Abstract
Soil respiration constitutes the second largest flux of carbon (C) between terrestrial ecosystems and the atmosphere. This study provides a synthesis of soil respiration (Rs) in 20 European grasslands across a climatic transect, including ten meadows, eight pastures and two unmanaged grasslands. Maximum rates of Rs (Rsmax), Rs at a reference soil temperature (10°C; Rs10) and annual Rs (estimated for 13 sites) ranged from 1.9 to 15.9 μmol CO2 m−2 s−1, 0.3 to 5.5 μmol CO2 m−2 s−1 and 58 to 1988 g C m−2 y−1, respectively. Values obtained for Central European mountain meadows are amongst the highest so far reported for any type of ecosystem. Across all sites Rsmax was closely related to Rs10.
Assimilate supply affected Rs at timescales from daily (but not necessarily diurnal) to annual. Reductions of assimilate supply by removal of aboveground biomass through grazing and cutting resulted in a rapid and a significant decrease of Rs. Temperature-independent seasonal fluctuations of Rs of an intensively managed pasture were closely related to changes in leaf area index (LAI). Across sites Rs10 increased with mean annual soil temperature (MAT), LAI and gross primary productivity (GPP), indicating that assimilate supply overrides potential acclimation to prevailing temperatures. Also annual Rs was closely related to LAI and GPP. Because the latter two parameters were coupled to MAT, temperature was a suitable surrogate for deriving estimates of annual Rs across the grasslands studied. These findings contribute to our understanding of regional patterns of soil C fluxes and highlight the importance of assimilate supply for soil CO2 emissions at various timescales.
Keywords: soil CO2 efflux, temperature, moisture, gross primary productivity, leaf area index, soil carbon, land use
Introduction
Soil respiration is the major source of CO2 released by terrestrial ecosystems and constitutes the second largest flux of carbon between ecosystems and the atmosphere (Raich and Schlesinger 1992). Current global estimates indicate that soils emit 68–80 Pg C per year (Raich and Potter 1995; Raich and others 2002), which exceeds emission rates from fossil fuel combustion by a factor of more than 10 (Schlesinger and Andrews 2000). In view of a growing interest concerning the role of ecosystems in the terrestrial carbon cycle and its feedbacks to climate change, increasing numbers of studies have explored soil respiration in relation to environmental factors and across bioclimatic gradients. Whilst soil respiration (Rs) has been well characterized for a range of forest ecosystems (for example, recent syntheses for bioclimatic transects by Janssens and others 2001; Kane and others 2003; Reichstein and others 2003; Hibbard and others 2005; Rodeghiero and Cescatti 2005), comparatively little is known for grasslands.
Depending on definition, grasslands cover 20–40% of the land surface. In Europe, grasslands play an important role in areas where climate and topography restrict a highly productive use of crops. Therefore, grasslands are frequent in mountain areas of Central, Southern and Eastern Europe and the uplands at higher latitudes. There is limited evidence that soil CO2 efflux from grasslands may be about 20% higher than that from comparable forest stands (Raich and Tufekcioglu 2000). Unfortunately, however, most annual estimates of soil CO2 efflux from temperate grasslands date back to the 1970s (compare reviews by Raich and Schlesinger 1992; Raich and Potter 1995), when primarily static-chamber methods were applied, which have been shown to underestimate Rs rates when fluxes are high (for example, Norman and others 1997). Recent estimates for annual Rs in grasslands based on dynamic chambers are largely restricted to seasonally dry, C4 dominated grasslands in North and South America (for example, Luo and others 1996; Bremer and others 1998; Davidson and others 2000; Wan and Luo 2003). These grassland systems are mostly unfertilized and prone to extended periods of water stress, and may therefore exhibit lower rates of Rs than could be expected, for example, for managed grasslands in temperate climates. Up to now no comparative study on Rs is available for temperate grasslands across a latitudinal transect. Thus, it is an aim of the present paper to provide an overview of soil CO2 efflux from differently managed and unmanaged temperate grasslands along a climatic gradient. We explore the hypotheses that with increasing mean annual temperature (1) annual soil CO2 efflux increases in the absence of extended droughts and (2) soil respiration at a reference temperature decreases, indicating a temperature acclimation of respiratory processes (as suggested by recent studies by Janssens and others 2003 and Rodeghiero and Cescatti 2005).
A second objective of this study is to analyze possible effects of assimilate supply on Rs at timescales from diurnal to annual. A number of recent studies have shown that assimilate supply may strongly affect Rs. Temperature-independent diurnal variations of Rs under trees have been suggested to result from changes in photosynthesis (Tang and others 2005; Baldocchi and others 2006; Liu and others 2006). An interruption of the transport of assimilates to the soil by tree girdling results in a substantial decrease of soil CO2 efflux (Högberg and others 2001). Likewise, reductions in assimilate supply by shading and/or clipping have been shown to reduce grassland Rs (for example, Craine and others 1999; Wan and Luo 2003; Bahn and others 2006). Estimates of the temperature sensitivity of Rs are often confounded by unidentified processes of substrate supply (compare reviews by Davidson and others 2006; Davidson and Janssens 2006). At an annual timescale Rs in forests is closely related to gross primary productivity (GPP) and leaf area index (LAI) (Janssens and others 2001; Reichstein and others 2003; Hibbard and others 2005), indicating a coupling between the amounts of CO2 assimilated by forest canopies and released from the soil. We expected that, similarly, in grasslands soil respiration responds to assimilate supply (1) at the diurnal scale, rates at a given temperature being higher during daytime than at night, (2) at the daily to weekly scale in relation to grassland management (cutting and grazing), and (3) at the annual scale in relation to GPP and LAI.
Methods
Sites
The sites were studied within the EU-Framework Program 5 projects CarboMont (Cernusca and others 2008) and GreenGrass (Soussana and others 2007) and cover a latitudinal transect from 41 °90′ N to 67°72′ N and an altitudinal range from 50 m (Carlow, Ireland) to almost 2000 m a.s.l. (Stubai, Austrian Alps) (Table 1), mean annual air and soil temperatures ranging from −1.0 to 10.1°C and from 3.3 to 11.8°C, respectively. Mean annual precipitation ranges from 500 to 1760 mm. Grasslands include meadows that are fertilized and cut at least twice a year (intensive use; Oensingen 2, Stubai 1), lightly used meadows that are unfertilized or slightly fertilized, cut once a year and are mostly grazed during short periods late in the season (all other meadows), a fertilized intensive pasture that is cut for silage and is grazed extensively from then onwards (Carlow), moderately grazed pastures, abandoned and natural (unmanaged) grassland. For details on site characteristics refer to Table 1.
Table 1.
