Abstract
Obesity is an epidemic problem affecting millions of people in the Western hemisphere and costs the United States economy more than $200 billion annually. Currently, there are no effective treatments to combat obesity. Recent studies have implicated the constitutive activity of estrogen receptor (ER) β as an important regulator of metabolic diseases. However, the potential of ER-β-selective ligands to offset obesity is not clear. We evaluated the pharmacological effect of ER-β-selective ligands (β-LGNDs) in animal models of high-fat diet- and ovariectomy-induced obesity. Ligand binding, transactivation, and uterotrophic studies with β-LGNDs demonstrated selectivity for ER-β over ER-α. Animals fed a high-fat diet showed a significant increase in body weight, and this weight gain was attenuated by β-LGNDs. High-fat diet-mediated increases in serum cholesterol, leptin, glucose, and fat accumulation in organs were also reduced by β-LGNDs. In addition, MRI scanning indicated that β-LGNDs altered body composition by reducing fat mass and increasing lean body mass. Organ weights and gene expression analyses demonstrated that adipose tissue is the center of action for β-LGNDs, and the reduction in body weight is likely due to increased energy expenditure. In vitro and in vivo mechanistic studies indicated that the anti-obesity effects of β-LGNDs were due to indirect peroxisome proliferator-activated receptor γ antagonistic actions requiring the ligand binding domain of ER-β and through abrogation of the ability of PGC-1 to coactivate peroxisome proliferator-activated receptor γ. In conclusion, these studies indicate that ligand-activated ER-β is a potential therapeutic target to combat obesity and obesity-related metabolic diseases.
Keywords: Drug Design, Metabolic Diseases, Obesity, Steroid Hormone, Steroid Hormone Receptor, Estrogen Receptor-beta
Introduction
Obesity is an epidemic disease affecting over 400 million people globally (1). Two-thirds of adults and children in the United States are either overweight or obese, making it a serious health risk and economic burden to society (2). Obesity is not a stand-alone disease, as its emergence leads to various complications, including type 2 diabetes mellitus (T2DM),3 hypertension, atherosclerosis, and other cardiovascular diseases, osteoporosis, and clinical depression (3, 4). The United States Food and Drug Administration required an anti-obesity drug to reduce the body weight by 5% and/or better results than placebo in 12 months, indicating that even a marginal reduction in body weight will cause a significant improvement in the welfare of these patients (5). Despite the exponentially growing global obesity pharmaceutical market, only two Food and Drug Administration-approved drugs are available for this indication: 1) amphetamines and sibutramine that act on the hypothalamus to control appetite stimulation in the central nervous system, and 2) Orlistat, which is a lipase inhibitor that blocks gastrointestinal absorption of fat and decreases energy uptake (6). Despite mediocre performance, these drugs are commonly associated with side effects such as tachycardia, hypertension, fecal incontinence, and/or cardiac valvopathy, thereby making anti-obesity drug development of paramount importance (6). The most effective weight reduction procedure is bariatric surgery, but it is restricted due to the risk of surgery and associated side effects (7). Readers are referred to two excellent reviews for more information on obesity research and drug development (6, 8).
Obesity is a heterogeneous disease that occurs when energy uptake exceeds energy expenditure. Although the etiology of obesity remains uncertain, several factors such as alterations in feeding behavior signals in the hypothalamus, levels of leptin, adipokines secreted by white adipose tissue (WAT), neuropeptides and neurotransmitters that control behavior, hormonal changes associated with age, inflammatory signals in adipose tissue, stress, and others trigger the onset of obesity (9–11).
Increases in the incidence of post-menopausal obesity, visceral obesity at andropause, and gender differences in the incidence of metabolic diseases indicate the importance of the nuclear hormone receptor (NHR) superfamily in regulating body weight (12, 13). The NHR family is composed of 48 members, of which 27 are ligand-regulated. Many of the NHRs play pivotal roles in regulating the emergence of metabolic diseases. Activation of bile acid NHRs such as farnesoid X receptor (FXR), constitutive androstane receptor, and pregnane X receptor promotes weight loss and also increases insulin sensitivity (14, 15). Similarly, estrogen-related receptors (ERR-α, ERR-β, and ERR-γ) play significant roles in increasing energy expenditure, reducing adipogenesis and body weight gain (16). Other members of the NHR belonging to the peroxisome proliferator-activated receptor (PPARs) and estrogen receptors (ERs) also play a role in maintenance of blood glucose and body fat, making the NHRs an attractive target to prevent/treat obesity and metabolic diseases (17–20).
Hormones are important regulators of adipose function, and epidemiological studies suggest that estrogens regulate metabolism and fat distribution. The presence of ER-α and ER-β, the two receptors that mediate the actions of estradiol, in adipose tissue implicates a direct role of ER ligands in adipose function. Moreover, the presence of more brown adipose tissue (BAT) in females points toward the possibility that circulating estradiol levels may be an important contributor toward the development of BAT (21). Studies with isoform-specific ER knock-out (KO) mice indicated the importance of these isoforms in maintaining lipid and glucose homeostasis (20, 22). ER-αKO mice exhibit insulin resistance, whereas high-fat diet-fed ER-βKO mice demonstrate a higher magnitude of obesity than wild type mice (23). Several other studies also speculate that ER-β might be the primary mediator of anti-obesity effects of circulating estrogens (24, 25).
In this study, we determined the role of ER-β in high-fat diet- and ovariectomy-induced obesity in mice using ER-β isoform-selective estrogen receptor ligands (β-LGNDs). β-LGNDs significantly reduced the high-fat diet-dependent increase in body weight. MRI scans demonstrated that the β-LGNDs altered body composition by reducing fat mass and increasing lean mass in both high-fat diet- and ovariectomy-induced obesity. β-LGNDs improved blood glucose and serum cholesterol profile, inhibited leptin, and reduced accumulation of fat in the liver. Collectively, these results suggest that β-LGNDs represent a new class of drugs to prevent/treat obesity and metabolic diseases.
MATERIALS AND METHODS
Ligand Binding Assay
Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]estradiol (PerkinElmer Life Sciences) in buffer A (10 mm Tris, pH 7.4, 1.5 mm disodium EDTA, 0.25 m sucrose, 10 mm sodium molybdate, 1 mm PMSF) to determine the equilibrium dissociation constant (Kd) of [3H]E2. Protein was incubated with increasing concentrations of [3H]E2 with and without a high concentration of unlabeled E2 at 4 °C for 18 h to determine total and nonspecific binding. Nonspecific binding was then subtracted from total binding to determine specific binding. Ligand binding curves were analyzed by nonlinear regression with one site saturation to determine the Kd of E2 (ER-α, 0.65 nm; ER-β, 1.83 nm). In addition, the concentration of [3H]E2 required to saturate ER-α and ER-β LBD was determined to be 1–3 nm. Increasing concentrations of two β-LGNDs (β-LGND1 and β-LGND2) (range, 10−11 to 10−6 m) were incubated with [3H]E2 (1–3 nm) and ER LBD (α or β) using the conditions described above.
Transient Transfection and Reporter Gene Assay
Human ERs (ER-α and ER-β) were cloned from prostate cDNA into a pCR3.1 plasmid vector backbone. PGC-1 was cloned into mammalian two-hybrid vector pACT. ER-β H475 was mutated to alanine using site-directed mutagenesis. Sequencing was performed to determine the absence of any nonspecific mutations. PPAR-α, PPAR-γ, and PPRE-LUC were kindly provided by Dr. Harish Srinivas (University of Pittsburgh). SHP promoter (−572 to +10) (26) was cloned into pGL3 basic luciferase reporter vector, and human FXR was cloned into pCR3.1. HEK-293 cells were plated at 100,000 cells per well of a 24-well plate in Dulbecco's minimal essential media (DMEM) + 5% charcoal-stripped fetal bovine serum. The cells were transfected using Lipofectamine (Invitrogen) with 0.25 μg of ERE-LUC (gift from Dr. Carolyn L. Smith, Baylor College of Medicine), 0.02 μg of CMV-LUC (Renilla luciferase), and 12.5 ng of rat ER-α or 25 ng of rat ER-β. The cells were treated 24 h after transfection with various concentrations of LGNDs or a combination of LGNDs and estradiol to determine the antagonistic activity. Luciferase assays were performed 48 h after transfection.
