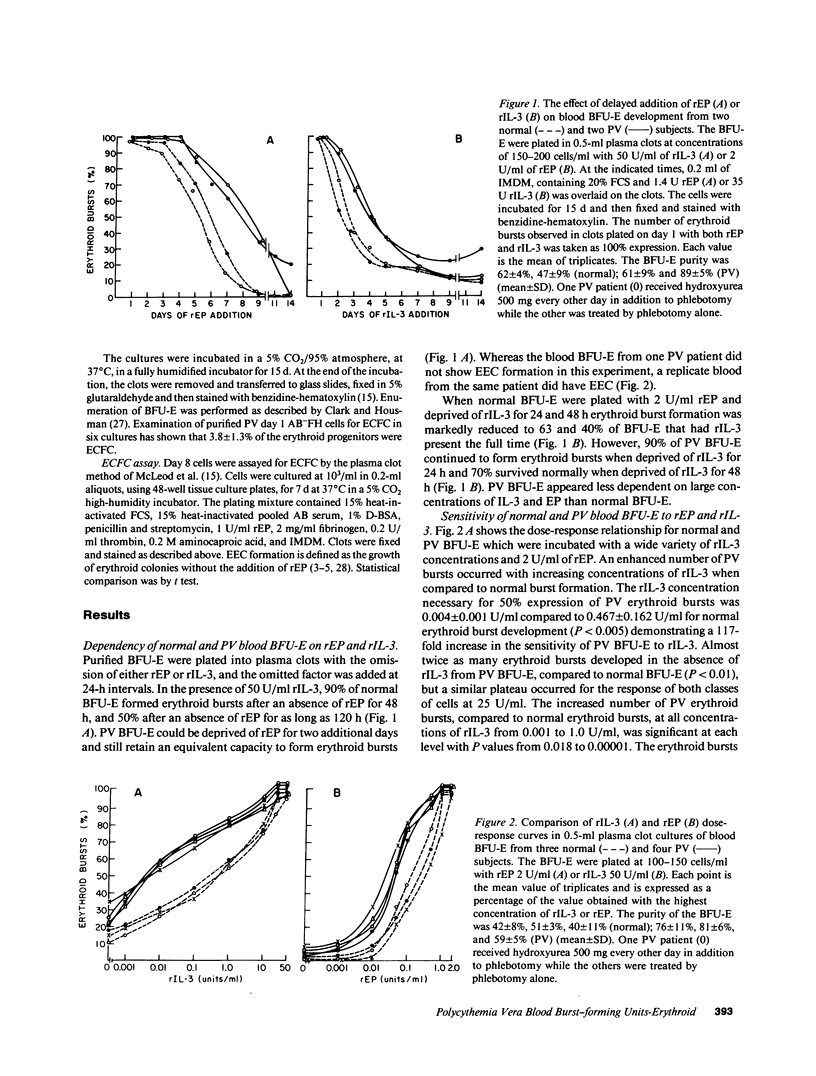
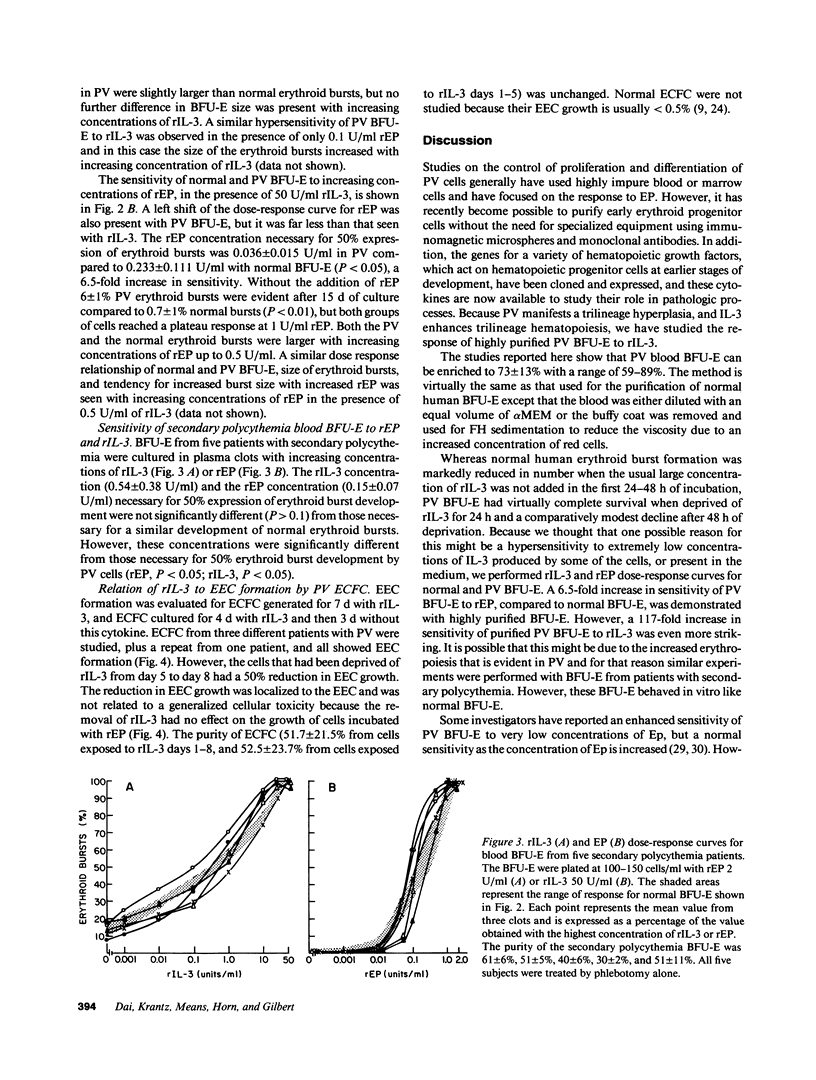
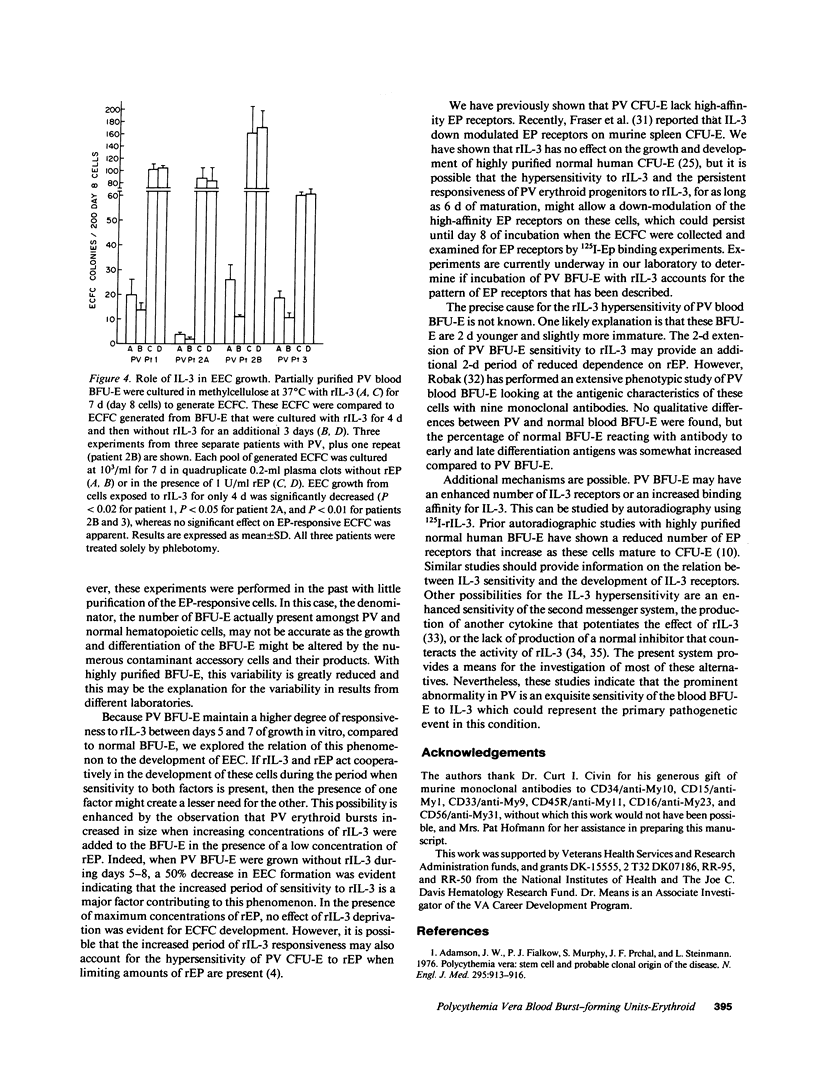
Because polycythemia vera (PV) is a clonal hematopoietic stem cell disease with a trilineage hyperplasia, and interleukin-3 (IL-3) stimulates trilineage hematopoiesis, we have studied the response of highly purified PV blood burst-forming units-erythroid (BFU-E) to recombinant human IL-3 (rIL-3). Whereas the growth of normal blood BFU-E in vitro rapidly declined by 40 and 60% after 24 and 48 h of incubation without 50 U/ml of rIL-3, the growth of PV BFU-E declined by only 10 and 30% under the same conditions, demonstrating a reduced dependence on rIL-3. A reduced dependence of PV BFU-E on recombinant human erythropoietin (rEP) was also present. Dose-response experiments showed a 117-fold increase in PV BFU-E sensitivity to rIL-3, and a 6.5-fold increase in sensitivity to rEP, compared to normal BFU-E, whereas blood BFU-E from patients with secondary polycythemia responded like normal BFU-E. Endogenous erythroid colony (EEC) formation, which is independent of the addition of rEP, was reduced by 50% after erythroid colony-forming cells were generated from PV BFU-E in vitro without rIL-3 for 3 d, whereas rEP-stimulated erythroid colonies were unaffected. These studies demonstrate a striking hypersensitivity of PV blood BFU-E to rIL-3, which may be the major factor in the pathogenesis of increased erythropoiesis without increased EP concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Fialkow P. J., Murphy S., Prchal J. F., Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976 Oct 21;295(17):913–916. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- Berlin N. I. Diagnosis and classification of the polycythemias. Semin Hematol. 1975 Oct;12(4):339–351. [PubMed] [Google Scholar]
- Casadevall N., Vainchenker W., Lacombe C., Vinci G., Chapman J., Breton-Gorius J., Varet B. Erythroid progenitors in polycythemia vera: demonstration of their hypersensitivity to erythropoietin using serum free cultures. Blood. 1982 Feb;59(2):447–451. [PubMed] [Google Scholar]