Site Characteristics and Data Acquisition
| Site | Land use |
Location (latitude/ longitude) |
Altitude (m) |
MAT air/soil (°C) |
MAP (mm) |
Soil type | Soil texture |
Soil pH |
|---|---|---|---|---|---|---|---|---|
| Amplero, Abruzzi (I) | Mc1g | 41°54′ N 13°36′ E | 900 | 10.0/11.8(5) | 1365 | Haplic phaeozem | C | 6.4 |
| Alinya (E) | P | 42°12′ N 1°27′ E | 1765 | 6.5/8.5(5) | 700 | Lythic cryrendoll | SC | 7.4 |
| Monte Bondone (I) | Mc1 | 46°02′ N 11°07′ E | 1550 | 5.5/9.7(5) | 1189 | Hapludalf | fine L | 5.3 |
| Passo Brocon (I) | P | 46°07′ N 11°41′ E | 1750 | 5.8/n.a. | 1200 | Alfisol | SL | n.a. |
| Seebodenalp (CH) | Mc1g | 47°05′ N 8°45′ E | 1025 | 7.3/n.a. | 1061 | Stagnic cambisol | n.a. | n.a. |
| Oensingen extensive (CH) |
Mc31 | 47°17′ N 7°44′ E | 450 | 9.0/10.5(5) | 1200 | Stagnic cambisol | SL | 7.5 |
| Stubai 1 (A) | Mc3f | 47°17′ N 11°32′ E | 970 | 6.3/9.0(5) | 850 | Fluvisol | SL | 6.8 |
| Stubai 2 (A) | Mc1fg | 47°17′ N 11°32′ E | 1750 | 3.0/6.9(5) | 1097 | Dystric cambisol | fine L | 5.5 |
| Stubai 3 (A) | P | 47°17′ N 11°32′ E | 1960 | 3.0/6.9(5) | 1097 | Dystric cambisol | fine L | 6.8 |
| Berchtesgaden 1 (D) | Ma2 | 47°37′ N 12°35′ E | 620 | 8.3/8.1(5) | 1665 | Cambisol | n.a. | 5.8 |
| Berchtesgaden 2 (D) | P | 47°37′ N 12°35′ E | 620 | 8.3/6.9(5) | 1665 | Cambisol | n.a. | 6.6 |
| Berchtesgaden 3 (D) | Mc2fg | 47°37′ N 12°35′ E | 620 | 8.3/7.6(5) | 1665 | Cambisol | n.a. | 6.6 |
| Berchtesgaden 4 (D) | P | 47°37′ N 12°35′ E | 1420 | 5.6/5.2(5) | 1761 | Rendzina | n.a. | 6.3 |
| Berchtesgaden 5 (D) | P | 47°37′ N 12°35′ E | 1425 | 5.6/5.3(5) | 1761 | Rendzina | n.a. | 6.7 |
| Berchtesgaden 6 (D) | Pr3 | 47°37′ N 12°35′ E | 1430 | 5.6/6.5(5) | 1761 | Rendzina | n.a. | 6.7 |
| Polana (SK) | Mc1g | 48°38′ N 19°32′ E | 850 | 5.8/n.a. | 853 | Eutric cambisol | LS | 4.7 |
| Carlow (IRL) | Pic1f | 52°85′ N, 6°54′ W | 50 | 10.1/10.2 | 974 | Calcic luvisol | SCL | 7.3 |
| Auchencorth Moss (GB) |
U | 55°79′ N 3°24′ W | 270 | 8.0/6.9(5) | 970 | Histosol | Peat | n.a. |
| Cow Park (GB) | Mc1f | 55°52′ N 3°12′ W | 200 | 8.3/8.9(10) | 849 | Gleysol | CL | 6.4 |
| Varriö (FIN) | U | 67°72′ N 29°60′ E | 480 | −1.0/3.3(1) | 500 | n.a. | S | n.a. |
| Site | Soil C (kg m−2) |
LAI (m−2 m2) |
Biomass (g m−2) |
Rs10 (μmol m−2 s−1) |
Rsmax (μmol m−2 s−1) |
GPP (g C m−2 y−1) |
System used |
Data set |
|---|---|---|---|---|---|---|---|---|
| Amplero, Abruzzi (I) | 5.42 | 1.93 | 125 | 3.47 | 9.5 | 1246 | CP4 | 2002–4 |
| Alinya (E) | n.a. | n.a. | 515 | 1.99 | 6.5 | 600 | OH, CP4 | 2003–4 |
| Monte Bondone (I) | 8.12 | 3.2 | 286 | 5.39 | 14.1 | 1358 | CL | 2002–4 |
| Passo Brocon (I) | n.a. | n.a. | n.a. | 3.94 | 14.8 | n.a. | CP2 | 2002–3 |
| Seebodenalp (CH) | n.a. | 5.0 | n.a. | n.a. | 14.2 | n.a. | CL | 2003 |
| Oensingen extensive (CH) |
5.99 | 5.9 | 490.0 | 3.58 | n.a. | 1750 | CL | 2003–4 |
| Stubai 1 (A) | 5.89 | 4.6 | 449 | 4.39 | 15.9 | 1697 | CP4 | 2002–6 |
| Stubai 2 (A) | 7.49 | 4.2 | 431 | 5.49 | 15.1 | n.a. | CP4 | 2002–4 |
| Stubai 3 (A) | n.a. | 1.7 | 157 | 2.20 | 7.3 | n.a. | OH, CP4 | 2002–4 |
| Berchtesgaden 1 (D) | 2.90 | 6.0 | 699 | 3.83 | 10.3 | n.a. | CH | 2003 |
| Berchtesgaden 2 (D) | 3.10 | 3.5 | 207 | 2.86 | 8.4 | n.a. | CH | 2003 |
| Berchtesgaden 3 (D) | 3.10 | 4.7 | 299 | 3.16 | 8.8 | n.a. | CH | 2003 |
| Berchtesgaden 4 (D) | 3.80 | 5.7 | 411 | 2.37 | 8.8 | n.a. | CH | 2003 |
| Berchtesgaden 5 (D) | 5.50 | 4.1 | 275 | 1.42 | 6.5 | n.a. | CH | 2003 |
| Berchtesgaden 6 (D) | n.a. | 4.6 | 438 | 1.48 | 8.1 | n.a. | CH | 2003 |
| Polana (SK) | 7.56 | 3.1 | 415 | n.a. | 12.0 | n.a. | CH | 2004 |
| Carlow (IRL) | n.a. | 4.1 | 680 | 3.19 | 8.4 | 1856 | CPC1 | 2002–4 |
| Auchencorth Moss (GB) |
n.a. | 3.6 | n.a. | 2.09 | 4.9 | 698 | CP3 | 2003–4 |
| Cow Park (GB) | n.a. | 5.7 | 540.4 | 5.06 | 13.6 | n.a. | CP2 | 2003 |
| Varriö (FIN) | 2.50 | 0.8 | n.a. | 0.25 | 1.9 | 200 | CP4 | 2002–3 |
Land use: M(eadow); P(asture); U(nmanaged); number of cuts: c1–5, f(ertilized), g(razed) late in the season; 1… sown in 2001; 2a(bandoned) in 2002; 3… reintroduced in 2002. MAT…mean annual air/soil temperature (in 2003, at the soil depth given in parentheses). MAP…Mean annual precipitation. Soil type according to FAO classification. Soil texture: c(lay), s(ilt), l(oam). Soil carbon (C) content refers to the uppermost 20–25 cm of the soil, leaf area index (LAI) and aboveground biomass refer to peak values. Rs10 (soil respiration at 10°C), Rsmax (maximum soil respiration rates). GPP…gross primary productivity (in 2003). Soil respiration system used: C(losed system), O(pen System), L(ICOR 6400-09) or P(PSystems upgraded SRC-1, EGM)2–4 or C(iras)1, H(omemade, compare text). Data set: 2002–4 refers to the years when soil respiration was measured.
Soil Respiration Measurements
The two major systems used in this study were the LI 6400-09 soil respiration chamber combined with a LI 6400 IRGA (Li-Cor, Lincoln, NE, USA) and the upgraded SRC-1 chamber in combination with an IRGA of the same company (EGM 1, 2 and 4, CIRAS-1) (PPSystems, Hitchin, Herts, UK) (Table 1). On several occasions these two systems were cross-compared (October 2002, Stubai Valley; April 2004, Innsbruck; August 2004, Seebodenalp; September 2004, Monte Bondone; summer 2005, Viterbo), indicating a good agreement (on the average less than 5% difference) between the systems applied on the same collars. At the Berchtesgaden sites a manually operated closed system (home-made system as described by Velthof and Oenema 1995, combining a chamber with a vent with IRGAs (Li-800 and Li-6262, Licor, Lincoln, NE, USA) was applied (compare also Pumpanen and others 2004). At Polana a home-made chamber was attached to an infrared gas analyzer (Li 6250, Li-Cor, USA). At Alinya and at the Stubai sites in addition to the closed chamber systems home-made open systems were installed. For measurements, all chambers were placed on collars, which had been inserted into the soil at least 24 h prior to measurement (Bahn and others 2008). Aboveground vegetation was removed from inside the collars before measurements were started. In parallel to soil respiration measurements soil temperature (using soil temperature probes) and soil moisture (using TDR probes) were recorded.
Additional Site Parameters
Continuous half-hourly means of soil temperature and moisture were obtained with microclimate stations using soil thermocouples and TDR sensors. Soil carbon stocks were measured on at least nine cores per sites, except at Värriö where fewer large soil monoliths were excavated. Sieved and root-free soil samples were analyzed for C using elemental analyzers. Leaf area index and standing biomass and were measured from samples cut at the soil surface. Leaf area was determined using leaf area meters. For biomass estimates samples were oven-dried at 70–80°C. Gross primary productivity was estimated from eddy covariance measurements, as described by Wohlfahrt and others (2008) and Gilmanov and others (2007).