Ishikawa Growth Assay
Ishikawa cells were plated at 15,000 cells/well in 24-well plates in DMEM/F-12 (1:1) + 5% charcoal-stripped fetal bovine serum without phenol red. The cells were maintained in this medium at 37 °C for 3 days. Medium was changed immediately prior to drug treatment for an additional 72 h. After 72 h, the cells were fixed with formalin, and the amount of alkaline phosphatase measured by para-nitrophenyl phosphate method.
Uterotrophic Assay
All animal studies were performed in accordance with the current guidelines for animal welfare. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Tennessee Health Science Center, Memphis.
Sprague-Dawley rats aged 18–20 days were randomized based on body weight into groups of seven animals and treated with either vehicle, 50 μg/kg/day estradiol subcutaneously (Sigma), 10 mg/kg/day tamoxifen orally, or 30 mg/kg/day β-LGND1 or β-LGND2 subcutaneously. Body weight was recorded at pretreatment (day 0) and before necropsy (day 4). Statistical differences among groups were evaluated by one-way analysis of variance followed by post hoc analysis. Rats were treated for 3 consecutive days and then sacrificed 24 h after the last dose. The body of the uterus was cut just above its junction with the cervix and at the junction of the uterine horns with the ovaries. The uterus was weighed with and without intrauterine fluid. Statistical comparisons were made between the weights of dry uteri.
Obesity Studies
C57BL/6 male mice (4 weeks old) were obtained from Harlan Laboratories (Indianapolis, IN). The animals were divided into different groups and were fed with a normal or high-fat diet (Harlan, IN). The composition of the diets is given in supplemental Table 1. For the prevention studies (studies 1 and 2), the animals were treated with vehicle, β-LGND1 or β-LGND2 (30 mg/kg/day subcutaneously) beginning on day 1 of the study and continuing for 12 weeks. For the treatment study (study 3), the animals were maintained on the respective diets for 6 weeks and then treated daily as indicated for an additional 12 weeks. Biweekly body weights and food consumption were measured. At the end of the studies, blood and tissues were collected for RNA isolation, histology, and protein estimation. Dual energy x-ray absorptiometry (DEXA; Piximus II, GE) scanning was performed at the end of the first obesity study with β-LGND1, and MRI scanning (EchoMRI, 4-in-1 composition analyzer, Echo Medical Systems, Houston, TX) was performed at weeks 0, 6, and 12 for the second obesity study performed with β-LGND1 and β-LGND2. For the treatment obesity study (where the animals were fed with high-fat diet for 6 weeks prior to beginning drug treatment for 12 weeks), MRI scans were performed at weeks 0, 6, 12, and 18. Cholesterol and leptin concentrations were measured in serum at the end of the study using ELISA-based methods. Luminex beads inflammation panel was used to detect cytokines in serum (Millipore, Billerica, MA). The list of cytokines is given in supplemental Table 2. Histology was performed on cryosections and stained with Oil Red O. Serum testosterone and follicle-stimulating hormone were measured using the Luminex beads method (Millipore).
Oral glucose tolerance tests were performed on 16-h fasted mice. Mice were administered 150 mg of glucose by oral gavage. Blood samples were taken at 0, 15, 30, 60, 90, and 120 min after glucose administration for glucose levels.
For the ovariectomy-induced obesity model, 6-week-old female C57BL/6 mice were sham-operated or ovariectomized, and various parameters such as body weight, feed consumption, and body composition were measured for 9 weeks. At the end of the study, the animals were sacrificed, and various measurements were performed in serum and tissues.
RNA extracted from WAT, BAT, liver, and muscle were reverse-transcribed using cDNA synthesis kit (Applied BioSystems, Foster City, CA). Real time PCR was performed for a selected list of genes involved in obesity and metabolic diseases (supplemental Table 3) using real time PCR TaqMan gene expression array cards (Applied Biosystems). Ucp2 and Ucp3 gene expressions were measured by real time PCR using TaqMan probes from Applied Biosystems. Western analysis was performed with UCP-1 antibody (Millipore) as indicated previously (27).
RESULTS
In Vitro Characterization of β-LGND1 and β-LGND2
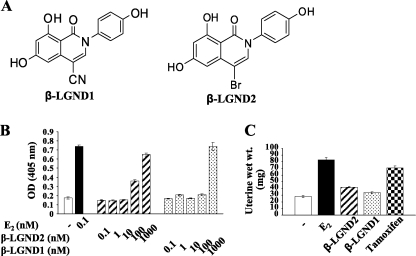
We selected two β-LGNDs from a library of isoform-selective ligands developed at GTx (Fig. 1A). β-LGND1 and β-LGND2 bound ER-β with high affinity with Ki values of 5.35 and 2.11 nm, respectively, which were comparable with the binding by E2 (Table 1). However, β-LGND1 and β-LGND2 bound to ER-α with much lower affinity than estradiol, with Ki values of 94 and 40 nm, respectively (Table 1). As such, β-LGND1 and β-LGND2 bound to ER-β with almost 100-fold selectivity compared with ER-α (Table 1).
FIGURE 1.
In vitro and in vivo characterization of ER-β-selective ligands. A, structure of β-LGND1 and β-LGND2. B, β-LGND1 and β-LGND2 weakly induce Ishikawa cell proliferation. Ishikawa cells plated at 15,000 cells/well in DMEM/F-12 + 5% charcoal-stripped fetal bovine serum medium were treated with various concentrations of β-LGND1 and β-LGND2 for 72 h. The cells were fixed, and alkaline phosphatase assay was performed to measure the cell number. C, β-LGND1 and β-LGND2 does not increase uterine weights. Immature female rats (n = 7) were treated for 3 days with estradiol (50 μg/kg/day subcutaneously), β-LGND2 (30 mg/kg/day subcutaneously), β-LGND1 (30 mg/kg/day subcutaneously), or tamoxifen (10 mg/kg/day orally). At the end of 3 days of treatment, the animals were sacrificed, and wet uterine weights were measured. Data are expressed as means ± S.E. RBA, relative binding affinity; ER-α, estrogen receptor α; ER-β, estrogen receptor β.
TABLE 1.
Binding and transactivation characteristics of ER-β ligands, β-LGND1, and β-LGND2
Ligand binding assays are shown in the 2nd to 6th columns. Binding affinity was determined using purified rat ER-α and ER-β LBD and [3H]estradiol as described under “Materials and Methods.” Transactivation assays are shown in the 7th and 8th columns. HEK293 cells were transfected with 0.25 μg of ERE-LUC, 0.5 ng of CMV-Renilla-LUC, and 25 ng of pCR3.1 ER-α or 50 ng pCR3.1 ER-β and treated with the ligands for 24 h. The cells were lysed, and firefly luciferase activity was measured and normalized to Renilla luciferase.
| Groups | Ki ER-α | Ki ER-β | RBA to E2 ER-α | RBA to E2 ER-β | Ratio RBA α/β | Agonist ER-α | Agonist ER-β |
|---|---|---|---|---|---|---|---|
| nm | nm | nm | nm | ||||
| E2 | 0.36 | 2.33 | 1 | 1 | 1 | 0.014 | 0.0942 |
| β-LGND1 | 93.92 | 5.35 | 235.56 | 3.05 | 77.26 | 132.70 | 7.90 |
| β-LGND2 | 39.74 | 2.11 | 112.41 | 1.16 | 96.91 | 38.09 | 1.38 |
To determine whether the selectivity in ER binding also translated into ER-β-selective activity, transient transactivation assays were performed in HEK-293 cells transfected with plasmids encoding ER-α or ER-β and ERE-LUC. The cells were treated with varying concentrations of the ligands, and their EC50 values were determined. Both β-LGND1 and β-LGND2 functioned as agonists to both ER-α and ER-β with a selectivity of 20–30-fold toward ER-β and with EC50 values of less than 10 nm for ER-β (Table 1).
