Abstract
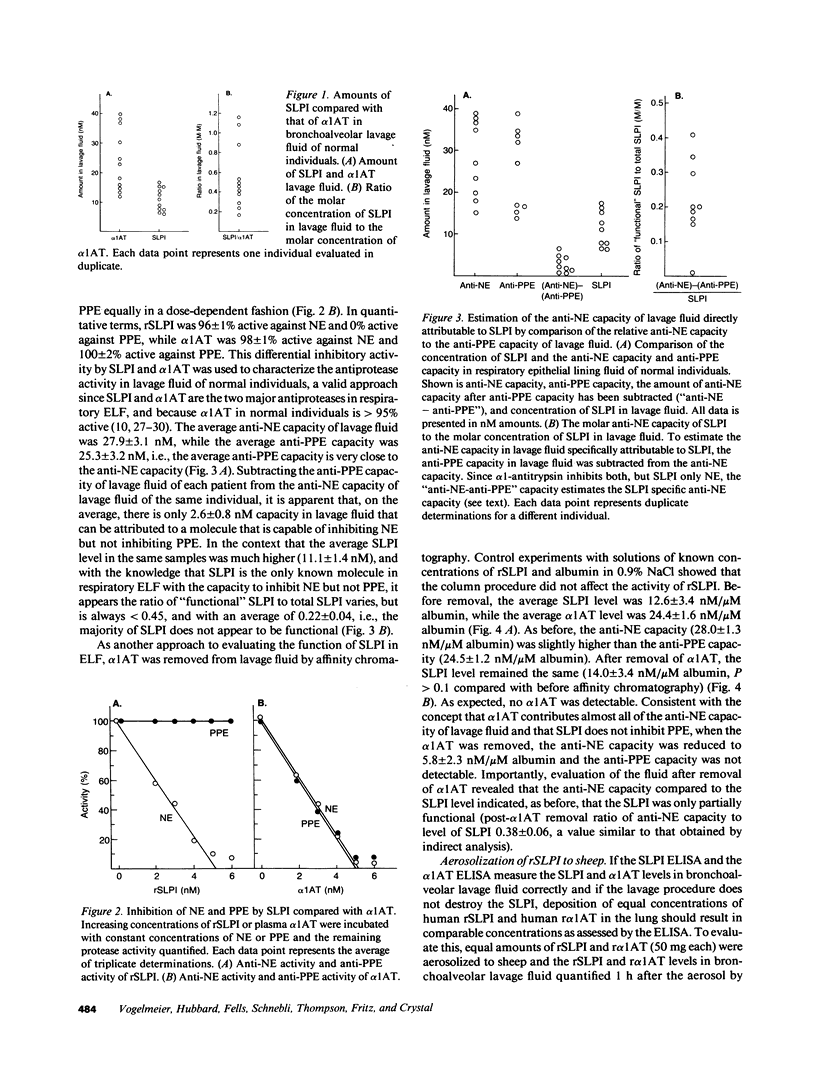
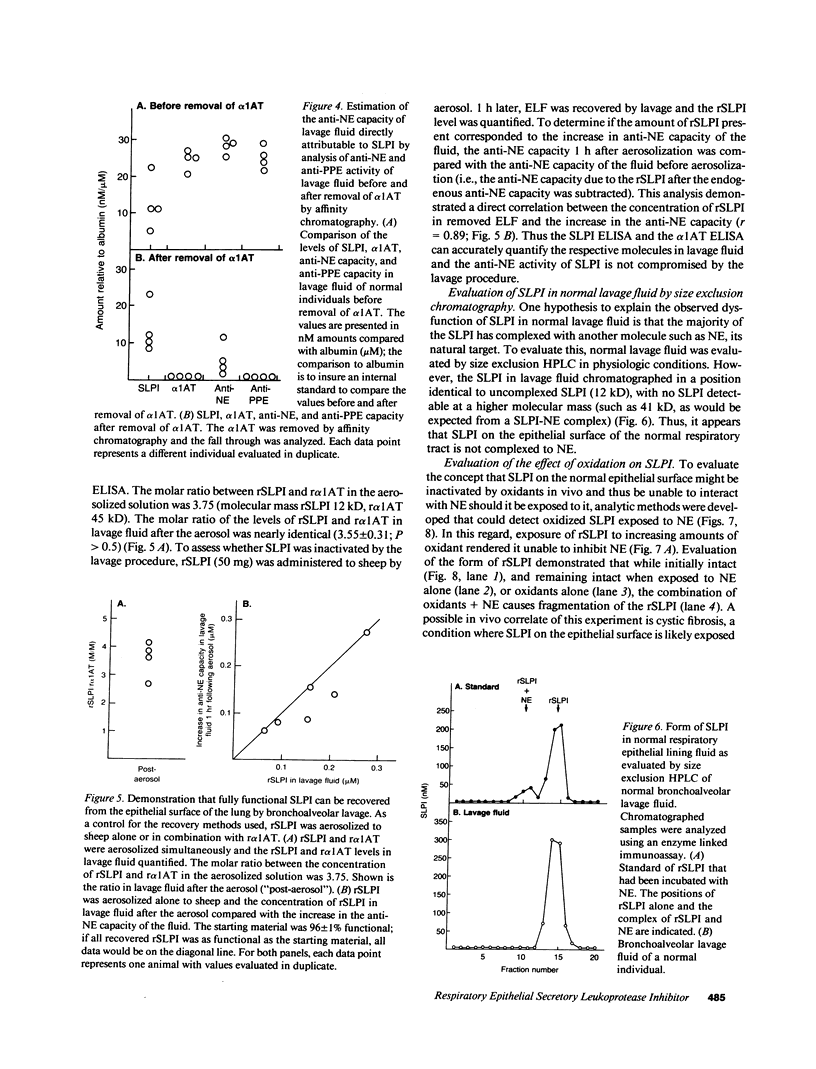
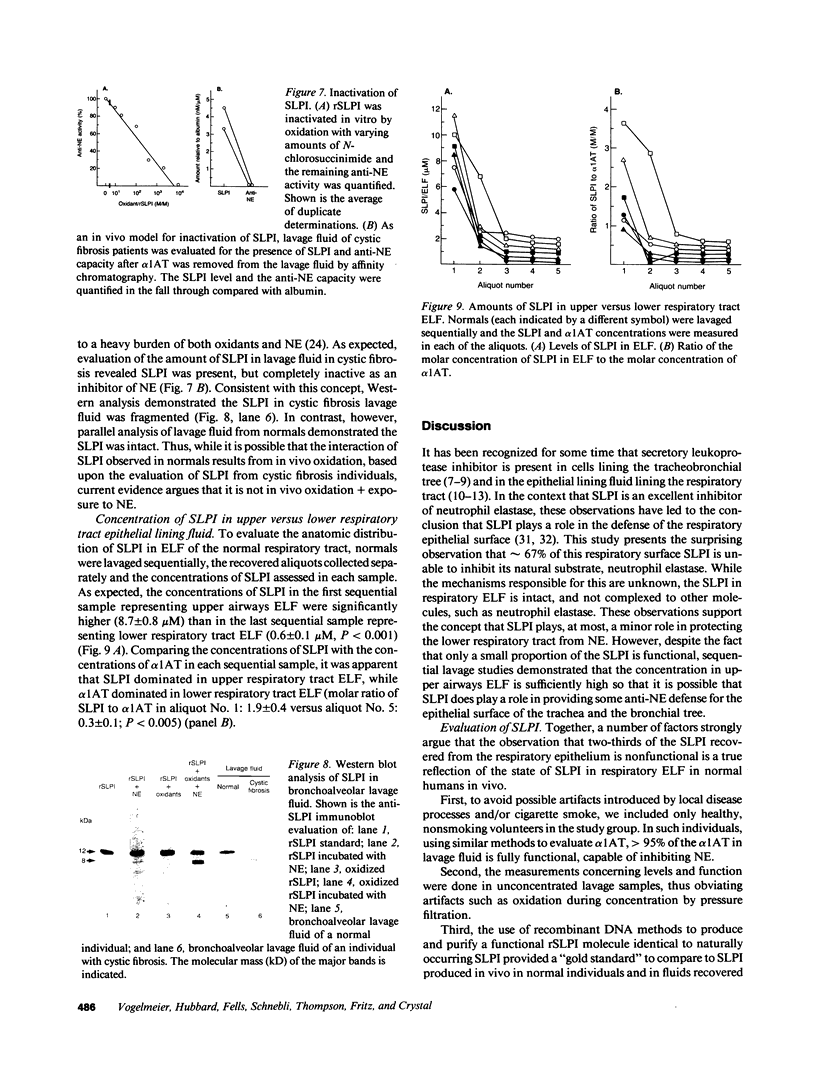
Secretory leukoprotease inhibitor (SLPI), a 12-kD nonglycosylated serine antiprotease with a high capacity for inhibiting neutrophil elastase (NE), is produced by cells of mucosal surfaces including the human lung. The molar concentrations of SLPI in total respiratory tract epithelial lining fluid (ELF) were 56 +/- 10% that of alpha 1-antitrypsin, suggesting SLPI may be more important for the anti-NE protection of the pulmonary epithelial surface than previously thought. However, evaluation demonstrated that SLPI in respiratory ELF was only one-third functional. Studies aerosolizing recombinant SLPI (rSLPI) to sheep demonstrated that in the short term, neither aerosolization and alveolar deposition nor the lavage procedure inactivated the SLPI molecule. In vitro studies with rSLPI demonstrated that exposure to oxidants did not modify the form of the molecule, while exposure to oxidants and NE caused the molecule to be cleaved from 12 to 8 kD. Consistent with this, evaluation of SLPI in lavage fluid of individuals with cystic fibrosis (a condition with oxidants and NE on the respiratory epithelium) showed that the SLPI was degraded. However, evaluation of SLPI in normal ELF by molecular sieve analysis and Western analysis demonstrated an intact 12-kD molecule, suggesting that the partial inactivation of SLPI in normals in vivo is not because it is complexed to NE or exposed to oxidants + NE. Together, these observations demonstrate that SLPI is present in large amounts in respiratory ELF, but since the majority of the SLPI is inactive, it likely does not play a significant role in protecting the normal respiratory epithelium, except perhaps in the upper airways where the levels of SLPI are the highest.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Boudier C., Pelletier A., Gast A., Tournier J. M., Pauli G., Bieth J. G. The elastase inhibitory capacity and the alpha 1-proteinase inhibitor and bronchial inhibitor content of bronchoalveolar lavage fluids from healthy subjects. Biol Chem Hoppe Seyler. 1987 Aug;368(8):981–990. doi: 10.1515/bchm3.1987.368.2.981. [DOI] [PubMed] [Google Scholar]
