Abstract
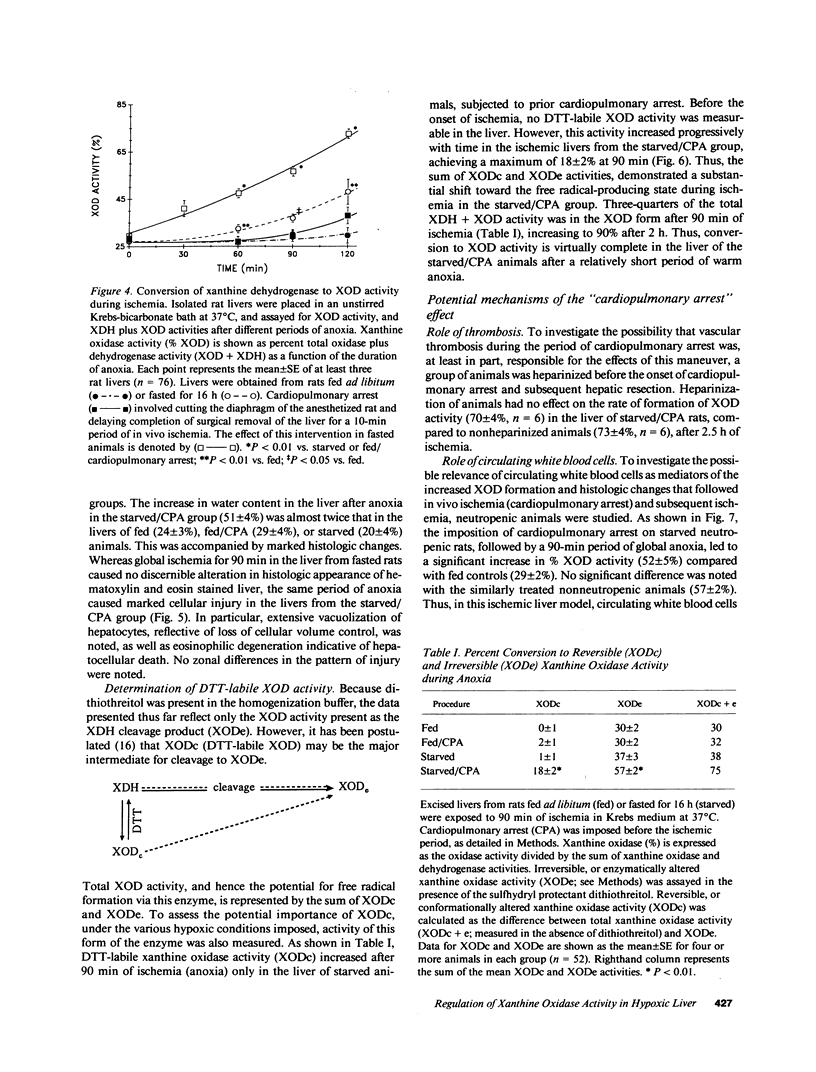
It has been widely proposed that conversion of xanthine dehydrogenase (XDH) to its free radical-producing form, xanthine oxidase (XOD), underlies ischemic/reperfusion injury, although the relationship of this conversion to hypoxia and its physiologic control have not been defined. This study details the time course and control of this enzymatic interconversion. In a functionally intact, isolated perfused rat liver model, mean % XOD activity increased as a function of both the duration (25 to 45% in 3 h) and degree (r = 0.97) of hypoxia. This process was markedly accelerated in ischemic liver by an overnight fast (45 vs. 30% at 2 h), and by imposing a short period of in vivo ischemia (cardiopulmonary arrest 72%). Moreover, only under these conditions was there a significant rise in the XOD activity due to the conformationally altered XDH molecule (XODc, 18%), as well as concomitant morphologic injury. Neither circulating white blood cells nor thrombosis appeared to contribute to the effects of in vivo ischemia on enzyme conversion. Thus, it is apparent that conversion to the free radical-producing state, with high levels of XOD activity and concurrent cellular injury, can be achieved during a relatively short period of hypoxia under certain well-defined physiologic conditions, in a time course consistent with its purported role in modulating reperfusion injury. These data also suggest that the premorbid condition of organ donors (e.g., nutritional status and relative state of hypoxia) is important in achieving optimal organ preservation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkison D., Höllwarth M. E., Benoit J. N., Parks D. A., McCord J. M., Granger D. N. Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand Suppl. 1986;548:101–107. [PubMed] [Google Scholar]
- Angermüller S., Bruder G., Völkl A., Wesch H., Fahimi H. D. Localization of xanthine oxidase in crystalline cores of peroxisomes. A cytochemical and biochemical study. Eur J Cell Biol. 1987 Dec;45(1):137–144. [PubMed] [Google Scholar]
- Anundi I., King J., Owen D. A., Schneider H., Lemasters J. J., Thurman R. G. Fructose prevents hypoxic cell death in liver. Am J Physiol. 1987 Sep;253(3 Pt 1):G390–G396. doi: 10.1152/ajpgi.1987.253.3.G390. [DOI] [PubMed] [Google Scholar]
- Anundi I., de Groot H. Hypoxic liver cell death: critical Po2 and dependence of viability on glycolysis. Am J Physiol. 1989 Jul;257(1 Pt 1):G58–G64. doi: 10.1152/ajpgi.1989.257.1.G58. [DOI] [PubMed] [Google Scholar]
- Atalla S. L., Toledo-Pereyra L. H., MacKenzie G. H., Cederna J. P. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985 Dec;40(6):584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Auscher C., Amory N., Pasquier C., Delbarre F. Localization of xanthine oxidase acitivty in hepatic tissue. A new histochemical method. Adv Exp Med Biol. 1977;76A:605–609. [PubMed] [Google Scholar]