Because members of the NHR superfamily have moderately homologous LBDs, transactivation assays were performed to determine the cross-reactivity with 13 other NHRs (receptors for progesterone, mineralocorticoids, androgens, glucocorticoids, FXR, pregnane X receptor, liver X receptor, retinoid X receptor, PPAR-α, PPAR-γ and ERR-α, ERR-β, and ERR-γ). β-LGND1 and β-LGND2 failed to cross-react with any of the above mentioned receptors even at concentrations as high as 10 μm (data not shown).
Activation of ER-α, but not ER-β, induces uterine proliferation (28). This effect is one potential concern in the development of ER-α agonists. As such, we examined the ability of β-LGND1 and β-LGND2 to stimulate in vitro growth of Ishikawa endometrial cells using varying concentrations of the ligands and an alkaline phosphatase assay. As shown in Fig. 1B, β-LGND1 and β-LGND2 induced the proliferation of Ishikawa cells only at the highest concentration tested (1 μm) or the concentration at which they cross-react with ER-α. On the other hand, E2 promoted the proliferation of the cells at very low concentrations (i.e. 0.1 nm).
We also examined the effects of β-LGND1 and β-LGND2 on the proliferation of uterus in vivo. β-LGND1 and β-LGND2 were administered subcutaneously at a dose of 30 mg/kg/day, whereas E2 was administered subcutaneously at a dose of 50 μg/kg/day and tamoxifen at a dose of 10 mg/kg/day orally for 3 days. Tamoxifen was used as a tissue-selective positive control ER-α ligand. E2 and tamoxifen stimulated the proliferation of the uterus significantly, as demonstrated by the increase in uterine weight, whereas both β-LGND1 and β-LGND2 failed to induce uterine growth (Fig. 1C). In addition to confirming the absence of uterotrophic activity in vivo, these studies also helped us in determining the dose (30 mg/kg/day subcutaneously) for the obesity studies.
β-LGND1 Represses High-fat Diet-induced Body Weight Gain (Study 1)
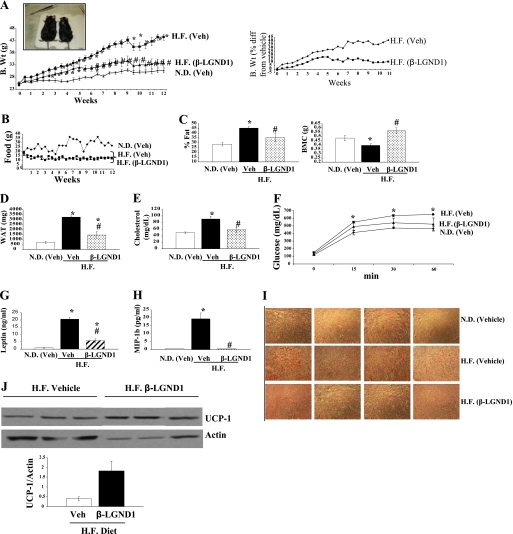
Four-week-old C57BL/6 mice were fed with a normal diet or high-fat diet. One group of the high-fat diet-fed animals was treated with 30 mg/kg/day β-LGND1 subcutaneously (high-fat treated), whereas the other groups received vehicle subcutaneously and either a normal (control) or high-fat diet (high-fat vehicle). Biweekly body weights and feed consumption were measured. As expected and published earlier, maintenance on a high-fat diet increased the body weight of the mice significantly compared with the control mice starting from week 3 (Fig. 2A). High-fat diet mice treated with β-LGND1 showed only a moderate increase in body weight and were statistically indistinguishable from control mice demonstrating the ability of β-LGND1 to repress the body weight gain induced by a high-fat diet. Fig. 2A (inset) shows representative pictures of mice in the high-fat groups that were treated with vehicle (left) or β-LGND1 (right). Mice in the high-fat diet groups that received vehicle alone gained 40% more weight than animals receiving a normal diet (Fig. 2A, right panel). However, mice in the high-fat diet group treated with β-LGND1 gained only 5% more weight than the normal diet-fed controls demonstrating a greater than 85% reduction in body weight by β-LGND1 compared with vehicle-treated animals receiving the high-fat diet.
FIGURE 2.
β-LGND1 represses diet induced obesity. Six-week-old C57BL6 mice (n = 5) were fed with normal diet or high-fat diet and treated with vehicle or β-LGND1 (30 mg/kg/day subcutaneously) for 12 weeks. Biweekly body weight (A) and feed consumption (B) were measured. A, right graph shows the percent difference (diff) in body weight of high-fat diet-fed groups from normal diet-fed group. A, inset shows a representative mouse. C, β-LGND1 reduces body fat and increases bone mineral content. Whole body DEXA scanning was performed on the C57BL6 mice (animals described in A; n = 5) to measure the body fat (left panel) and bone mineral content (right panel). Body fat content is expressed as percent fat of body weight. D, E, G, and H, β-LGND1-reduced metabolic disease markers. C57BL6 mice (animals described in A; n = 5) were sacrificed after 12 weeks of treatment and the weight of WAT (D) was measured. Blood was collected, and cholesterol (E), leptin (G), and inflammatory marker MIP-1b (H) were measured in the serum as described under “Materials and Methods.” F, β-LGND1 reduces serum glucose after glucose tolerance test. Glucose tolerance test was performed at the end of 12 weeks of treatment (animals described in A; n = 5) by administering 150 mg of glucose orally and measuring blood glucose levels at 0, 15, 30, and 60 min of glucose administration. I, β-LGND1 reduces lipid accumulation in liver. Sections were performed on cryopreserved liver from C57BL6 mice (animals described in A; n = 5) and stained for lipid accumulation using Oil Red O staining. J, β-LGND-1 increases UCP-1 protein levels in brown adipose tissue. UCP-1 expression at protein levels were determined by Western blot analysis of protein extracts from brown adipose tissue and hybridizing the proteins with an antibody for UCP-1 and normalized to actin. Representative images of n = 5 are shown. Bar graph shows the quantification of UCP-1 and normalization to actin levels. N.D, normal diet; H.F., high-fat diet. MIP, 1b-macrophage inflammatory protein; veh, vehicle; B.Wt, body weight; UCP-1, uncoupling protein-1; *, significance at p < 0.05 from normal diet-fed vehicle-treated animals; #, significance at p < 0.05 from high-fat diet-fed vehicle-treated animals. Values are expressed as means ± S.E. with n = 5 animals.
Although the feed consumption of both groups of high-fat diet-fed animals was lower than that observed for the control mice, β-LGND1 treatment did not affect total caloric intake, indicating that alteration in feed consumption or satiety was not the mechanism for the observed body weight reduction (Fig. 2B).
β-LGND1 Alters Metabolic Disease Markers
We used DEXA to examine the changes in body composition that accompanied the body weight difference observed in mice that received the high-fat diet and β-LGND1. As expected, animals that received the high-fat diet and vehicle had significantly higher body fat than animals in the normal diet (control) group or those receiving β-LGND1 (Fig. 2C, left panel).
As obesity inversely correlates with bone mineral density and content (29), we also examined the effects of diet and β-LGND1 on total body bone mineral content in these mice using DEXA. Maintenance on a high-fat diet reduced body bone mineral content significantly compared with controls. Treatment of high-fat diet-fed mice with β-LGND1 prevented the loss in body bone mineral content (Fig. 2C, right panel), suggesting that secondary beneficial effects on bone accompany reduced obesity. Future studies will be conducted to determine any direct beneficial effects of β-LGND1 on bone.
Organ weight measurements indicate that WAT weight was significantly increased by 2–2.5-fold in animals maintained on the high-fat diet treated with vehicle compared with normal diet controls (Fig. 2D). This increase in WAT weight was significantly reduced in β-LGND1-treated mice. Serum cholesterol (Fig. 2E) and leptin levels (Fig. 2G) were significantly increased in animals fed the high-fat diet and treated with vehicle as compared with normal diet controls, and this increase was significantly reversed by β-LGND1.
One of the many pathological conditions associated with obesity is insulin resistance resulting in T2DM (30). Glucose tolerance tests were performed to determine whether high-fat diet-fed animals exhibited signs of insulin resistance and T2DM. Administration of glucose increased the blood sugar level as early as 15 min in all the groups. Animals fed the high-fat diet and treated with vehicle demonstrated a significant increase in blood glucose levels compared with normal diet controls (Fig. 2F). However, the blood glucose levels of high-fat diet-fed mice treated with β-LGND1 were not statistically different from the normal diet control groups.
