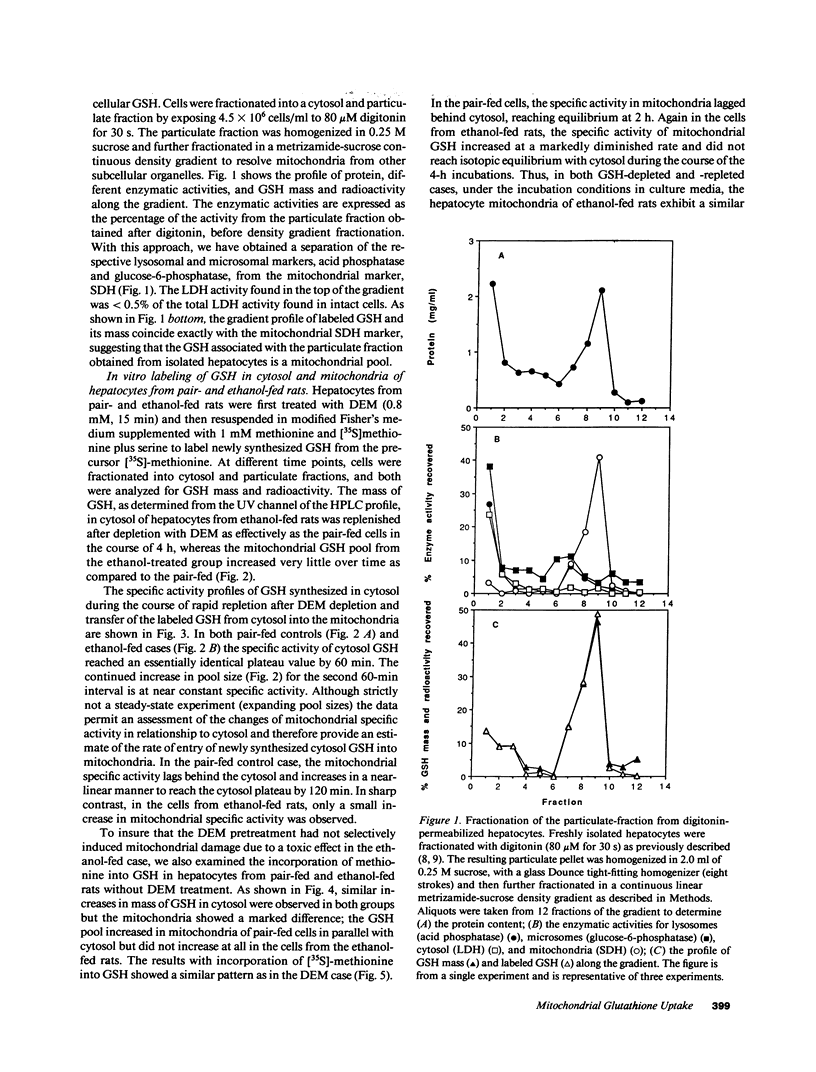
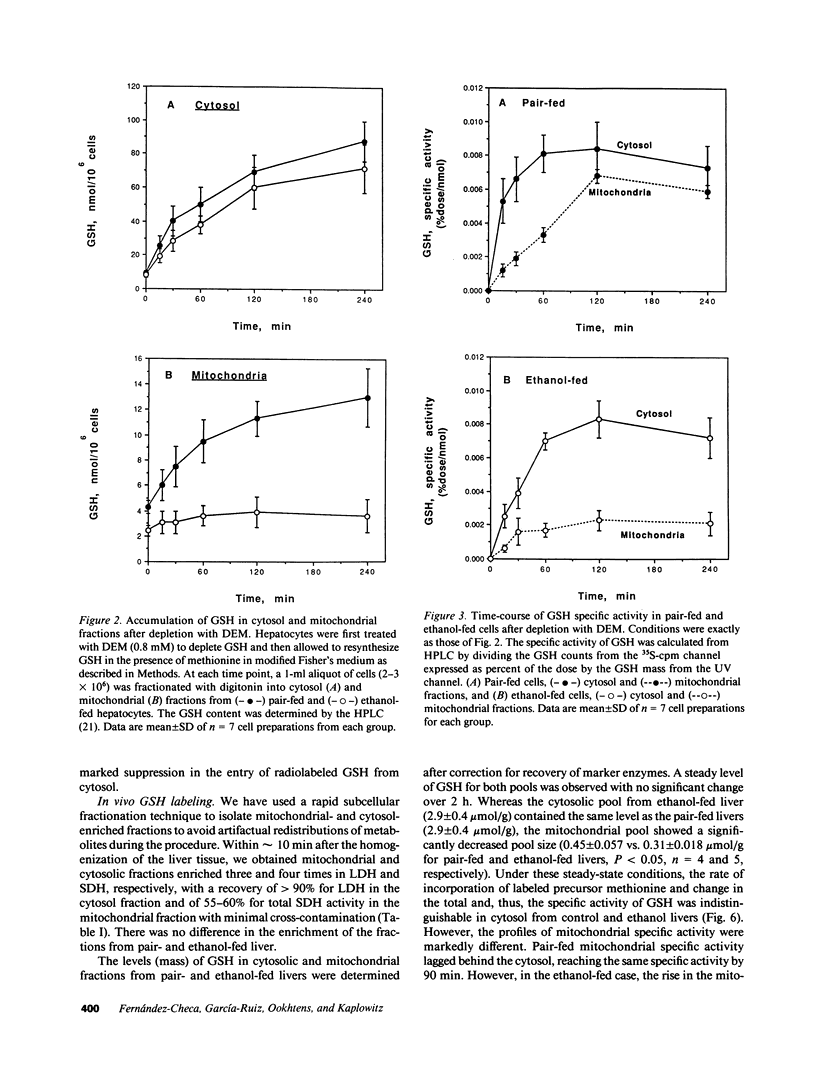
Abstract
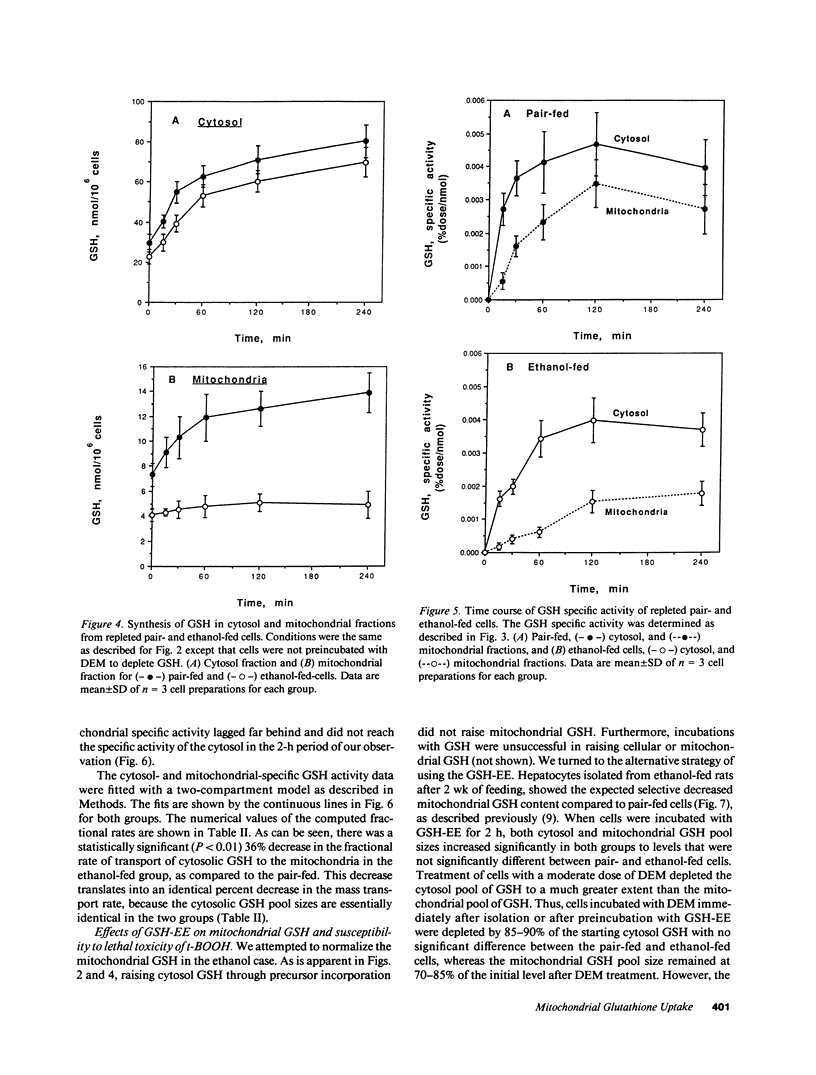
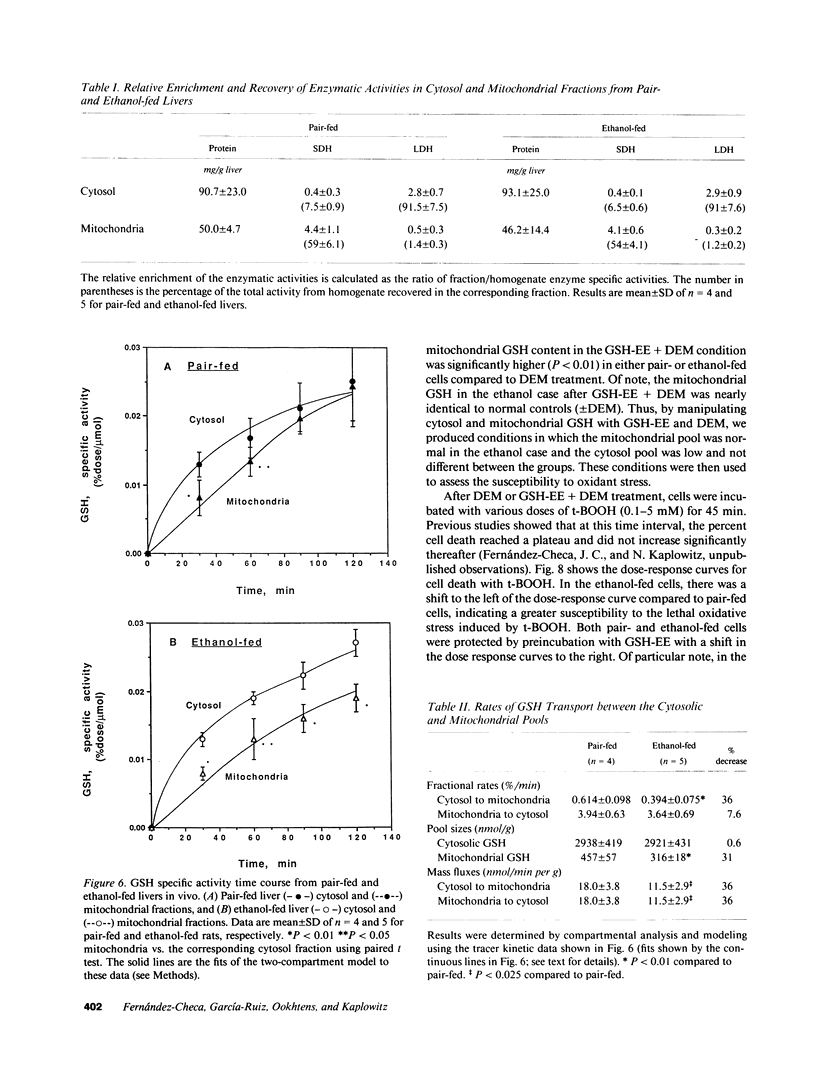
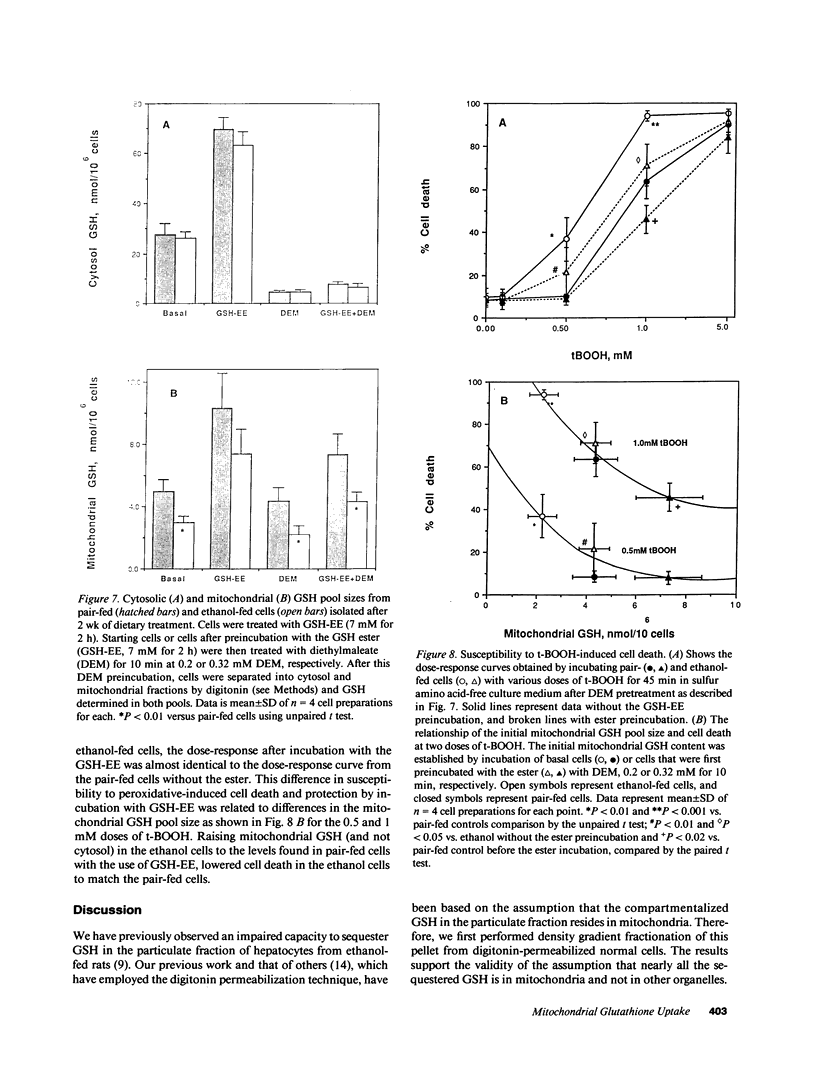
Isolated hepatocytes incubated with [35S]-methionine were examined for the time-dependent accumulation of [35S]-glutathione (GSH) in cytosol and mitochondria, the latter confirmed by density gradient purification. In GSH-depleted and -repleted hepatocytes, the increase of specific activity of mitochondrial GSH lagged behind cytosol, reaching nearly the same specific activity by 1-2 h. However, in hepatocytes from ethanol-fed rats, the rate of increase of total GSH specific radioactivity in mitochondria was markedly suppressed. In in vivo steady-state experiments, the mass transport of GSH from cytosol to mitochondria and vice versa was 18 nmol/min per g liver, indicating that the half-life of mitochondrial GSH was approximately 18 min in controls. The fractional transport rate of GSH from cytosol to mitochondria, but not mitochondria to cytosol, was significantly reduced in the livers of ethanol-fed rats. Thus, ethanol-fed rats exhibit a decreased