- Barnwell S. G., Lowe P. J., Coleman R. Effect of taurochenodeoxycholate or tauroursodeoxycholate upon biliary output of phospholipids and plasma-membrane enzymes, and the extent of cell damage, in isolated perfused rat livers. Biochem J. 1983 Oct 15;216(1):107–111. doi: 10.1042/bj2160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky S. A., Popp J. A., Kauffman F. C., Thurman R. G. Trypan blue uptake as a new method to investigate hepatotoxicity in periportal and pericentral regions of the liver lobule: studies with allyl alcohol in the perfused liver. J Pharmacol Exp Ther. 1984 Sep;230(3):755–760. [PubMed] [Google Scholar]
- Bradford B. U., Marotto M., Lemasters J. J., Thurman R. G. New, simple models to evaluate zone-specific damage due to hypoxia in the perfused rat liver: time course and effect of nutritional state. J Pharmacol Exp Ther. 1986 Jan;236(1):263–268. [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Frederiks W. M., Fronik G. M., Marx F., James J. Influence of nutritional state on ischemic damage in rat liver. Liver. 1985 Dec;5(6):342–347. doi: 10.1111/j.1600-0676.1985.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Narang N. Ouabain-insensitive K-adenosine triphosphatase in distal nephron segments of the rabbit. J Clin Invest. 1988 Apr;81(4):1204–1208. doi: 10.1172/JCI113436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan J., Hammaker L., Licko V., Schmid R. Bilirubin kinetics in intact rats and isolated perfused liver. Evidence for hepatic deconjugation of bilirubin glucuronides. J Clin Invest. 1981 Apr;67(4):1003–1015. doi: 10.1172/JCI110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Jennische E. Effects of ischemia on the hepatic cell membrane potential in the rat. Differences between fed and fasted animals. Acta Physiol Scand. 1983 May;118(1):69–73. doi: 10.1111/j.1748-1716.1983.tb07242.x. [DOI] [PubMed] [Google Scholar]
- Jennische E. Possible influence of glutathione on postischemic liver injury. Acta Pathol Microbiol Immunol Scand A. 1984 Jan;92(1):55–64. doi: 10.1111/j.1699-0463.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- Kato S., Kawase T., Alderman J., Inatomi N., Lieber C. S. Role of xanthine oxidase in ethanol-induced lipid peroxidation in rats. Gastroenterology. 1990 Jan;98(1):203–210. doi: 10.1016/0016-5085(90)91311-s. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Shanley P. F., Whittenburg D., Berger E., Repine J. E. Neutrophils accentuate ischemia-reperfusion injury in isolated perfused rat kidneys. Am J Physiol. 1988 Oct;255(4 Pt 2):F728–F735. doi: 10.1152/ajprenal.1988.255.4.F728. [DOI] [PubMed] [Google Scholar]
- Marotto M. E., Thurman R. G., Lemasters J. J. Early midzonal cell death during low-flow hypoxia in the isolated, perfused rat liver: protection by allopurinol. Hepatology. 1988 May-Jun;8(3):585–590. doi: 10.1002/hep.1840080325. [DOI] [PubMed] [Google Scholar]