- Boudier C., Pelletier A., Pauli G., Bieth J. G. The functional activity of alpha 1-proteinase inhibitor in bronchoalveolar lavage fluids from healthy human smokers and non-smokers. Clin Chim Acta. 1983 Aug 31;132(3):309–315. doi: 10.1016/0009-8981(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Inactivation of bronchial mucous proteinase inhibitor by cigarette smoke and phagocyte-derived oxidants. Exp Lung Res. 1980 Aug;1(3):225–237. doi: 10.3109/01902148009065462. [DOI] [PubMed] [Google Scholar]
- De Water R., Willems L. N., Van Muijen G. N., Franken C., Fransen J. A., Dijkman J. H., Kramps J. A. Ultrastructural localization of bronchial antileukoprotease in central and peripheral human airways by a gold-labeling technique using monoclonal antibodies. Am Rev Respir Dis. 1986 May;133(5):882–890. [PubMed] [Google Scholar]
- Fritz H. Human mucus proteinase inhibitor (human MPI). Human seminal inhibitor I (HUSI-I), antileukoprotease (ALP), secretory leukocyte protease inhibitor (SLPI). Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):79–82. [PubMed] [Google Scholar]
- Fryksmark U., Ohlsson K., Polling A., Tegner H. Distribution of antileukoprotease in upper respiratory mucosa. Ann Otol Rhinol Laryngol. 1982 May-Jun;91(3 Pt 1):268–271. doi: 10.1177/000348948209100308. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Gauthier F., Fryksmark U., Ohlsson K., Bieth J. G. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982 Jan 18;700(2):178–183. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- Goldstein W., Döring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986 Jul;134(1):49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Fendrich G., Huber R., Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988 Feb;7(2):345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser K., Reichert R., Schwarz S., Werle E. isolierung und Charakterisierung eines Proteaseninhibitors aus menschlichen Bronchialsekret. Hoppe Seylers Z Physiol Chem. 1972 Feb;353(2):221–226. [PubMed] [Google Scholar]