- Cashman J. D., Eaves C. J., Eaves A. C. Unregulated proliferation of primitive neoplastic progenitor cells in long-term polycythemia vera marrow cultures. J Clin Invest. 1988 Jan;81(1):87–91. doi: 10.1172/JCI113315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin C. I., Loken M. R. Cell surface antigens on human marrow cells: dissection of hematopoietic development using monoclonal antibodies and multiparameter flow cytometry. Int J Cell Cloning. 1987 Jul;5(4):267–288. doi: 10.1002/stem.5530050403. [DOI] [PubMed] [Google Scholar]
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Clarke B. J., Housman D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1105–1109. doi: 10.1073/pnas.74.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves C. J., Eaves A. C. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978 Dec;52(6):1196–1210. [PubMed] [Google Scholar]
- Eid J., Ebert R. F., Gesell M. S., Spivak J. L. Intracellular growth factors in polycythemia vera and other myeloproliferative disorders. Proc Natl Acad Sci U S A. 1987 Jan;84(2):532–536. doi: 10.1073/pnas.84.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. G., Thomas S., Ferrara J. L., Greenstein J. L. Developmental regulation of erythropoiesis by hematopoietic growth factors: analysis on populations of BFU-E from bone marrow, peripheral blood, and fetal liver. Blood. 1989 Jul;74(1):49–55. [PubMed] [Google Scholar]
- Eridani S., Dudley J. M., Sawyer B. M., Pearson T. C. Erythropoietic colonies in a serum-free system: results in primary proliferative polycythaemia and thrombocythaemia. Br J Haematol. 1987 Dec;67(4):387–391. doi: 10.1111/j.1365-2141.1987.tb06158.x. [DOI] [PubMed] [Google Scholar]
- Fraser J. K., Nicholls J., Coffey C., Lin F. K., Berridge M. V. Down-modulation of high-affinity receptors for erythropoietin on murine erythroblasts by interleukin 3. Exp Hematol. 1988 Oct;16(9):769–773. [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Cline M. J. Polycythemia vera: hormonal modulation of erythropoiesis in vitro. Blood. 1977 Mar;49(3):399–405. [PubMed] [Google Scholar]