Annual Totals of Soil Respiration
Annual totals of soil respiration were estimated by applying the following functions, which were fitted against the measured values.
| (1) |
where Rs denotes soil respiration rate (μmol CO2 m−2 s−1); a, b and k are fitting parameters and Ts is soil temperature (°C) (compare Rodeghiero and Cescatti 2005).
| (2) |
where Rs10 is the soil respiration rate at 10°C and Eo is the activation energy (Lloyd and Taylor 1994, Eqn. 1).
For the sites Alinya, Monte Bondone and Carlow inclusion of soil water content as a separate parameter resulted in a distinctly better model fit; therefore, a Gompertz function (Janssens and others 2003) was added to Eqn. 2:
where a and b are fitting parameters and Ms is the soil moisture (relative volumetric soil water content, %).
A nonlinear estimation least squares method (STATISTICA, StatSoft, Inc., 2005) was used to fit the models and derive model parameters. For estimating the generalization error of the applied models (Eqs. 1, 2, 3), and testing their performance, we applied a holdout validation method (Shao 1993). For each site the original dataset was separated in two datasets called the “training set” and the “testing set”. The testing set was selected by randomly sampling 20% of the original data, according to their temperature frequency distribution. The remaining 80% of the data belonging to the training set was used for model fitting and to derive the model parameters with a nonlinear estimation least square method (STATISTICA, Statsoft, Inc., 2005). We preferred the holdout validation method to a more complex k-fold cross validation (Shao 1993), because the datasets were large enough to allow for extraction of 20% of the data, leaving at the same time enough data for model fitting (more than 21; Table 2). Table 2 summarizes the model parameters and r2, as well as linear regression statistics of the validation. At all sites residuals of predicted versus observed values of Rs were plotted against Ms, r2 values of the linear regressions are reported in Table 2. The models (1)–(3) were compared with respect to r2, mean absolute error (MAE) and model efficiency (ME) (compare for example, Medlyn and others 2005; Richardson and Hollinger 2005), and the best performing model was selected for estimating annual totals of soil respiration for each site (Table 2).
Table 2.
Annual Soil Respiration: Model Parameters, r2 of Model Fit, Number of Observations, Validation (r2, Slopes and Intercepts of Linear Fits between Modelled and Independently Observed Rates of Soil Respiration), Mean Absolute Error (MAE) and Model Efficiency (ME), r2 of Residuals of Modelled Versus Observed Soil Respiration in Relation to Soil Water Content (SWC), Modelled Annual Soil Respiration Based on Single and on Bin-Averaged Data and Confidence Interval of Annual Soil Respiration (CI, in % Percent of Annual Total Obtained from Bin-Averaged Data)
| Site | Model | Model parameters | r 2 | n | Validation |
MAE | ME | Residuals versus SWC r2 |
Annual Rs (g m−2 y−1) Total |
CI (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r 2 | Slope | Intercept | ||||||||||
| Amplero | 1 | a = 6.05, b = 18.18, k = 0.32 | 0.37 | 120 | 0.39 | 1.35 | −0.71 | 1.59 | 0.37 | 0.29 | 1305 | 40.9 |
| Alinya | 3 | Rs10 = 1.99, Eo = 236.9, c = −11.187, d = 0.859 | 0.47 | 806 | 0.50 | 0.82 | 0.36 | 7.85 | 0.35 | 0.00 | 494 | 17.1 |
| Monte Bondone | 3 | Rs10 = 5.39, Eo = 384.3, c = −0.0059, d = 0.035 | 0.74 | 430 | 0.82 | 1.09 | −0.70 | 7.55 | 0.68 | 0.01 | 1743 | 17.1 |
| Oensingen 1 | 1 | a = 14.84, b = 17.22, k = 0.17 | 0.45 | 131 | 0.44 | 1.02 | 1.38 | 2.76 | 0.75 | 0.00 | 1988 | 87.4 |
| Stubai 1 | 1 | a = 12.69, b = 23.29, k = 0.25 | 0.66 | 380 | 0.71 | 1.01 | −0.10 | 1.22 | 0.97 | 0.04 | 1792 | 11.2 |
| Stubai 3 | 1 | a = 5.33, b = 8.95, k = 0.18 | 0.48 | 1066 | 0.51 | 1.03 | −0.15 | 0.81 | 0.83 | 0.10 | 729 | 9.4 |
| Berchtesgaden 1 | 2 | Rs10 = 3.83, Eo = 310.2 | 0.80 | 55 | 0.68 | 0.86 | 0.77 | 0.95 | 0.96 | 0.03 | 1378 | 22.0 |
| Berchtesgaden 2 | 2 | Rs10 = 2.86, Eo = 260.2 | 0.77 | 68 | 0.67 | 0.93 | 0.34 | 0.82 | 0.97 | 0.05 | 907 | 32.0 |
| Berchtesgaden 3 | 2 | Rs10 = 3.16, Eo = 288.7 | 0.77 | 55 | 0.84 | 0.98 | 0.65 | 0.90 | 0.96 | 0.01 | 1070 | 19.7 |
| Carlow | 3 | Rs10 = 3.19, Eo = 269.8, c = −7.109, d = 0.422 | 0.44 | 69 | 0.34 | 0.90 | 0.22 | 8.70 | 0.50 | 0.06 | 1166 | 69.7 |
| Auchencorth Moss | 2 | Rs10 = 2.09, Eo = 541.3 | 0.65 | 22 | 0.47 | 0.69 | 0.53 | 0.46 | 0.91 | 0.06 | 529 | 62.9 |
| Cow Park | 1 | a = 5.65, b = 520.23, k = 0.84 | 0.23 | 1044 | 0.19 | 0.81 | 0.98 | 1.84 | 0.82 | 0.09 | 1246 | 12.5 |
| Varriö | 1 | a = 1.71, b = 17.15, k = 0.11 | 0.45 | 26 | 0.39 | 0.88 | 0.08 | 0.13 | 0.83 | n.a. | 58 | 23.9 |
Annual soil CO2 efflux was calculated as the sum of single half-hourly fluxes obtained from model outputs based on continuously recorded soil temperature and moisture data. There are several approaches to estimating the uncertainty of modelled annual totals of fluxes, including Monte Carlo and bootstrap simulations (Richardson and Hollinger 2005). We decided to adopt a simpler approach, which is more conservative than the ones mentioned above and thus likely provides comparatively higher uncertainty estimates. We binned the data to equally sized Ts classes. For each class the average and the 95% confidence interval of Rs were calculated, which were each used for parameterizing the equation previously applied for the site (compare Lavigne and others 1997) and calculating annual Rs and its confidence interval as described above. Following this approach the uncertainty estimate for a selected site (Stubai 1) is approximately five times higher (11.2%, Table 2) than when applying a bootstrap analysis (2.2%), and about half the value when the bootstrap analysis is combined with a Monte Carlo simulation (assuming a Gaussian uncertainty of 20%) of soil temperature (24%).
Results
Rates of soil respiration at a reference temperature of 10°C (Rs10) ranged from 0.25 to 5.49 μmol m−2 s−1 (Table 1) and increased with increasing mean annual soil temperature, C content of the upper soil layers, peak leaf area index and annual gross primary productivity across sites (Figure 1A–D). Maximum Rs exceeded 14 μmol m−2 s s−1 for a number of Central European sites, reaching values of up to 15.9 μmol m−2 s−1 on warm summer days (Table 1, Figure 2). Across sites maximum Rs rates were not correlated with associated soil temperatures, but generally increased with increasing Rs10 of a site (Figure 2A, B). The temperature sensitivity of Rs (Q10) based on the pooled data sets and calculated for a common temperature range (at 5 cm depth) from 10 to 15°C ranged from 2.0 to 4.9 and was not related to mean annual soil temperature (r2 = 0.01; not shown).
Figure 1.
Soil respiration at a reference temperature (10°C) in relation to A mean annual soil temperature (r2 = 0.69, P < 0.001), B soil C content in the uppermost 20–25 cm of the soil (r2 = 0.50, P < 0.05), C peak leaf area index (r2 = 0.55, P < 0.01) and D annual gross primary productivity (r2 = 0.88, P = 0.001). Meadows (▲), pastures (●), unmanaged Northern grasslands (◇).
Figure 2.