Inflammation is a central component of obesity, and recent studies emphasize that obesity is an inflammatory disease (31, 32). To determine the role of inflammation in high-fat diet-induced obesity, a panel of 32 inflammatory cytokines was measured in serum using Luminex beads from Millipore (supplemental Table 2). Of the 32 cytokines measured, only macrophage inflammatory protein-1β (MIP-1β) was significantly increased by the high-fat diet. However, this increase was completely reversed by β-LGND1 (Fig. 2H).
One of the perilous secondary effects of obesity and hypercholesterolemia is fatty liver disease (33). Liver cryosections were obtained from the mice and stained with Oil Red O to determine the accumulation of fat in liver. Photographs shown in Fig. 2I demonstrate that maintenance on a high-fat diet increased the accumulation of fat in liver sections as evident from the increased Oil Red O staining. However, liver sections obtained from high-fat diet-fed mice treated with β-LGND1 did not stain for oil red suggesting that β-LGND1 completely prevented the accumulation of fat in the liver.
β-LGND1 Alters the Expression of Genes Involved in Energy Homeostasis, Adipogenesis, and Anti-oxidant Pathways
A subset of 32 genes that are implicated in lipogenesis, lipolysis, anti-oxidant, and other related pathways was selected, and the effect of β-LGND1 on these genes was evaluated using TaqMan PCR-based arrays. RNA from liver, muscle, WAT, and BAT was applied to these arrays. Genes for which their expression was more than 2-fold different and significant at p < 0.01 in β-LGND1-treated mice compared with high-fat diet animals treated with vehicle are summarized in Table 2.
TABLE 2.
β-LGND1 alters the gene expression involved in lipid homeostasis
RNA from brown adipose tissue, white adipose tissue, and liver was extracted from C57BL6 mice (animals described in Fig 2; n = 5) and cDNA-synthesized, and the expressions of 32 genes (complete list of genes given in supplemental Table 1) were measured using TaqMan gene expression array cards. Genes that were significantly different in high-fat diet-fed β-LGND1-treated animals from high-fat diet-fed vehicle-treated animals at p < 0.01 and having more than 2-fold difference are expressed. Values are expressed as -fold change from high-fat diet-fed and vehicle-treated animals with − indicating a decrease in gene expression and + indicating an increase in gene expression in the β-LGND1-treated samples. Numbers in the parentheses are corresponding reference numbers to corroborate the statements.
| Gene name | Fold increase(+)/decrease (−) | Function |
|---|---|---|
| Brown adipose tissue | ||
| Ddit3 (DNA damage inducible transcript III) | (−)2.01 | Promotes obesity, oxidative stress, β-cell damage (42) |
| GPx-3 (glutathione peroxidase) | +2.25 | Prevents obesity, oxidative stress, insulin resistance, and inflammation (41) |
| LPL (lipoprotein lipase) | (−)2.50 | High level increase of insulin resistance and T2DM. High fat diet increase in lipoprotein lipase (36) |
| PLTP (phospholipid transfer protein) | (−)3.76 | Involved in atherogenesis, hypercholesterolemia, and atherosclerosis (39) |
| ER-β (estrogen receptor β) | +3.50 | |
| Dhcr24 (dehydrocholesterol reductase) | (−)3.33 | Encodes cholesterol-synthesizing enzyme Seladin-1 (40) |
| UCP-1 (uncoupled protein-1) | +6.90 | Promotes energy expenditure, reduces cholesterol (34) |
| UCP-2 (uncoupled protein-2) | +3.48 | Promotes energy expenditure, reduces cholesterol (35) |
| White adipose tissue | ||
| SREBP1 (Sterol regulatory element-binding protein 1) | (−)6.14 | Increases fatty acid synthesis and cholesterol (38) |
| FASN (fatty-acid synthase) | (−)7.92 | Fatty acid synthesis. Mostly in association with SREBP (37) |
| Ddit3 (DNA damage-inducible transcript III) | (−)3.05 | Promotes obesity, oxidative stress, β-cell damage (42) |
| LPL (lipoprotein lipase) | (−)3.15 | High levels increase insulin resistance and T2DM. High-fat diet increased LPL in tissues (36) |
| Liver | ||
| GPx-3 (glutathione peroxidase) | +2.60 | Prevents obesity, oxidative stress, insulin resistance, inflammation, and major antioxidant in plasma (41) |
| CIDEA (Cell death-inducing DNA fragmentation factor) | (−)5.50 | Very important factor in adipose cell function and obesity |
Uncoupling protein-1 (Ucp-1) (34), a thermogenic mitochondrial protein and a marker for BAT, was decreased in animals that received the high-fat diet and vehicle compared with normal diet controls. However, β-LGND1 reversed and, in fact, demonstrated a robust increase in Ucp-1 gene expression, which is suggestive of increased energy expenditure. A 7-fold increase in Ucp-1 by β-LGND1 indicates a tremendous increase in the energy expenditure, which could be one of the important processes triggered by ligands activating ER-β. The increased expression of Ucp-1 was confirmed at the protein level by Western blot analysis, which demonstrated that β-LGND-1 augmented the expression by a minimum of 2-fold (Fig. 2J). Ucp-2 (35) and Ucp-3 were also measured in RNA extracted from BAT, and β-LGND1 increased the expression of Ucp-2 significantly by 3.5-fold and Ucp-3 by 2.2-fold.
The expression of genes that promote lipogenesis, such as lipoprotein lipase (Lpl) (36), fatty-acid synthase (Fasn) (37), sterol regulatory element-binding protein-1 (Srebp-1) (38), phospholipid transfer protein (Pltp) (39), and dehydrocholesterol reductase (Dhcr24) (40), was increased in BAT and WAT isolated from high-fat diet-fed mice treated with vehicle. These increases were reversed efficiently by the administration of β-LGND1. In addition, genes such as glutathione peroxidase (GPx-3) (41) and DNA damage-inducible transcript III (Ddit3) (42), which are involved in the anti-oxidant and oxidative stress pathways, were significantly altered by β-LGND1. Cumulatively, these results suggest that β-LGND1 mediates its anti-obesity effects by synergistically acting on brown adipose tissue to increase the energy expenditure and on white adipose tissue to inhibit lipogenesis.
β-LGND1 and β-LGND2 Inhibit Body Weight Gain and Alter Fat Composition of High-fat Diet-fed Animals (Study 2)
To determine whether this anti-obesity effect is unique to β-LGND1 or it is an ER-β ligand class effect, another ER-β agonist, β-LGND2, with almost similar binding, transactivation, and isoform selectivity profile (Fig. 1A) was chosen and compared with β-LGND1 in the high-fat diet study. In this study, MRI was performed instead of DEXA to determine the effects on the whole body fat and lean mass.
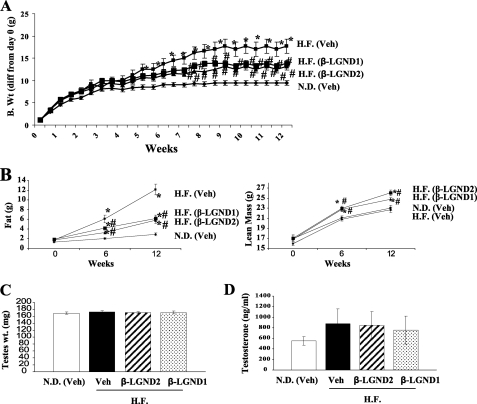
Treatment with β-LGND1 replicated the effects observed in the prior study shown in Fig. 2A with significant reduction in body weight (Fig. 3A) without altering the feed consumption (data not shown). β-LGND2 also reduced the body weight of high-fat diet-fed mice, with results comparable with those observed with β-LGND1. Both ligands prevented the body weight increase caused by the high-fat diet by more than 50%. The body weights of mice treated with β-LGND1 and β-LGND2 were statistically indistinguishable from the normal diet controls.
FIGURE 3.