- Lacombe C., Casadevall N., Varet B. Polycythaemia vera: in vitro studies of circulating erythroid progenitors. Br J Haematol. 1980 Feb;44(2):189–199. doi: 10.1111/j.1365-2141.1980.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Means R. T., Jr, Krantz S. B., Sawyer S. T., Gilbert H. S. Erythropoietin receptors in polycythemia vera. J Clin Invest. 1989 Oct;84(4):1340–1344. doi: 10.1172/JCI114303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal J. F., Adamson J. W., Murphy S., Steinmann L., Fialkow P. J. Polycythemia vera. The in vitro response of normal and abnormal stem cell lines to erythropoietin. J Clin Invest. 1978 Apr;61(4):1044–1047. doi: 10.1172/JCI109003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal J. F., Axelrad A. A. Letter: Bone-marrow responses in polycythemia vera. N Engl J Med. 1974 Jun 13;290(24):1382–1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- Reid C. D. The significance of endogenous erythroid colonies (EEC) in haematological disorders. Blood Rev. 1987 Jun;1(2):133–140. doi: 10.1016/0268-960x(87)90008-7. [DOI] [PubMed] [Google Scholar]
- Robak T. Antigenic characteristics of erythropoietin dependent and independent erythroid progenitors (BFU-E and CFU-E) in polycythaemia vera and idiopathic myelofibrosis defined by monoclonal antibodies. Arch Immunol Ther Exp (Warsz) 1988;36(6):733–747. [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dai C. H., Koury S. T., Horn S. T., Glick A. D., Civin C. I. Purification of human blood burst-forming units-erythroid and demonstration of the evolution of erythropoietin receptors. J Cell Physiol. 1990 Feb;142(2):219–230. doi: 10.1002/jcp.1041420202. [DOI] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dessypris E. N., Koury S. T., Sawyer S. T. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest. 1989 May;83(5):1701–1709. doi: 10.1172/JCI114070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Kans J. S., Dessypris E. N., Sawyer S., Glick A. D., Civin C. I. Purification of human erythroid colony-forming units and demonstration of specific binding of erythropoietin. J Clin Invest. 