Maximum rates of soil respiration in relation to A the associated soil temperature and B soil respiration at a reference temperature (10°C) (r2 = 0.87, P < 0.001). Meadows (▲), pastures (●), unmanaged Northern grasslands (◇). Error bars denote standard deviations obtained for the highest spatially replicated soil respiration rates (n = 3–9) recorded at a single point of time.
Soil moisture constrained Rs at higher soil temperatures at most sites. This is reflected by the logistic model, whose sigmoid shape levels Rs at higher Ts when limitations due to low soil moisture are frequent, and the Lloyd and Taylor model combined with a Gompertz-function, which modifies the temperature response of Rs in relation to soil moisture. Reductions of Rs by low soil moisture were most pronounced at the Spanish site Alinya, which—with the exception of the northernmost site Värriö—was characterized by the lowest amounts of annual precipitation (Table 1). Two time series illustrate the course of Rs at Alinya during two summer periods immediately before and after short rainfall events. In July 2003 Rs was low and its diurnal variation was minor until after a rain event when soil moisture increased from less than 10 to more than 20 vol%. After a short time lag following the rain pulse soil CO2 efflux doubled and then followed a distinct diurnal pattern (Figure 3A). As soil moisture dropped below 10 vol% during the subsequent days, Rs was again reduced to values occurring before the rain, with a decreasing response to Ts fluctuations. In June 2004, soil moisture never decreased to less than 20 vol%, and an increase in soil moisture after rain did not alter Rs rates and their response to Ts (Figure 3B).
Figure 3.
Soil respiration (◆), soil temperature (dashed line) and soil moisture (solid line) before and after rainfall during summer periods in A 2003 and B 2004 at Alinya. Bars indicate the timing and the amount rainfall. Soil respiration was measured using a continuous system on five collars, which were changed on DOY 206 in 2003, as indicated by different symbols. Note the different scales in A and B.
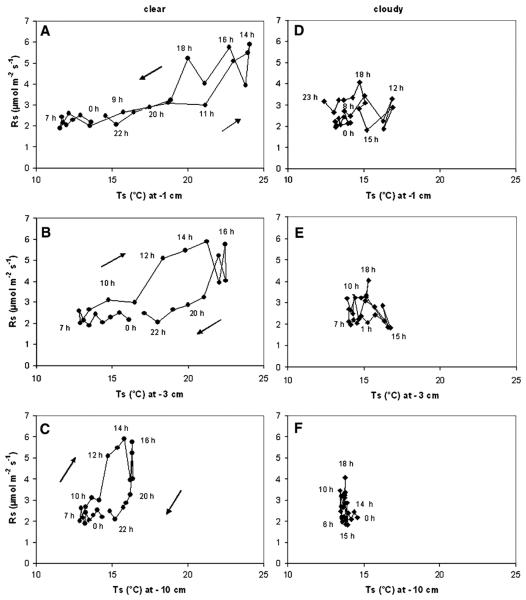
The temperature response of Rs was not only influenced by thresholds of soil moisture, but varied under non-limiting water supply in the course of the day. This was particularly obvious on clear days, when a hysteresis effect occurred, which became more apparent when relating soil CO2 efflux to temperature at increasing soil depth (Figure 4A–C). When related to a given Ts at 1 cm soil depth Rs was higher in the late afternoon as compared to the morning hours (Figure 4A), whereas in relation to a given Ts at deeper soil layers Rs was highest during morning hours and lowest at night (Figure 4B–C). On a cloudy day at a given Ts at 1 cm evening and early night-time values of Rs were slightly higher than during the rest of the day (Figure 4D), whereas no clear temperature response and temperature-independent pattern of Rs were observed in relation to temperatures at deeper soil layers (Figure 4E–F).
Figure 4.
Diurnal changes of soil respiration (Rs) in response to temperature at 1, 3 and 10 cm soil depth on a clear (18 June 2004; A–C) and a subsequent cloudy day (19 June 2004; D–F) at Alinya. Consecutive hourly means have been connected by lines, inserts indicate time of the day. For further details on the time course of soil respiration, soil temperature and soil water content refer to Figure 3B.
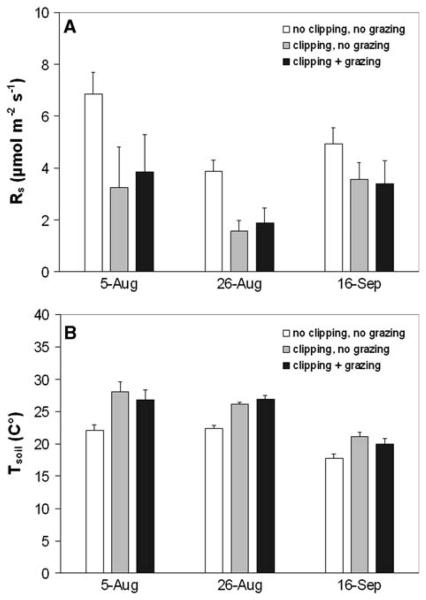
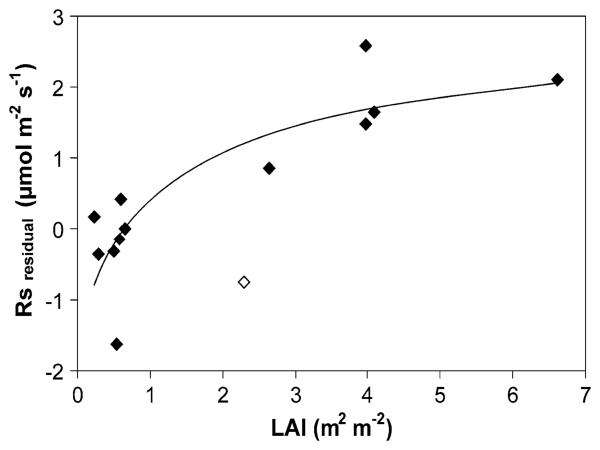
At a daily to weekly timescale land use affected Rs and its response to Ts. At Monte Bondone Rs of clipped plots was reduced by approximately 10% relative to adjacent unclipped plots, and recovered after about 2 weeks (data not shown). At Amplero clipping resulted in a reduction of Rs by more than 50% at two periods during August, and by almost 30% later in the season, whereas it caused an increase in soil temperature during all periods (Figure 5). Additional grazing caused a further significant reduction of Rs only in late August (Figure 5). At Carlow a silage cut early in the season followed by subsequent extensive grazing resulted in distinct seasonal fluctuations of LAI, which explained much of the variation of observed versus predicted Rs values, as based on the seasonal relationship between Ts and Rs (Figure 6).
Figure 5.
Effects of clipping and grazing on A soil CO2 efflux and B soil temperature at Amplero during the summer of 2004 (for each treatment n = 9).
Figure 6.
Residuals of observed minus predicted values of soil respiration at Carlow in relation to seasonal values of leaf area index (LAI) in 2003 (r2 = 0.70, P < 0.001, when excluding the data point indicated with an open symbol from the regression).
Annual soil CO2 efflux from the grasslands ranged from 58 to 1988 g C m−2 y−1 (Table 2), the average being 1108 g C m−2 y−1. The highest values were found for meadows in the Alps (Oensingen, Stubai Valley, Monte Bondone), the lowest occurred at the northernmost site in Finland (Värriö). The uncertainty of the annual estimates was 9–25% for most sites, but as high as 40 to almost 90% for three sites in different parts of the European transect (Table 2). Across sites annual Rs was closely related to mean annual Ts (Figure 7A). Without the two Mediterranean sites (Amplero, Alinya), whose annual totals were distinctly below the regression line, r2 of the regression increased from 0.81 to 0.92 (P < 0.001). Across sites annual Rs increased with increasing soil C content in the upper soil layers (r2 = 0.65; not shown) and peak leaf area index (Figure 7B, without values for Auchencorth Moss r2 = 0.77), and was very well correlated with gross primary productivity (Figure 7C), but exhibited no relationship with peak aboveground biomass (r2 = 0.02; not shown).
Figure 7.
Annual soil respiration in relation to A mean annual soil temperature (r2 = 0.81, P < 0.001), B peak leaf area index (r2 = 0.74, P < 0.001) and C annual gross primary productivity (r2 = 0.94, P < 0.001). Meadows (▲), pastures (●), unmanaged Northern grasslands (◇).