β-LND1 and β-LGND2 represses high-fat diet-induced obesity. A, 6-week-old C57BL6 mice (n = 12) were fed with normal diet or high-fat diet and treated with vehicle, β-LGND1 (30 mg/kg/day subcutaneously) or β-LGND2 (30 mg/kg/day subcutaneously) for 12 weeks. Body weight was measured biweekly and represented as body weight difference from day 0. B, β-LGND1 and β-LGND2 reduce fat mass and increase muscle mass. Before the initiation of the study, at 6 and 12 weeks after treatment, MRI scan was performed on the C57BL6 mice (n = 12) to measure the fat (left panel) and lean body mass (right panel). C and D, β-LGND1 and β-LGND2 does not inhibit the hypothalamus/pituitary/gonadal (HPG) axis. At sacrifice, testes weight (C) was measured and blood collected for serum testosterone measurements (D). N.D., normal diet; H.F., high-fat diet; *, significance at p < 0.05 from normal diet-fed vehicle-treated animals; #, significance at p < 0.05 from high-fat diet-fed vehicle-treated animals.
MRI demonstrated a significant reduction in fat mass in both β-LGND1- and β-LGND2-treated groups compared with animals receiving the high-fat diet and vehicle (Fig. 3B, left panel). Both ligands prevented the increase in body fat by more than 50%, comparable with the reduction in body weight. Maintenance on a high-fat diet and vehicle did not alter the lean mass significantly compared with normal diet controls (Fig. 3B, right panel). Both β-LGND1 and β-LGND2 increased the lean mass in high-fat diet-fed animals by ∼2 g in 12 weeks, indicating that ER-β-selective ligands not only repress body weight in high-fat diet-fed mice but do so by promoting favorable changes in body composition (i.e. by decreasing fat mass and increasing lean mass). These changes were obvious as early as 6 weeks into treatment, and the differences were magnified by 12 weeks of treatment. Tissue weights indicated that both β-LGND1 and β-LGND2 comparably decreased WAT weight and increased gastrocnemius muscle weight without altering the weights of other tissues such as liver, heart, and brain (data not shown), reproducing the results demonstrated in Fig. 2A.
To ensure that the effects on body composition and weight were not mediated by cross-reactivity with ER-α, parameters in the hypothalamus/pituitary/gonadal axis were measured (43). As ER-α is associated with a variety of side effects such as thromboembolism, cardiovascular problems, breast cancer, and others, any functional cross-reactivity of the ER-β ligands in vivo with this receptor isoform might be considered undesirable and preclude its use for a chronic medical condition like obesity. Testes weights (Fig. 3C) and serum testosterone (Fig. 3D) levels were not altered by β-LGND1 or β-LGND2 in animals fed with the high-fat diet and treated with vehicle, β-LGND1, or β-LGND2 for 12 weeks. Follicle-stimulating hormone, another hormone in the hypothalamus/pituitary/gonadal axis, was also not altered by diet or drug treatment (data not shown). These results suggest that the anti-obesity effects of the β-LGNDs were not mediated through cross-reactivity with ER-α or effects on sex hormone levels.
β-LGND2 Inhibits Body Weight and Fat Mass in Obese Animals (Study 3, Treatment Phase)
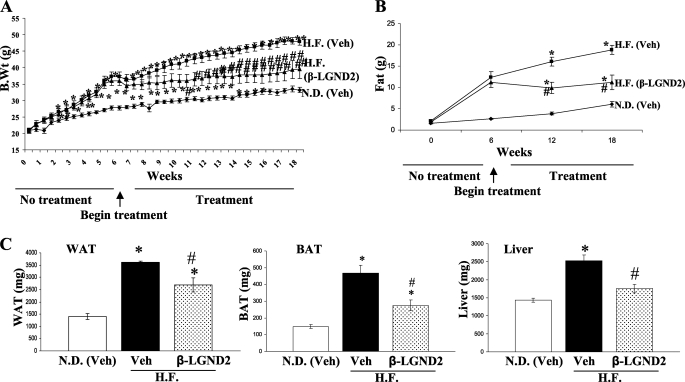
As the first two studies were designed to prevent obesity (i.e. animals were fed with a high-fat diet and treated simultaneously), a subsequent study was conducted to evaluate the ability of β-LGND2 to affect body composition in mice that were already obese. Mice were divided into three groups consisting of one group fed a normal diet (control) and the other two groups fed with the high-fat diet for 6 weeks. After 6 weeks, the animals were treated daily with vehicle or 30 mg/kg/day β-LGND2 subcutaneously for another 12 weeks. All the animals were maintained on their respective diets during the entire course of the study. Maintenance on the high-fat diet significantly increased the body weight by 3 weeks compared with normal diet controls. Initiation of β-LGND2 treatment at week 6 prevented further gains in body weight throughout the remainder of the study. By week 16, the body weight of high-fat diet-fed animals treated with β-LGND2 was not significantly different from normal diet control mice (Fig. 4A). MRI demonstrated that the body fat increase observed in animals on the high-fat diet was reduced by treatment with β-LGND2 (Fig. 4B). Consistent with the prevention studies, β-LGND2 effectively opposed the high-fat diet-dependent increase in WAT, BAT, and liver weights (Fig. 4C).
FIGURE 4.
β-LGND2 efficiently treats high-fat diet-induced obesity. Six-week-old C57BL6 mice (n = 8) were maintained in normal diet or high-fat diet for 6 weeks. After 6 weeks, the animals were treated with vehicle or β-LGND2 (30 mg/kg/day) subcutaneously for 12 weeks. Biweekly body weight was measured (A). MRI scan was performed at day 0 and at weeks 6, 12, and 18 to determine the fat mass (B). At sacrifice, weights of WAT, BAT, and liver were measured and expressed as bar graphs (C). N.D., normal diet; H.F., high-fat diet. *, significance at p < 0.05 from normal diet-fed vehicle-treated animals; #, significance at p < 0.05 from high-fat diet-fed vehicle-treated animals.
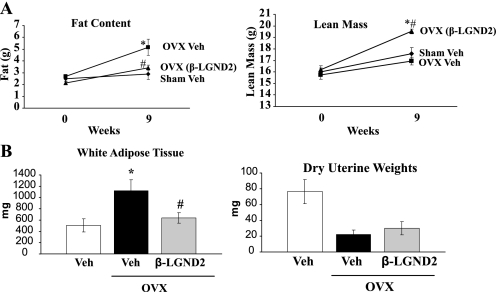
β-LGNDs Also Alter Body Composition in an Animal Model of Postmenopausal Obesity
Postmenopausal obesity increases the susceptibility of women to cardiovascular risks (44). Because our β-LGNDs affected body composition in an animal model of high-fat diet-induced obesity, we hypothesized that they might also be effective in an animal model of postmenopausal obesity. As we saw in the high-fat diet model, MRI scan demonstrated that ovariectomy (OVX) increased the fat mass significantly and that β-LGND2 completely prevented the increase in fat mass (Fig. 5A, left panel). β-LGND2 also significantly increased lean mass (Fig. 5A, right panel) indicating that β-LGND2 caused consistent changes in body composition in the high-fat diet- and OVX-induced animal models of obesity. Measurement of WAT and uterus weights indicated that β-LGND2 completely inhibited the WAT accrued due to OVX without affecting uterine weight, indicating absence of ER-α cross-reactivity (Fig. 5B).
FIGURE 5.
β-LGND2 alters body composition of OVX mice. Six-week-old C57BL6 female mice (n = 8) were either sham-operated and treated with vehicle on ovariectomized mice and treated with vehicle or 30 mg/kg/day β-LGND2 subcutaneously for 9 weeks. MRI scan was performed at day 0 and week 9 to determine the fat mass (A, left panel) and lean mass (A, right panel). After 9 weeks, the mice were sacrificed and weights of white adipose tissue and uterus (B) were recorded. Veh, vehicle; *, significance at p < 0.05 from normal diet-fed vehicle-treated animals; #, significance at p < 0.05 from high-fat diet-fed vehicle-treated animals.