mitochondrial GSH pool size due to an impaired entry of cytosol GSH into mitochondria. Hepatocytes from ethanol-fed rats exhibited a greater susceptibility to the oxidant stress-induced cell death from tert-butylhydroperoxide. Incubation with glutathione monoethyl ester normalized the mitochondrial GSH and protected against the increased susceptibility to t-butylhydroperoxide, which was directly related to the lowered mitochondrial GSH pool size in ethanol-fed cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Andersson B. S., Jones D. P. Use of digitonin fractionation to determine mitochondrial transmembrane ion distribution in cells during anoxia. Anal Biochem. 1985 Apr;146(1):164–172. doi: 10.1016/0003-2697(85)90411-7. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Kaplowitz N. Inhibition of glutathione efflux from isolated rat hepatocytes by methionine. J Biol Chem. 1984 Aug 10;259(15):9355–9358. [PubMed] [Google Scholar]
- Fariss M. W., Reed D. J. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ookhtens M., Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987 Jul;80(1):57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ookhtens M., Kaplowitz N. Effects of chronic ethanol feeding on rat hepatocytic glutathione. Relationship of cytosolic glutathione to efflux and mitochondrial sequestration. J Clin Invest. 1989 Apr;83(4):1247–1252. doi: 10.1172/JCI114008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ren C., Aw T. Y., Ookhtens M., Kaplowitz N. Effect of membrane potential and cellular ATP on glutathione efflux from isolated rat hepatocytes. Am J Physiol. 1988 Oct;255(4 Pt 1):G403–G408. doi: 10.1152/ajpgi.1988.255.4.G403. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L. Preparation and properties of succinate: ubiquinone oxidoreductase (complex II). Methods Enzymol. 1978;53:21–27. doi: 10.1016/s0076-6879(78)53008-5. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Aw T. Y., Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Eberle D. E., Petrini J., Touloukian J., Corvasce M. C., Kuhlenkamp J. Factors influencing the efflux of hepatic glutathione into bile in rats. J Pharmacol Exp Ther. 1983 Jan;224(1):141–147. [PubMed] [Google Scholar]