Land use of the grasslands studied was generally related to mean annual soil temperature (Figure 7A), which decreased from meadows (mean annual Ts across sites 9.5 ± 0.6°C) to pastures (8.3 ± 0.7°C), and the natural grasslands (5.1 ± 1.8°C). Thus it is not possible to separate effects of land use on annual Rs from those related to temperature. Average annual Rs pooled for meadows, pastures and natural grasslands was 1520 ± 151, 865 ± 155 and 293 ± 236 g C m−2 y−1, respectively.
Discussion
Soil Respiration in Relation to Climate and Site Productivity
Raich and Schlesinger (1992) and Raich and Potter (1995) showed that at a global scale temperature is the single best predictor of annual Rs in the absence of water stress. More recently, several authors have pointed out that a consideration of productivity (for example, gross primary productivity, GPP) or surrogates of productivity (for example, peak leaf area index, LAI) improves predictions of annual Rs of forests (Janssens and others 2001; Reichstein and others 2003; Hibbard and others 2005), especially in cases when GPP does not increase consistently with temperature (Janssens and others 2001). Dornbush and Raich (2006) have demonstrated for central Iowa grasslands that, in contrast, aboveground net primary productivity (ANPP) is less suited for predicting intra-annual variations of Rs than soil temperature.
All these observations are well supported by our data. We found that across sites annual Rs was closely related to GPP and LAI (Figure 7C, D). At the same time peak standing biomass, a proxy for ANPP, did not explain intersite variations of annual Rs. This, however, was not unexpected, as peak standing biomass underestimates productivity in grasslands that are cut more than once a year or that are intensively grazed, and it likely overestimates productivity in unmanaged sites. Moreover, estimates of site productivity from aboveground biomass may be strongly biased, as they ignore belowground productivity as well as dying and decomposing biomass. Mean annual Ts was a very good predictor of annual Rs across sites, except for the two Mediterranean mountain grasslands, which exhibited distinctly lower annual Rs than expected from its relationship to mean annual Ts. For these two cases proxies or direct measures of soil moisture (compare Table 2; Figure 3) would need to be included in models accounting for the variability of Rs at larger scales (Raich and others 2002; Reichstein and others 2003). The fact that the slope of the temperature–respiration relationship of the grasslands studied (Figure 7A) was best described by a power rather than a linear (Raich and Schlesinger 1992) function may be explained by the fact that land use changed from pastures to more productive, fertilized meadows as annual temperatures increased.
There is some disagreement between studies on whether (Smith 2003; Rodeghiero and Cescatti 2005) or not (Janssens and others 2003; Reichstein and others 2003) soil C content influences Rs. Rodeghiero and Cescatti (2005) suggested that a lack of standardized protocols for soil C sampling and a confusion of the terms concentration and content may obscure a possible relationship of soil C content and respiration. Our data indicate an increase in annual Rs with soil C content in the uppermost soil layers. It should, however, be noted that sites with high soil C content were also characterized by higher productivity. The degree to which soil efflux is coupled to soil C content may be largely determined by the proportions of labile versus recalcitrant C (Gu and others 2004; Davidson and Jansssens 2006), as well as priming effects on soil organic matter decomposition through fresh organic C from litter-fall and root exudation (Kuzyakov 2002; Pendall and others 2003; Subke and others 2004), which may be coupled to short-term assimilate supply as related to gross primary productivity.
Soil respiration at a reference temperature (Rsref) has often been used as an input for temperature dependent models of Rs and as a key parameter for comparing Rs across bioclimatic transects (Janssens and others 2003; Reichstein and others 2003; Hibbard and others 2005; Rodeghiero and Cescatti 2005). Our study indicates that Rsref is also well suited for predicting maximum rates of Rs occurring across sites (Figure 2B). Recently some authors interpreted an observed decrease of Rsref with increasing mean annual temperature along bioclimatic transects as an indication of acclimation of the respiration of roots and microorganisms (Janssens and others 2003; Rodeghiero and Cescatti 2005). This interpretation is not supported by our results, which show that Rsref increases with mean annual Ts (Figure 1A). As Rsref increased with site productivity (GPP, LAI) across all these mentioned studies (compare also Reichstein and others 2003; Hibbard and others 2005) it appears that site productivity rather than acclimation to a mean annual temperature determines Rsref, indicating that substrate supply overrides potential acclimation.
Magnitude of Grassland Soil Respiration and Its Significance for Partitioning Ecosystem Carbon Fluxes
Soil CO2 efflux varied considerably across the grasslands studied. Peak values, as regards instantaneous maximum flux rates, rates at a reference temperature and annual totals, were amongst the highest reported in the literature (Table 3). Especially Central European meadows exhibited distinctly higher rates and annual totals of soil CO2 efflux than has previously been documented for most forests and grasslands (Table 3). These meadows are characterized by comparatively high Ts during summer coupled with only minor restrictions due to low soil moisture, by fertilization and, in consequence, by a comparatively high peak leaf area index and gross primary productivity. Comparatively low values of CO2 efflux of less than 1 μmol m−2 s−1 during winter were compensated by very high respiration rates during summer (up to 14–16 μmol m−2 s−1) yielding annual totals in the range of 1743–1988 g C m−2 y−1 (Tables 1, 2). For a lightly grazed grassland on the Tibetan plateau a similarly high annual soil CO2 efflux was estimated (Cao and others 2004, Table 3).
Table 3.
Soil Respiration at a Reference Temperature, Maximum and Annual Soil Respiration in Grasslands from Different Regions in Relation to Ranges Reported for Forests Compared Along Bioclimatic Transects, and Some Selected Forest Sites
| Ecosystem type, region |
R (at Tref) (μmol m−2 s−1) |
Rmax (μmol m−2 s−1) |
Rannual (g C m−2 y−1) |
References |
|---|---|---|---|---|
| Grasslands | ||||
| Temperate grasslands | 187–8302 | Various sources in Raich and Schlesinger (1992) | ||
| Tropical grasslands and savannas | 380–9002 | Various sources in Raich and Schlesinger (1992) | ||
| Tropical pastures (Amazonia) | 5.1 (25°) | 11.01 | 1000–1500 | Davidson and others (2000) |
| Tallgrass prairie (North America) | 9.5 | 1090–1347 | Bremer and others (1998), Bremer and Ham (2002) | |
| Tallgrass prairie (North America) | 3 (18°)1 | 11001 | Wan and Luo (2003) | |
| Tallgrass prairie (North America) | 1700 | Mielnick and Dougas (2000) | ||
| Konza prairie (North America) | 15 (18–22)3 | 1300–2100 | Knapp and others (1998); Harper and others (2005) | |
| Central Iowa C3 grassland (North America) | 730 | Dornbush and Raich (2006) | ||
| Central Iowa C4 grassland (North America) | 1300 | Dornbush and Raich (2006) | ||
| California grassland (North America) | 3.8 | 344–485 | Luo and others (1996) | |
| Shortgrass steppe (North America) | 4.6 | 530 | Pendall and others (2003) | |
| Great Basin western wheatgrass–cheatgrass | 0.5–6.51 (15°) | 3.3–5.7 | 400–6501 | Hibbard and others (2005) |
| Oklahoma grassland (North America) | 704–881 | Zhou and others (2007) | ||
| Pasture (lightly–heavily grazed) (Tibet) | 1.7–3.9 (10°) | 3.2–6.5 | 1533–2035 | Cao and others (2004) |
| Meadows, latitudinal transect (Europe) | 3.2–5.5 (10°) | 9.5–15.9 | 1070–1988 | This study (range, for details refer to Tables 1 and 2) |
| Pastures, latitudinal transect (Europe) | 1.4–3.2 (10°) | 6.5–14.8 | 494–1166 | This study (range, for details refer to Tables 1 and 2) |
| Ungrazed, Northern Europe | 0.3–2.1 (10°) | 1.9–4.9 | 58–529 | This study (range, for details refer to Tables 1 and 2) |
| Forests | ||||
| Forests, latitudinal transect (Europe) | 0.7–4.9 (10°) | 281–1456 | Janssens and others (2001, 2003) | |
| Forests, latitudinal transect (Europe, US) | 1.7–12.5 | 427–1805 | Hibbard and others (2005) and sources therein | |
| Temperate forests | 250–14142 | Various sources in Raich and Schlesinger (1992)2 | ||
| Tropical forests | 345–14502 | Various sources in Raich and Schlesinger (1992)2 | ||
| Tropical forest, Amazonia | 11.61 | 2000 | Davidson and others (2000) | |
| Douglas-fir forest | 1920 | Drewitt and others (2002) |
Estimated from figure.