ER-β Ligands Inhibit PPAR-γ Function in a Receptor-dependent Manner
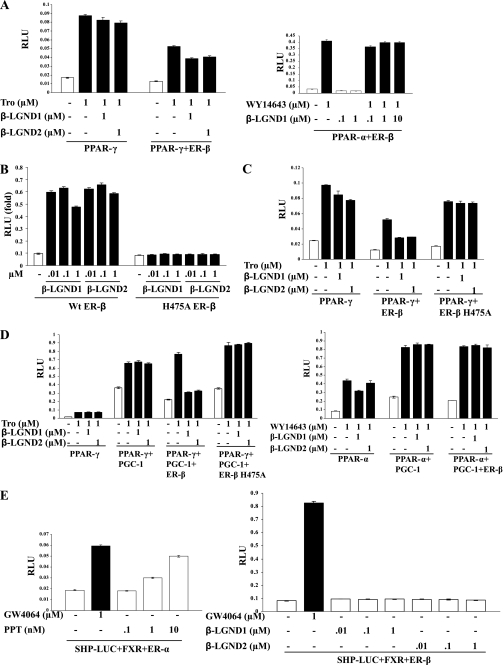
Foryst-Ludwig et al. (23) previously demonstrated that ER-β ligand independently inhibits PPAR-γ through N-terminal interactions. PPAR-γ was also demonstrated to be a proadipogenic transcription factor (45). In addition, one of the genes completely repressed by β-LGND1 in BAT and WAT (i.e. Lpl) is a PPAR-γ target gene (46) (Table 2). Transactivation studies were thus performed in HEK-293 cells transfected with ER-β, PPAR-γ, or PPAR-α and PPRE-LUC to determine the direct or indirect effects of β-LGND1 and β-LGND2 on PPAR activity. Both β-LGNDs partially inhibited troglitazone-induced PPAR-γ activity when cotransfected with ER-β (Fig. 6A, left panel) but did not affect WY14643 induced PPAR-α transactivation (Fig. 6A, right panel). Ligand-independent or constitutive inhibition of PPAR-γ by ER-β was also observed, confirming an earlier report (23).
FIGURE 6.
ER-β ligand-dependently inhibits PPAR-γ function through LBD. A, HEK-293 cells were transfected with 0.25 μg of PPRE-LUC, 5 ng of CMV-Renilla luciferase, and the indicated receptors (PPAR-γ and ER-β for the left panel and PPAR-α and ER-β for the right panel). The cells were treated 24 h after transfection with the indicated ligands and harvested 48 h after transfection, and firefly luciferase activity was measured and normalized to Renilla luciferase. B, His475 in ER-β LBD is important for its function. His475 in ER-β LBD was mutated to alanine (A) using site-directed mutagenesis kit. Transactivation assay was performed as described in A in HEK-293 cells with a titration of ER-β ligands in wild type or ER-β H475A. C, ER-β H475A fails to inhibit PPAR-γ transactivation. HEK-293 cells were transfected with 0.25 μg of PPRE-LUC, 5 ng of CMV-Renilla luciferase, and 50 ng of the indicated receptors (PPAR-γ or PPAR-γ and wild type ER-β or PPAR-γ and ER-β H475A). The cells were treated 24 h after transfection with the indicated ligands and harvested 48 h after transfection, and firefly luciferase activity was measured and normalized to Renilla luciferase. D, ER-β ligand-dependently inhibits PGC-1 coactivated PPAR-γ but not PPAR-α transactivation. HepG2 cells were transfected with 0.25 μg of PPRE-LUC, 5 ng of CMV-Renilla luciferase, 0.5 μg of PGC-1 or vector backbone, and 100 ng of the indicated receptors (PPAR-γ or PPAR-γ and wild type ER-β or PPAR-γ and ER-β H475A for left panels and PPAR-α or PPAR-α and ER-β for right panel). The cells were treated 24 h after transfection with the indicated ligands and harvested 48 h after transfection, and firefly luciferase activity was measured and normalized to Renilla luciferase. E, SHP-1 is an ER-β-specific target. HEK-293 cells were transfected with 0.25 μg of SHP-LUC, 5 ng of CMV-Renilla luciferase, and 50 ng of the indicated receptors (FXR and ER-α for the left panel and FXR and ER-β for the right panel). The cells were treated 24 h after transfection with the indicated ligands and harvested 48 h after transfection, and firefly luciferase activity was measured and normalized to Renilla luciferase. PPAR, peroxisome proliferator and activated receptor; ER, estrogen receptor; H, histidine; A, alanine; RLU, relative luciferase units; Tro, troglitazone; PGC-1, PPAR-γ coactivator; SHP, small heterodimer partner; FXR, farsenoid X receptor.
To determine whether the LBD of ER-β was required to inhibit PPAR-γ transactivation, we mutated histidine 475 in the ER-β LBD to alanine. This residue is critical for ligand binding to ER-β (47, 48). We confirmed this by mutating His475 to alanine and compared its transactivation to wild type ER-β. Transfection of HEK-293 cells with ERE-LUC, ER-β, or H475A ER-β confirmed that mutation of His475 to alanine abrogated the ability of β-LGND1 and β-LGND2 to activate ER-β (Fig. 6B).
Because H475A impaired β-LGND-dependent ER-β transactivation, the ability of this mutant receptor to inhibit PPAR-γ transactivation was determined and compared with wild type. As shown in Fig. 6C, wild type ER-β inhibited ligand-dependently and -independently the troglitazone-induced PPAR-γ transactivation, whereas H475A ER-β failed to inhibit PPAR-γ transactivation indicating the importance of ligand binding and ER-β-LBD to inhibit PPAR-γ transactivation.
PPAR-γ coactivator-1 (PGC-1) functions selectively as a PPAR-γ coactivator in many tissues such as WAT, BAT, and pancreatic islets. To determine whether ER-β ligands inhibit the ability of PGC-1 to coactivate PPAR-γ, PPAR-γ transactivation studies were performed in the presence or absence of PGC-1. In the absence of ER-β, troglitazone activated PPAR-γ, although PGC-1 robustly increased both the basal and ligand-dependent activity (Fig. 6D, left panel). However, wild type ER-β, but not H475A ER-β, ligand-dependently abolished the troglitazone-dependent PPAR-γ transactivation, indicating that ER-β not only inhibits basal PPAR-γ but also inhibits PGC-1 coactivated PPAR-γ transactivation. Conversely, coactivation of PPAR-α by PGC-1 was not inhibited by ER-β (Fig. 6D, right panel) confirming the selectivity of inhibition and lack of cross-reactivity.
SHP is an orphan member of the NHR family that is also known to play a role in metabolic diseases (49). The SHP promoter contains an estrogen-response element (ERE), and its activity was increased by estradiol through ER-α (50). We used HEK-293 cells transfected with SHP promoter-luciferase, FXR, and ER-β plasmids to determine whether β-LGND1 and β-LGND2 activate SHP through ER-β.The right panel of Fig. 6E demonstrates that neither of the ligands activated SHP, whereas FXR ligand GW4064 increased its activity significantly. The left panel of Fig. 6E shows that an ER-α selective ligand PPT increased SHP activity reproducing the earlier published results that SHP is an ER-α target gene.
DISCUSSION
Obesity is a complex disease that has created an increasing demand for drugs that reduce body weight and also treat conditions associated with obesity such as T2DM, osteoporosis, inflammation, muscle weakness, and others. Most of the drugs in development to treat obesity target the G protein-coupled receptor class and are associated with side effects ranging from nausea to depression (51, 52). Drugs acting through the G protein-coupled receptor class control appetite through central nervous system intervention, eventually leading to various neurological and psychological problems (53), as recently exemplified by the discontinuation of a cannabinoid antagonist due to severe psychological problems leading to suicide (54).
Discovery of ER-β in the prostate 12 years ago fostered the development of isoform-selective estrogen receptor modulators (55). Various in vivo and in vitro studies have unraveled the potential of ER-β as a therapeutic target. ER-β is currently suggested as a target for a variety of disorders, including prostate cancer, benign prostatic hyperplasia, inflammation, neuroprotection, and others (56–59). Here, we show for the first time that ligand-activated ER-β is a potential target to treat obesity and associated diseases.
Knock-out models and clinical studies revealed that ER-α is the primary mediator of reproductive and bone effects of estrogens, whereas ER-β mediates nonreproductive effects such as neurological, anti-inflammatory, and anti-proliferative effects. These studies mostly expound the beneficial effects of ER-β.