- Marubayashi S., Dohi K., Ochi K., Kawasaki T. Role of free radicals in ischemic rat liver cell injury: prevention of damage by alpha-tocopherol administration. Surgery. 1986 Feb;99(2):184–192. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McKelvey T. G., Höllwarth M. E., Granger D. N., Engerson T. D., Landler U., Jones H. P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988 May;254(5 Pt 1):G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- Metzger J., Lauterburg B. H. Effect of allopurinol on oxidant stress and hepatic function following ischemia and reperfusion in the rat. Liver. 1988 Dec;8(6):344–349. doi: 10.1111/j.1600-0676.1988.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Smith C. V., Hughes H., Lenz M. L., Jaeschke H., Shappell S. B., Michael L. H., Entman M. L. No evidence for reactive oxygen damage in ischemia-reflow injury. Trans Assoc Am Physicians. 1987;100:54–61. [PubMed] [Google Scholar]
- Nordström G., Seeman T., Hasselgren P. O. Beneficial effect of allopurinol in liver ischemia. Surgery. 1985 Jun;97(6):679–684. [PubMed] [Google Scholar]
- Owens M. L., Lazarus H. M., Wolcott M. W., Maxwell J. G., Taylor J. B. Allopurinol and hypoxanthine pretreatment of canine kidney donors. Transplantation. 1974 Apr;17(4):424–427. doi: 10.1097/00007890-197404000-00015. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen free radicals in shock, ischemia, and organ preservation. Surgery. 1983 Sep;94(3):428–432. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986 Jun;250(6 Pt 1):G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Parks D. A., Williams T. K., Beckman J. S. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988 May;254(5 Pt 1):G768–G774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll F. J., Bissell D. M., Perez H. D. Human hepatocytes metabolizing ethanol generate a non-polar chemotactic factor for human neutrophils. Biochem Biophys Res Commun. 1986 Jun 13;137(2):688–694. doi: 10.1016/0006-291x(86)91133-2. [DOI] [PubMed] [Google Scholar]
- Schaper J., Schaper W. Reperfusion of ischemic myocardium: ultrastructural and histochemical aspects. J Am Coll Cardiol. 1983 Apr;1(4):1037–1046. doi: 10.1016/s0735-1097(83)80106-5. [DOI] [PubMed] [Google Scholar]
- Schlayer H. J., Karck U., Ganter U., Hermann R., Decker K. Enhancement of neutrophil adherence to isolated rat liver sinusoidal endothelial cells by supernatants of lipopolysaccharide-activated monocytes. Role of tumor necrosis factor. J Hepatol. 1987 Dec;5(3):311–321. doi: 10.1016/s0168-8278(87)80037-5. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Mickelson J. K., Lucchesi B. R. Free radical scavengers in myocardial ischemia. Fed Proc. 1987 May 15;46(7):2413–2421. [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976 Feb;172(2):354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- Younes M., Strubelt O. The involvement of reactive oxygen species in hypoxic injury to rat liver. Res Commun Chem Pathol Pharmacol. 1988 Mar;59(3):369–381. [PubMed] [Google Scholar]
- de Groot H., Littauer A. Hypoxia, reactive oxygen, and cell injury. Free Radic Biol Med. 1989;6(5):541–551. doi: 10.1016/0891-5849(89)90047-6. [DOI] [PubMed] [Google Scholar]
- van Dyke R. W., Gollan J. L., Scharschmidt B. F. Oxygen consumption by rat liver: effects of taurocholate and sulfobromophthalein transport, glucagon, and cation substitution. Am J Physiol. 1983 May;244(5):G523–G531. doi: 10.1152/ajpgi.1983.244.5.G523. [DOI] [PubMed] [Google Scholar]