Most data based on closed static chambers.
Peak fluxes after rainfall.
Attempts to quantify soil CO2 emissions at scales from ecosystems to the globe (Raich and Schlesinger 1992; Raich and Potter 1995; Schlesinger and Andrews 2000; Raich and others 2002) rely very much on the accuracy of estimates of annual totals and their underlying parameters. However there is still a considerable uncertainty in such estimates (for example, Hibbard and others 2005; this study), which may result from inevitable tradeoffs between temporal and spatial data coverage (Savage and Davidson 2003), the large amount of abiotic and biotic drivers and their interactions that may vary, and often co-vary, in the course of the year (for example, Davidson and others 2006) and interannually (Raich and others 2002) and the vast diversity of C substrates and enzymes, which so far constrain mechanistic approaches to modelling soil respiration (Davidson and Janssens 2006). Such uncertainties not only reduce the value of estimates of the magnitude of individual fluxes, but also limit their usefulness for partitioning ecosystem carbon fluxes. A particularly critical aspect concerning estimates of ecosystem CO2 component fluxes is related to systematic differences between different methods. This is a major constraint when, for example, relating absolute values of annual Rs (based on chamber measurements) to those of GPP (derived from eddy covariance measurements) (Table 1, Figure 7D). Generally, chamber-based estimates of fluxes (as mostly applied for soil respiration measurements) tend to yield distinctly (20–40%) higher values than those based on eddy covariance measurements (for example, Goulden and others 1996; Lavigne and others 1997; Drewitt and others 2002; Bolstad and others 2004; Wohlfahrt and others 2005a). There are a number of major reasons for these discrepancies. These include: (1) differences in spatial sampling/footprint area (Lavigne and others 1997; Drewitt and others 2002; Davidson and others 2002); (2) overestimation of dynamic chamber-based fluxes through underpressure created in chamber headspace by surface wind (Bain and others 2005); (3) underestimation of EC-based respiration fluxes through advection or insufficient turbulence/mixing at night (Goulden and others 1996; Lavigne and others 1997). (4) Daytime respiration rates may be overestimated when extrapolating from EC-based nighttime data respiration fluxes due to a neglected reduction of leaf respiration in light (Atkin and others 1997; Amthor and Baldocchi 2001; Wohlfahrt and others 2005b). (5) Daytime respiration rates extrapolated from nighttime data usually do not account for possible temperature-independent effects on Rs (Tang and others 2005; Liu and others 2006; this study, but see discussion below). Therefore, care should be taken when using soil respiration data based on chamber measurements for partitioning ecosystem, regional or global fluxes of CO2, which are based on different methodologies.
Soil Respiration and Short-Term Changes in Assimilate Supply
In recent years it has become increasingly evident that soil respiration is closely related to canopy photosynthesis at various timescales. As discussed above, GPP and its surrogate LAI are generally well correlated with annual Rs. Also at the seasonal timescale we observed in an intensively managed pasture that the residuals of the temperature–respiration relationship could be well explained by changes in LAI (Figure 6). Likewise, Reichstein and others (2003) found that across a range of forest ecosystems residual Rs (based on a model using temperature and precipitation as a predictor) was closely related to LAI. Bremer and Ham (2002) found for an intensively grazed Konza prairie that LAI needed to be considered to obtain realistic estimates of annual Rs. Our cutting and grazing experiments indicate that removal of the leaf mass may result in a significant reduction of Rs (Figure 5) and its temperature sensitivity. This is in agreement with observations from tallgrass prairies and a pasture on the Tibetan plateau (Bremer and others 1998; Johnson and Matchett 2001; Wan and Luo 2003; Cao and others 2004). In contrast, it has also been observed that soil warming following clipping may override effects of reduced assimilate supply and lead to an increase in Rs (Bahn and others 2006). However, when corrected for such temperature effects Rs is consistently reduced by clipping within 1–2 days (Wan and Luo 2003; Bahn and others 2006; this study), which is arguably the effect of a lack of assimilate supply by photosynthesis.
These findings strongly favor the view of a close short-term coupling of photosynthesis and Rs. Evidence also comes from isotopic studies demonstrating that in forests Rs is largely driven by freshly produced photosynthates and that time-lags for the isotopic signal from tree photosynthesis to appear in Rs are in the range of 1–10 days (Ekblad and Högberg 2001; Bowling and others 2002; Ekblad and others 2005; Steinmann and others 2004). It remains, however, somewhat speculative to what extent Rs is affected by photosynthesis at a diurnal timescale. Recently, it has been shown that temperature-independent diel variations of Rs occur, which have been interpreted as modulating effects of photosynthesis (Tang and others 2005; Baldocchi and others 2006; Liu and others 2006). Our results indicate, similarly, that there is a diurnal hysteresis in the temperature–Rs relationship (Figure 4). However, especially on sunny days the pattern changes depending on the soil depth at which temperature is measured (Figure 4A–C). Such a diel hysteresis, which can also be depicted by the residuals of observed versus predicted values of the temperature–respiration relationship (Liu and others 2006), may be determined by a number of factors that produce a time lag between the rate of CO2 flux emitted from the soil and the Ts at a given depth, the most predominant ones being (1) shifts in phase and amplitude of soil temperature with depth, (2) diurnal changes of soil moisture close to a critical threshold value, (3) time lags between CO2 production in various soil layers and the diffusion of CO2 out of the soil (which may be more relevant when soil moisture is high) and (4) possible effects of diurnal changes in the supply of newly produced photosynthates on root and rhizosphere respiration. In the case of Figure 4 soil moisture is unlikely to have determined the observed hystereses (compare Figure 3 and section “Results”). However, it is not possible to clearly separate potential effects of progressive changes in Ts and of photosynthesis. Even when assuming (1) a constant basal respiratory activity and (2) a constant temperature response across all soil layers as well as (3) no short-term effects of photosynthesis on soil respiration, a diurnal hysteresis in the relationship between soil temperature at any fixed depth and soil respiration would occur, because shifts in phase and amplitude of soil temperature with depth during the day create time lags in the relationship (Reichstein and others 2005). However, it is likely that respiratory activity and its temperature response are not evenly distributed in the soil, being higher in soil layers with high root activity and lower in deeper soil layers where little or no rhizosphere priming of the soil organic matter decomposition occurs (for example, Boone and others 1998; Kuzyakov 2002; Pendall and others 2003; Fontaine and others 2004). In that case effects will be much more confounded and preclude a clear interpretation of an apparent diurnal relationship of canopy photosynthesis versus soil respiration (as described for example, by Tang and others 2005). A way forward in understanding the short-term coupling between canopy photosynthesis and Rs would be to monitor diurnal changes in CO2 production in the main rooting horizon together with the associated Ts and moisture, together with canopy photosynthesis, and/or to carry out factorial manipulation experiments including isotopic pulse labelling.
Conclusions
This study provides the first synthesis of soil respiration in temperate (mostly mountain) grasslands along a climatic transect, documenting that soil CO2 efflux from Central European meadows is amongst the highest so far reported for any type of ecosystem. The study indicates that in and across grasslands soil respiration is closely related to assimilate supply for time scales from daily (but not necessarily diurnal) to annual. Across sites Rs10increased with mean annual soil temperature (MAT), LAI and gross primary productivity (GPP) indicating that assimilate supply overrides potential acclimation to prevailing temperatures. Also annual Rs was closely related to LAI and GPP. Because the latter two parameters were coupled to MAT, temperature was a suitable surrogate for deriving estimates of annual Rs across the grasslands studied. Thus, our study contributes to an understanding of regional patterns of soil C fluxes, and highlights the importance of assimilate supply as a major driver of soil CO2 emissions and the necessity of future work examining more explicitly a direct short-term coupling between canopy photosynthesis and soil respiratory fluxes.