This report demonstrates that ER-β ligands not only prevent but also treat obesity. The ligands alter body composition by reducing fat mass and increasing lean mass. A variety of mechanisms can be proposed from the gene expression studies. The observed increase in Ucp-1 suggests that β-LGNDs increase energy expenditure through uncoupled respiration, which ultimately reduces body weight (60). Although there was no difference observed in the treated mice in BAT weight, the tissue responsible for uncoupled respiration, the observed changes in body composition and gene expression suggest that it may be a valuable predictor for changes that occur in other tissues. The expression of several important genes in the lipogenesis pathways, such as Srebp, Lpl, and others, was repressed, indicating that β-LGNDs inhibit lipid synthesis in addition to increasing uncoupled respiration. Earlier studies demonstrated an increase in lipogenesis when rats were ovariectomized, implicating a role for ERs in direct regulation of lipid synthesis (61). Similarly, another ERβ-selective ligand, genistein, reduced Srebp-1 expression in liver cells (62), corroborating the results obtained in this study. Likewise, genes in the oxidative stress and anti-oxidant pathways were significantly regulated in this study, suggesting that β-LGNDs promote weight loss through multiple pathways. Other pathways involving mast cells and angiogenesis are also implicated in obesity suggesting the complexity of the disease and the potential to utilize these pathways to target the disease (63, 64).
The reduction in feed consumption in the high-fat diet-fed animals correlates well with the increase in serum leptin and with previous publications (65, 66). Leptin has dual functions; one is to induce satiety, and the other is to serve as a marker for adipose tissue mass and obesity. In our studies, increased leptin in high-fat diet-fed mice caused a reduction in feed consumption. Simultaneously, β-LGNDs reduced body fat and body weight resulting in partial reduction in leptin levels (Fig. 2G). Although high-fat diet reduced the total food consumption, it increased the fat calories consumed by almost 10-fold (supplemental Fig. 1), indicating that the increase in body weight is due to the consumption of increased fat calories.
Earlier reports indicate that one of the coactivators of PPAR-γ, PRDM16, promotes the formation of BAT through PPAR-γ and PGC-1 activation (60). Knockdown of PRDM16 in BAT inhibits PPAR-γ function leading to the transformation of BAT to muscle (67). It is possible that ER-β inhibits PPAR-γ function indirectly by inhibiting the ability of PRDM16 to activate PPAR-γ in BAT. Although we have demonstrated the effect of ER-β on PPAR-γ activity with another coactivator, PGC-1 (Fig. 6D), future studies will be performed to demonstrate the interaction between ER-β, PRDM16, and PPAR-γ. Although directly antagonizing PPAR-γ with antagonists such as SR-202 and GW-9662 prevents high-fat diet-induced obesity, this may cause unwarranted side effects associated with manipulating PPAR-γ (68, 69). Hence, antagonizing PPAR-γ through indirectly acting agents like ER-β agonists by altering coactivator function could potentially perform the desired functions without adverse side effects.
The adipose tissue lipogenic gene expression data and the PPAR-γ transactivation data correlate well. We speculate that ER-β sequesters PGC-1 away from PPAR-γ to inhibit PPAR-γ function. PGC-1 is a coactivator for many steroid receptors such as PPARs and ERRs, and earlier studies have demonstrated that these receptors cross-talk at the level of their common coactivators (70). In fact, these receptors compete for a limiting pool of coactivators. In this scenario, ER-β competes with PPAR-γ for the limiting amount of PGC-1, subsequently inhibiting PPAR-γ function.
We ruled out the possibility that the pharmacological effects of β-LGNDs are mediated through cross-talk with ER-α and other NHRs and are now treating ER-αKO and ER-βKO mice with a high-fat diet and β-LGNDs to corroborate our findings. In addition, we also tested the effect of these ligands on action of AMP-activated kinase. The beneficial effects observed in the current studies (e.g. reducing cholesterol levels, alleviating diabetes, and increasing muscle mass) might also be mediated by AMP-activated kinase activation (71). Phosphorylation of AMP-activated kinase at Thr172 was measured in WAT, liver, and C2C12 muscle cell culture. No significant increase in phosphorylation of AMP-activated kinase was observed with β-LGND1 or β-LGND2 (data not shown) both in vitro and in vivo in target tissues. As nonspecific effects cannot be proved by process of elimination, the ongoing knock-out studies will be the appropriate path to prove the ER-β-specific effects.
MIP-1 protein orchestrates inflammatory responses at sites of injury mainly by recruiting pro-inflammatory cells. Thus, MIP-1 protein is a key player in the pathogenesis of several diseases (72). Earlier publications and preclinical studies from Wyeth clearly indicated the anti-inflammatory effects of ER-β ligands (58). As obesity is an inflammatory disease (9), induction of MIP-1 was not surprising considering the level of fat mass and body weight increase in vehicle-treated high-fat diet-fed animals. However, ER-β ligands reduced MIP-1 in these obese mice providing protection against further inflammatory diseases (Fig. 2H). It is surprising that out of 32 cytokines tested, only one cytokine was regulated by obesity and by ER-β ligands. These results were evaluated in different tissues at mRNA level to identify the tissue of origin. Unfortunately, this regulation in cytokines that was observed in serum was not observed in the examined tissues (data not shown).
We demonstrated that β-LGNDs can be used in diet- and ovariectomy-induced obesity. The mechanistic and characteristic aspects of obesity associated with both these conditions are entirely different. Diet induced-obesity and obesity in people undergoing prolonged rest arise due to increased energy consumption and decreased energy expenditure resulting in a significant accumulation of adipose tissue. On the other hand, postmenopausal obesity emanates because of reduced circulating estrogens and lost repression of adipose tissue proliferation and adipokine synthesis. The deposition of fat mass, and particularly central fat mass in postmenopausal obesity due to the lack of estrogens, is also responsible for an increase in circulating adipocytokines, which have implications for insulin resistance and cardiovascular diseases. β-LGNDs excelled in their ability to inhibit obesity associated with multiple etiologies.
There are four known strategies to prevent and/or treat obesity, including appetite suppression, inhibition of nutrient digestion and absorption, stimulation of fat mobilization, and increase in energy expenditure (73). β-LGNDs appear to offer a unique and comprehensive strategy to treat obesity by increasing energy expenditure, reducing fat synthesis, increasing muscle mass, and reducing inflammation. Targeting obesity peripherally without involving the central nervous system is a safe strategy, if effective weight reduction is demonstrated. Collectively, these studies demonstrate that β-LGNDs can repress body weight, improve insulin sensitivity, alleviate hypercholesterolemia, reduce leptin levels, improve lean mass, and reduce fat mass without any visible side, toxic, or reproductive effects validating ER-β as a bona fide target to combat obesity and metabolic diseases.
Supplementary Material
Acknowledgments
We thank Deanna Parke, Stacey Lindsey, Terrence Costello, and Katy Kail for the help rendered toward the animal studies. We also thank Jessica Grabowski for help with Western analysis.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Tables 1–3.
- T2DM
- type 2 diabetes mellitus
- ER
- estrogen receptor
- ERR
- estrogen-related receptor
- β-LGND
- ER-β-selective ligand
- WAT
- white adipose tissue
- BAT
- brown adipose tissue
- NHR
- nuclear hormone receptor
- FXR
- farnesoid X receptor
- PPAR
- peroxisome proliferator-activated receptor
- KO
- knock-out
- LBD
- ligand binding domain
- OVX
- ovariectomy
- E2
- estradiol
- SHP
- small heterodimeric partner
- DEXA
- dual energy x-ray absorptiometry.