1987 Aug;80(2):357–366. doi: 10.1172/JCI113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Sawyer S. T., Civin C. I. Quantitation of specific binding of erythropoietin to human erythroid colony-forming cells. J Cell Physiol. 1988 Nov;137(2):337–345. doi: 10.1002/jcp.1041370218. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Emerson S. G., Mufson A., Gesner T. G., Nathan D. G. Dependence of highly enriched human bone marrow progenitors on hemopoietic growth factors and their response to recombinant erythropoietin. J Clin Invest. 1986 Jan;77(1):74–81. doi: 10.1172/JCI112305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C. A., Niemeyer C. M., Nathan D. G., Ekern S. C., Bieber F. R., Yang Y. C., Wong G., Clark S. C. Stimulation of human hematopoietic colony formation by recombinant gibbon multi-colony-stimulating factor or interleukin 3. J Clin Invest. 1987 Sep;80(3):818–823. doi: 10.1172/JCI113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H., Loken M. R., Civin C. I. Antigenic analysis of hematopoiesis. IV. The My-11 hematopoietic cell surface antigen is expressed by myelomonocytic and lymphoid, but not erythroid, progenitor cells. Exp Hematol. 1986 Nov;14(10):935–945. [PubMed] [Google Scholar]
- Strauss L. C., Rowley S. D., La Russa V. F., Sharkis S. J., Stuart R. K., Civin C. I. Antigenic analysis of hematopoiesis. V. Characterization of My-10 antigen expression by normal lymphohematopoietic progenitor cells. Exp Hematol. 1986 Oct;14(9):878–886. [PubMed] [Google Scholar]
- Zaentz S. D., Luna J. A., Baker A. S., Krantz S. B. Detection of cytotoxic antibody to erythroblasts. J Lab Clin Med. 1977 Apr;89(4):851–860. [PubMed] [Google Scholar]
- Zanjani E. D., Lutton J. D., Hoffman R., Wasserman L. R. Erythroid colony formation by polycythemia vera bone marrow in vitro. Dependence on erythropoietin. J Clin Invest. 1977 May;59(5):841–848. doi: 10.1172/JCI108706. [DOI] [PMC free article] [PubMed] [Google Scholar]