ACKNOWLEDGEMENTS
The study was funded by the EU FP5 projects CarboMont (EVK-2001-00125) and GreenGrass (EVK-2001-00105); data analysis was supported by the Austrian National Science Fund (FWF) project P18756-B16. We acknowledge assistance in data collection by Nadine Pfahringer, Anton Pallua, Anton Stefan Schwarz, Robert Bajo, Christian Skublics, Yuelin Li and Silvia Baronti.
REFERENCES
- Amthor JS, Baldocchi DD. Terrestrial higher plant respiration and net primary productivity. In: Roy J, Saugier B, Mooney HA, editors. Terrestrial global productivity. Academic Press San Diego; San Diego: 2001. pp. 33–59. [Google Scholar]
- Atkin OK, Westbeek MHM, Cambridge ML, Lambers H, Pons TL. Leaf respiration in light and darkness. Plant Physiol. 1997;113:961–5. doi: 10.1104/pp.113.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A. Root respiration in temperate mountain grasslands differing in land use. Glob Chang Biol. 2006;12:995–1006. [Google Scholar]
- Bahn M, Kutsch W, Heinemeyer A, Janssens I. Towards a standardised protocol for the assessment of soil CO2 fluxes. In: Kutsch W, Bahn M, Heinemeyer A, editors. Soil carbon fluxes. An integrated methodology. Cambridge University Press; 2008. (in press) [Google Scholar]
- Bain WG, Hutyra L, Patterson DC, Bright AV, Daube BC, Munger JW, Wofsy SC. Wind-induced error in the measurement of soil respiration using closed dynamic chambers. Agric For Meteorol. 2005;131:225–32. [Google Scholar]
- Baldocchi D, Tang J, Xu L. How switches and lags in biophysical regulators affect spatio-temporal variation of soil respiration in an oak-grass savanna. J Geophys Res. 2006;111 doi: 101029/2005JG000063. [Google Scholar]
- Bolstad PV, Davis KJ, Martin J, Cook BD, Wang W. Component and whole-system respiration fluxes in northern deciduous forests. Tree Physiol. 2004;24:493–504. doi: 10.1093/treephys/24.5.493. [DOI] [PubMed] [Google Scholar]
- Boone RD, Nadelhoffer KJ, Canary JD, others Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature. 1998;396:570–2. [Google Scholar]
- Bowling DR, McDowell NG, Bond BJ, Law BE, Ehleringer JR. 13C content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia. 2002;131:113–24. doi: 10.1007/s00442-001-0851-y. [DOI] [PubMed] [Google Scholar]
- Bremer DJ, Ham JM. Measurement and modelling of soil CO2 flux in a temperate grassland under mowed and burned regimes. Ecol Appl. 2002;12:1318–28. [Google Scholar]
- Bremer DJ, Ham JM, Owensby CE, Knapp AK. Responses of soil respiration to clipping and grazing in a tallgrass prairie. J Environ Qual. 1998;27:1539–48. [Google Scholar]
- Cao G, Tang Y, Mo W, Mo W, Wang Y, Li Y, Zhao X. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol Biochem. 2004;36:237–43. [Google Scholar]
- Cernusca A, Bahn M, Berninger F, Tappeiner U, Wohlfahrt G. Effects of land-use changes on sources, sinks and fluxes of carbon in European mountain grasslands. Ecosystems. 2008 (this issue) [Google Scholar]
- Craine JM, Wedin DA, Chapin FSIII. Predominance of ecophysiological controls on soil CO2 flux in a Minnesota grassland. Plant Soil. 1999;207:77–86. [Google Scholar]
- Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–73. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- Davidson EA, Janssens IA, Luo Y. On the variability of respiration in terrestrial ecosystems moving beyond Q10. Glob Chang Biol. 2006;12:154–64. [Google Scholar]
- Davidson EA, Savage K, Verchot LV, Navarro R. Minimizing chamber artifacts and biases in chamber-based measurements of soil respiration. Agric For Meteorol. 2002;113:21–37. [Google Scholar]
- Davidson EA, Verchot LV, Cattanio JH, Ackermann IL, Carvalho JEM. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry. 2000;48:43–69. [Google Scholar]
- Dornbush ME, Raich JW. Soil temperature, not aboveground plant productivity, best predicts intra-annual variations of soil respiration in Central Iowa grasslands. Ecosystems. 2006;9:909–20. [Google Scholar]
- Drewitt GB, Black TA, Nesic Z, Humphreys ER, Jork EM, Swanson R, Ethier GJ, Griffis Morgenstern TK. Measuring forest floor CO2 fluxes in a Douglas-fir forest. Argric For Meteorol. 2002;110:229–317. [Google Scholar]
- Ekblad A, Högberg P. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between photosynthesis and root respiration. Oecologia. 2001;127:305–8. doi: 10.1007/s004420100667. [DOI] [PubMed] [Google Scholar]
- Ekblad A, Boström B, Holm A. Forest soil respiration rate and δ13C is regulated by recent above ground weather conditions. Oecologia. 2005;143:136–42. doi: 10.1007/s00442-004-1776-z. [DOI] [PubMed] [Google Scholar]
- Fontaine S, Bardoux G, Abbadie L, Mariotti A. Carbon input to soil may decrease soil carbon content. Ecol Lett. 2004;87:314–20. [Google Scholar]
- Gilmanov TG, Sousanna JF, Aires L, Allard V, Ammann C, Balzarolo M, Barcza Z, Bernhofer C, Campell CL, Cernusca A, Cescatti A, Clifton-Brown J, Dirks BOM, Dore S, Eugster W, Fuhrer J, Gimeno C, Gruenwald T, Haszpra L, Hensen A, Ibrom A, Jacobs AFG, Jones MB, Lanigan Laurila GT, Lohila A, Manca G, Marcolla B, Nagy Z, Pilegaard K, Pinter K, Pio C, Raschi A, Rogiers N, Sanz MJ, Stefani Sutton PM, Tuba Z, Valentini R, Williams ML, Wohlfahrt G. Partitioning European grassland net ecosystem CO2 exchange into gross primary productivity and ecosystem respiration using light response function analysis. Agric Ecosyst Environ. 2007;121:93–120. [Google Scholar]
- Goulden ML, Munger JW, Fan SM, Daube BC, Wofsy SC. Exchange of carbon dioxide by a deciduous forest response to interannual climate variability. Science. 1996;271:1576–8. [Google Scholar]
- Gu L, Post WM, King AW. Fast labile carbon turnover obscures sensitivity of heterotrophic respiration from soil to temperature: a model analysis. Global Biochem Cycles. 2004;18:1–11. [Google Scholar]
- Harper W, Blair JM, Fay PA, Knapp AK, Carlisle JD. Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Glob Chang Biol. 2005;11:322–34. [Google Scholar]
- Hibbard KA, Law BE, Reichstein M, Sulzman J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry. 2005;73:29–70. [Google Scholar]
- Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Loefvenius M, Read DJ. Large-scale forest girdling shows that current photo-synthesis drives soil respiration. Nature. 2001;411:789–91. doi: 10.1038/35081058. [DOI] [PubMed] [Google Scholar]
- Janssens IA, Lankreijer H, Matteucci G, Kowalski AS, Buchmann N, Epron D, Pilegaard K, Kutsch W, Longdoz B, Grünwald T, Montagnani L, Dore S, Rebmann C, Moors EJ, Grelle A, Rannik Ü , Morgenstern K, Oltchev S, Clement R, Gudmundsson J, Minerbi S, Berbigier P, Iborm A, Moncrieff J, Aubinet M, Bernhofer C, Jensen NO, Vesala T, Granier A, Schulze ED, Lindroth A, Dolman AJ, Jarvis PG, Ceulemans R, Valentini R. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Chang Biol. 2001;7:269–78. [Google Scholar]
- Janssens IA, Dore S, Epron D, Lankreijer H, Buchmann N, Longdoz B, Brossaud J, Montagnani L. Climatic influences on seasonal and spatial differences in soil CO2 efflux. In: Baldwin IT, Caldwell MM, Heldmaier G, Lange OL, Mooney HA, Schulze ED, Sommer U, editors. Fluxes of carbon, water and energy of European forests. Ecological studies. Vol. 163. Springer-Verlag; Berlin-Heidelberg: 2003. [Google Scholar]