- Ku R. H., Billings R. E. The role of mitochondrial glutathione and cellular protein sulfhydryls in formaldehyde toxicity in glutathione-depleted rat hepatocytes. Arch Biochem Biophys. 1986 May 15;247(1):183–189. doi: 10.1016/0003-9861(86)90547-3. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Depletion in vitro of mitochondrial glutathione in rat hepatocytes and enhancement of lipid peroxidation by adriamycin and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Biochem Pharmacol. 1983 Apr 15;32(8):1383–1388. doi: 10.1016/0006-2952(83)90451-3. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982 Apr 10;257(7):3747–3753. [PubMed] [Google Scholar]
- Moldéus P., Andersson B., Norling A., Ormstad K. Effect of chronic ethanol administration on drug metabolism in isolated hepatocytes with emphasis on paracetamol activation. Biochem Pharmacol. 1980 Jun 15;29(12):1741–1745. doi: 10.1016/0006-2952(80)90134-3. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson J. L., Mitchell M. C. Increased hepatic efflux of glutathione after chronic ethanol feeding. Biochem Pharmacol. 1986 May 1;35(9):1533–1537. doi: 10.1016/0006-2952(86)90121-8. [DOI] [PubMed] [Google Scholar]
- Reed D. J. Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H., Robertson D. E., Rubin E. The effect of ethanol on the temperature dependence of respiration and ATPase activities of rat liver mitochondria. Lab Invest. 1980 Mar;42(3):318–326. [PubMed] [Google Scholar]
- Solheim A. E., Seglen P. O. Subcellular distribution of proteolytically generated valine in isolated rat hepatocytes. Eur J Biochem. 1980 Jun;107(2):587–596. doi: 10.1111/j.1432-1033.1980.tb06067.x. [DOI] [PubMed] [Google Scholar]
- Thayer W. S., Rubin E. Effects of chronic ethanol intoxication on oxidative phosphorylation in rat liver submitochondrial particles. J Biol Chem. 1979 Aug 25;254(16):7717–7723. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tirmenstein M. A., Nelson S. D. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989 Jun 15;264(17):9814–9819. [PubMed] [Google Scholar]
- Vendemiale G., Jayatilleke E., Shaw S., Lieber C. S. Depression of biliary glutathione excretion by chronic ethanol feeding in the rat. Life Sci. 1984 Mar 12;34(11):1065–1073. doi: 10.1016/0024-3205(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Wahlländer A., Soboll S., Sies H., Linke I., Müller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979 Jan 1;97(1):138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]