- Hubbard R. C., Casolaro M. A., Mitchell M., Sellers S. E., Arabia F., Matthay M. A., Crystal R. G. Fate of aerosolized recombinant DNA-produced alpha 1-antitrypsin: use of the epithelial surface of the lower respiratory tract to administer proteins of therapeutic importance. Proc Natl Acad Sci U S A. 1989 Jan;86(2):680–684. doi: 10.1073/pnas.86.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps J. A., Franken C., Dijkman J. H. ELISA for quantitative measurement of low-molecular-weight bronchial protease inhibitor in human sputum. Am Rev Respir Dis. 1984 Jun;129(6):959–963. doi: 10.1164/arrd.1984.129.6.959. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Franken C., Dijkman J. H. Quantity of anti-leucoprotease relative to alpha 1-proteinase inhibitor in peripheral airspaces of the human lung. Clin Sci (Lond) 1988 Oct;75(4):351–353. doi: 10.1042/cs0750351. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van Twisk C., Klasen E. C., Dijkman J. H. Interactions among stimulated human polymorphonuclear leucocytes, released elastase and bronchial antileucoprotease. Clin Sci (Lond) 1988 Jul;75(1):53–62. doi: 10.1042/cs0750053. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Evans R. J., Eisenberg S. P., Thompson R. C. Secretory leukocyte protease inhibitor binding to mRNA and DNA as a possible cause of toxicity to Escherichia coli. J Bacteriol. 1989 Apr;171(4):2166–2172. doi: 10.1128/jb.171.4.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren H. W., Kramps J. A., Franken C., Meijer C. J., Dijkman J. A. Localisation of a low-molecular-weight bronchial protease inhibitor in the peripheral human lung. Thorax. 1983 Mar;38(3):180–183. doi: 10.1136/thx.38.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H. M., Kramps J. A., Dijkman J. H., Stockley R. A. Comparison of concentrations of two proteinase inhibitors, porcine pancreatic elastase inhibitory capacity, and cell profiles in sequential bronchoalveolar lavage samples. Thorax. 1986 Jun;41(6):435–441. doi: 10.1136/thx.41.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogushi F., Fells G. A., Hubbard R. C., Straus S. D., Crystal R. G. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987 Nov;80(5):1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrines M., Schneider-Pozzer M., Bieth J. G. Inhibition of neutrophil elastase by alpha-1-proteinase inhibitor oxidized by activated neutrophils. Am Rev Respir Dis. 1989 Mar;139(3):783–790. doi: 10.1164/ajrccm/139.3.783. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Ghafouri M., Thompson A. B., Linder J., Vaughan W., Jones K., Ertl R. F., Christensen K., Prince A., Stahl M. G. Fractional processing of sequential bronchoalveolar lavage to separate bronchial and alveolar samples. Am Rev Respir Dis. 1990 Jan;141(1):208–217. doi: 10.1164/ajrccm/141.1.208. [DOI] [PubMed] [Google Scholar]
- Seemüller U., Arnhold M., Fritz H., Wiedenmann K., Machleidt W., Heinzel R., Appelhans H., Gassen H. G., Lottspeich F. The acid-stable proteinase inhibitor of human mucous secretions (HUSI-I, antileukoprotease). Complete amino acid sequence as revealed by protein and cDNA sequencing and structural homology to whey proteins and Red Sea turtle proteinase inhibitor. FEBS Lett. 1986 Apr 7;199(1):43–48. doi: 10.1016/0014-5793(86)81220-0. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Traber L. D., Traber D. L., Spragg R. G. Pulmonary deposition and clearance of aerosolized alpha-1-proteinase inhibitor administered to dogs and to sheep. J Clin Invest. 1989 Oct;84(4):1145–1154. doi: 10.1172/JCI114278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. F., Guz A., Winning A. J., Cooke N. T., Burton G. H., Tetley T. D. Comparison of human lung surface protein profiles from the central and peripheral airways sampled using two regional lavage techniques. Eur Respir J. 1988 Oct;1(9):792–800. [PubMed] [Google Scholar]
- Stockley R. A. Chronic bronchitis: the antiproteinase/proteinase balance and the effect of infection and corticosteroids. Clin Chest Med. 1988 Dec;9(4):643–656. [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Crystal R. G. Comparison of alpha-1-antitrypsin levels and antineutrophil elastase capacity of blood and lung in a patient with the alpha-1-antitrypsin phenotype null-null before and during alpha-1-antitrypsin augmentation therapy. Am Rev Respir Dis. 1987 Mar;135(3):539–543. doi: 10.1164/arrd.1987.135.3.539. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Herzyk D. J., Gadek J. E. Comparison of smoker and nonsmoker lavage fluid for the rate of association with neutrophil elastase. Am J Respir Cell Mol Biol. 1989 Nov;1(5):423–429. doi: 10.1165/ajrcmb/1.5.423. [DOI] [PubMed] [Google Scholar]