- Johnson LC, Matchett JR. Fire and grazing regulate belowground processes in tallgrass prairie. Ecology. 2001;82:3377–89. [Google Scholar]
- Kane ES, Pregitzer KS, Burton AJ. Soil respiration along environmental gradients in Olympic National Park. Ecosystems. 2003;6:326–35. [Google Scholar]
- Kuzyakov Y. Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci. 2002;165:382–96. [Google Scholar]
- Lavigne MB, Ryan MG, Anderson DE, Baldocchi DD, Crill PM, Fitzjarrald DR, Goulden ML, Gower ST, Massheder JM, McCaughey JH, Rayment M, Striegl RG. Comparing nocturnal eddy covariance measurements to the estimates of ecosystems respiration made by scaling chamber measurements at six coniferous boreal sites. J Geophys Res. 1997;102:28977–85. [Google Scholar]
- Liu Q, Edwards NT, Post WM, Gu L, Ledford J, Lenhart S. Temperature-independent diel variation in soil respiration observed from a temperate deciduous forest. Glob Chang Biol. 2006;12:1–10. [Google Scholar]
- Lloyd J, Taylor JA. On the temperature dependence of soil respiration. Funct Ecol. 1994;8:315–23. [Google Scholar]
- Luo Y, Jackson RB, Field CB, Mooney HA. Elevated CO2 increase belowground respiration in California grasslands. Oecologia. 1996;108:130–7. doi: 10.1007/BF00333224. [DOI] [PubMed] [Google Scholar]
- Medlyn B, Robinson AP, Clement R, McMurtrie RE. On the validation of models of forest CO2 exchange using eddy covariance data: some perils and pitfalls. Tree Physiol. 2005;25:839–57. doi: 10.1093/treephys/25.7.839. [DOI] [PubMed] [Google Scholar]
- Norman JM, Kucharik CJ, Gower ST, Baldocchi DD, Crill PM, Rayment M, Savage K, Striegl RG. A comparison of six methods for measuring soil-surface carbon dioxide fluxes. J Geophys Res. 1997;102:28771–7. [Google Scholar]
- Pendall E, DelGrosso S, King JY, LeCain DR, Milchunas DG, Morgan JA, Mosier AR, Ojima DS, Parton WA, Tans PP, White JWC. Elevated atmospheric CO2 effects and soil water feedbacks on soil respiration components in a Colorado grassland. Global Biogeochem Cycles. 2003;17 doi:10.1029/2001GB001821. [Google Scholar]
- Pumpanen J, Kolari P, Ilvesniemi H, Minkkinen K, Vesala T, Niinistö S, Lohila A, Larmola T, Morero M, Pihlatie M, Janssens I, Yuste JC, Grünzweig JM, Reth S, Subke JA, Savage K, Kutsch W, Østreng G, Ziegler W, Anthoni P, Lindroth A, Hari P. Comparison of different chamber techniques for measuring soil CO2 efflux. Agric For Meteorol. 2004;3–4:159–76. [Google Scholar]
- Raich JW, Schlesinger WH. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus. 1992;44B:81–99. [Google Scholar]
- Raich JW, Potter CS. Global patterns of carbon dioxide emissions from soils. Global Biogeochem Cycles. 1995;9:23–36. [Google Scholar]
- Raich JW, Tufekcioglu A. Vegetation and soil respiration: correlations and controls. Biogeochemistry. 2000;48:71–90. [Google Scholar]
- Raich JW, Potter CS, Bhagawati D. Interannual variability in global soil respiration, 1980–94. Glob Chang Biol. 2002;8:800–12. [Google Scholar]
- Reichstein M, Rey A, Freibauer A, Tenhungen J, Valentini R, Banza J, Casals P, Cheng Y, Grünzweig JM, Irvine J, Joffre R, Law BE, Loustau D, Miglietta F, Oechel W, Ourcival JM, Pereira JS, Peressotti A, Ponti F, Qi Y, Rambal S, Rayment M, Romanya J, Rossi F, Tedeschi V, Tirone G, Xu M, Yakir D. Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Global Biogeochem Cycles. 2003;17(4):1104. [Google Scholar]
- Reichstein M, Subke JA, Angeli AC, Tenhunen J. Does the temperature sensitivity of decomposition of soil organic matter depend upon water content, soil horizon, or incubation time? Glob Chang Biol. 2005;11:1–14. [Google Scholar]
- Richardson AD, Hollinger DY. Statistical modeling of ecosystem respiration using eddy covariance data: maximum likelihood parameter estimation, and Monte Carlo simulation of model and parameter uncertainty, applied to three simple models. Agric For Meteorol. 2005;131:191–208. [Google Scholar]
- Rodeghiero M, Cescatti A. Main determinants of forest soil respiration along an elevation/temperature gradient in the Italian Alps. Glob Chang Biol. 2005;11:1024–41. [Google Scholar]
- Savage KE, Davidson EA. A comparison of manual and automated systems for soil CO2 flux measurements: trade-offs between spatial and temporal resolution. J Exp Bot. 2003;54:891–9. doi: 10.1093/jxb/erg121. [DOI] [PubMed] [Google Scholar]
- Schlesinger WH, Andrews JA. Soil respiration and the global carbon cycle. Biogeochemistry. 2000;58:7–20. [Google Scholar]
- Shao J. Linear model selection by cross validation. J Am Stat Assoc. 1993;88:486–94. [Google Scholar]
- Smith VR. Soil respiration and its determinants on a sub-Antarctic island. Soil Biol Biochem. 2003;35:77–91. [Google Scholar]
- Sousanna JF, Allard V, Pilegaard K, Ambus P, Amman C, Campbell C, Ceschia E, Clifton-Brown J, Czobel S, Domingues R, Flechard C, Fuhrer J, Hensen A, Horvath L, Jones M, Kasper G, Martin C, Nagy Z, Neftel A, Raschi A, Baronti S, Rees RM, Skiba U, Stefani P, Manca G, Sutton M, Tuba Z, Valentini R. Full accounting of the greenhouse gas (CO2, N2O, CH4) budget of nine European grassland sites. Agric Ecosyst Environ. 2007;121:121–34. [Google Scholar]
- Steinmann K, Siegwolf RTW, Saurer M, Körner C. Carbon fluxes to the soil in a mature temperate forest assessed by 13C isotope tracing. Oecologia. 2004;141:489–501. doi: 10.1007/s00442-004-1674-4. [DOI] [PubMed] [Google Scholar]
- Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF. Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia. 2004;139:551–9. doi: 10.1007/s00442-004-1540-4. [DOI] [PubMed] [Google Scholar]
- Tang J, Baldocchi D, Xu L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob Chang Biol. 2005;11:1298–304. [Google Scholar]
- Velthof GL, Oenema O. Nitrous oxide fluxes from grassland in the Netherlands: statistical analysis of flux chamber measurements. Eurasian J Soil Sci. 1995;46:533–40. [Google Scholar]
- Wan S, Luo Y. Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochem Cycles. 2003;17:1–12. [Google Scholar]
- Wohlfahrt G, Anfang C, Bahn M, Haslwanter A, Newesely C, Schmitt M, Drösler M, Pfadenhauer J, Cernusca A. Quantifying ecosystem respiration of a mountain meadow using eddy covariance, chambers and modelling. Agric For Meteorol. 2005;128:141–62. [Google Scholar]
- Wohlfahrt G, Bahn M, Haslwanter A, Newesely C, Cernusca A. Estimation of daytime ecosystem respiration to determine gross primary production of a mountain meadow. Agric For Meteorol. 2005;130:13–25. [Google Scholar]
- Wohlfahrt G, Anderson-Dunn M, Bahn M, Balzarolo M, Bern-inger F, Campbell C, Carrara A, Cescatti A, Christensen T, Dore S, Eugster W, Friborg T, Furger M, Gianelle D, Gimeno C, Hargreaves K, Hari P, Haslwanter A, Johansson T, Marcolla B, Milford C, Nagy Z, Nemitz E, Rogiers N, Sanz MJ, Siegwolf RTW, Susiluoto S, Sutton M, Tuba Z, Ugolini F, Valentini R, Zorer R, Cernusca A. Biotic, abiotic and anthropogenic controls on the net ecosystem CO2 exchange of European mountain grasslands. Ecosystems. 2008 (this issue) [Google Scholar]
- Zhou X, Wan S, Luo Y. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob Chang Biol. 2007;13:761–75. [Google Scholar]