REFERENCES
- 1.Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010) JAMA 303, 235–241 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen D. M., El-Serag H. B. (2010) Gastroenterol. Clin. North Am. 39, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie C. J., Milani R. V., Ventura H. O. (2009) J. Am. Coll. Cardiol. 53, 1925–1932 [DOI] [PubMed] [Google Scholar]
- 4.Fabricatore A. N., Wadden T. A. (2006) Annu. Rev. Clin. Psychol. 2, 357–377 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan. L. M. (2010) Gastroenterol. Clin. North Am. 39, 69–79 [DOI] [PubMed] [Google Scholar]
- 6.Cooke D., Bloom S. (2006) Nat. Rev. Drug Discov. 5, 919–931 [DOI] [PubMed] [Google Scholar]
- 7.Blackburn G. L., Hutter M. M., Harvey A. M., Apovian C. M., Boulton H. R., Cummings S., Fallon J. A., Greenberg I., Jiser M. E., Jones D. B., Jones S. B., Kaplan L. M., Kelly J. J., Kruger R. S., Jr., Lautz D. B., Lenders C. M., Lonigro R., Luce H., McNamara A., Mulligan A. T., Paasche-Orlow M. K., Perna F. M., Pratt J. S., Riley S. M., Jr., Robinson M. K., Romanelli J. R., Saltzman E., Schumann R., Shikora S. A., Snow R. L., Sogg S., Sullivan M. A., Tarnoff M., Thompson C. C., Wee C. C., Ridley N., Auerbach J., Hu F. B., Kirle L., Buckley R. B., Annas C. L. (2009) Obesity 17, 842–862 [DOI] [PubMed] [Google Scholar]
- 8.Chang P. (2009) Nat. Rev. Drug Discov. 8, 529. [DOI] [PubMed] [Google Scholar]
- 9.Yu R., Kim C. S., Kang J. H. (2009) Forum Nutr. 61, 95–103 [DOI] [PubMed] [Google Scholar]
- 10.Rother E., Jordan S. D., Brüning J. C. (2009) Dtsch. Med. Wochenschr. 134, 1057–1059 [DOI] [PubMed] [Google Scholar]
- 11.Reisin E., Jack A. V. (2009) Med. Clin. North Am. 93, 733–751 [DOI] [PubMed] [Google Scholar]
- 12.Allende-Vigo M. Z. (2008) P. R. Health Sci. J. 27, 190–195 [PubMed] [Google Scholar]
- 13.Geer E. B., Shen W. (2009) Gend. Med. 6, Suppl. 1, 60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. (2008) Nat. Rev. Drug Discov. 7, 678–693 [DOI] [PubMed] [Google Scholar]
- 15.Cariou B., Staels B. (2007) Trends Pharmacol. Sci. 28, 236–243 [DOI] [PubMed] [Google Scholar]
- 16.Ariazi E. A., Jordan V. C. (2006) Curr. Top. Med. Chem. 6, 203–215 [DOI] [PubMed] [Google Scholar]
- 17.Kintscher U., Goebel M. (2009) Curr. Opin. Investig. Drugs 10, 381–387 [PubMed] [Google Scholar]
- 18.van Beekum O., Fleskens V., Kalkhoven E. (2009) Obesity 17, 213–219 [DOI] [PubMed] [Google Scholar]
- 19.Billin A. N. (2008) Expert Opin. Investig. Drugs 17, 1465–1471 [DOI] [PubMed] [Google Scholar]
- 20.Barros R. P., Machado U. F., Gustafsson J. A. (2006) Trends Mol. Med. 12, 425–431 [DOI] [PubMed] [Google Scholar]
- 21.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris H. A. (2007) Mol. Endocrinol. 21, 1–13 [DOI] [PubMed] [Google Scholar]
- 23.Foryst-Ludwig A., Clemenz M., Hohmann S., Hartge M., Sprang C., Frost N., Krikov M., Bhanot S., Barros R., Morani A., Gustafsson J. A., Unger T., Kintscher U. (2008) PLoS Genet. 4, e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallottini V., Bulzomi P., Galluzzo P., Martini C., Marino M. (2008) Infect. Disord. Drug Targets 8, 52–60 [DOI] [PubMed] [Google Scholar]
- 25.Liang Y. Q., Akishita M., Kim S., Ako J., Hashimoto M., Iijima K., Ohike Y., Watanabe T., Sudoh N., Toba K., Yoshizumi M., Ouchi Y. (2002) Int. J. Obes. Relat. Metab. Disord. 26, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 26.Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., Maloney P. R., Willson T. M., Kliewer S. A. (2000) Mol. Cell 6, 517–526 [DOI] [PubMed] [Google Scholar]
- 27.Narayanan R., Adigun A. A., Edwards D. P., Weigel N. L. (2005) Mol. Cell. Biol. 25, 264–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morani A., Warner M., Gustafsson J. A. (2008) J. Intern. Med. 264, 128–142 [DOI] [PubMed] [Google Scholar]
- 29.Zhao L. J., Liu Y. J., Liu P. Y., Hamilton J., Recker R. R., Deng H. W. (2007) J. Clin. Endocrinol. Metab. 92, 1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freemantle N., Holmes J., Hockey A., Kumar S. (2008) Int. J. Clin. Pract. 62, 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emilsson V., Thorleifsson G., Zhang B., Leonardson A. S., Zink F., Zhu J., Carlson S., Helgason A., Walters G. B., Gunnarsdottir S., Mouy M., Steinthorsdottir V., Eiriksdottir G. H., Bjornsdottir G., Reynisdottir I., Gudbjartsson D., Helgadottir A., Jonasdottir A., Jonasdottir A., Styrkarsdottir U., Gretarsdottir S., Magnusson K. P., Stefansson H., Fossdal R., Kristjansson K., Gislason H. G., Stefansson T., Leifsson B. G., Thorsteinsdottir U., Lamb J. R., Gulcher J. R., Reitman M. L., Kong A., Schadt E. E., Stefansson K. (2008) Nature 452, 423–428 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Zhu J., Lum P. Y., Yang X., Pinto S., MacNeil D. J., Zhang C., Lamb J., Edwards S., Sieberts S. K., Leonardson A., Castellini L. W., Wang S., Champy M. F., Zhang B., Emilsson V., Doss S., Ghazalpour A., Horvath S., Drake T. A., Lusis A. J., Schadt E. E. (2008) Nature 452, 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurevich-Panigrahi T., Panigrahi S., Wiechec E., Los M. (2009) Curr. Med. Chem. 16, 506–521 [DOI] [PubMed] [Google Scholar]
- 34.Jezek P., Garlid K. D. (1998) Int. J. Biochem. Cell Biol. 30, 1163–1168 [DOI] [PubMed] [Google Scholar]
- 35.Andrews Z. B., Erion D. M., Beiler R., Choi C. S., Shulman G. I., Horvath T. L. (2010) Endocrinology 151, 2078–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Eckel R. H. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E271–E288 [DOI] [PubMed] [Google Scholar]
- 37.Wakil S. J. (1989) Biochemistry 28, 4523–4530 [DOI] [PubMed] [Google Scholar]
- 38.Capeau J. (2008) Diabetes Metab. 34, 649–657 [DOI] [PubMed] [Google Scholar]
- 39.Quintão E. C., Cazita P. M. (2010) Atherosclerosis 209, 1–9 [DOI] [PubMed] [Google Scholar]
- 40.Stevenson J., Brown A. J. (2009) Biochem. J. 420, e1–4 [DOI] [PubMed] [Google Scholar]
- 41.Maeda K., Okubo K., Shimomura I., Mizuno K., Matsuzawa Y., Matsubara K. (1997) Gene 190, 227–235 [DOI] [PubMed] [Google Scholar]
- 42.Oyadomari S., Mori M. (2004) Cell Death Differ. 11, 381–389 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T., Katsu Y., Watanabe H., Iguchi T. (2008) Toxicology 253, 117–124 [DOI] [PubMed] [Google Scholar]
- 44.Turgeon J. L., Carr M. C., Maki P. M., Mendelsohn M. E., Wise P. M. (2006) Endocr. Rev. 27, 575–605 [DOI] [PubMed] [Google Scholar]
- 45.Tontonoz P., Spiegelman B. M. (2008) Annu. Rev. Biochem. 77, 289–312 [DOI] [PubMed] [Google Scholar]
- 46.Kersten S. (2008) PPAR Res. 2008,132960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh R. W., Rajan S. S., Sharma S. K., Guo Y., DeSombre E. R., Mrksich M., Greene G. L. (2006) J. Biol. Chem. 281, 17909–17919 [DOI] [PubMed] [Google Scholar]
- 48.Bhat R. A., Stauffer B., Unwalla R. J., Xu Z., Harris H. A., Komm B. S. (2004) J. Steroid Biochem. Mol. Biol. 88, 17–26 [DOI] [PubMed] [Google Scholar]
- 49.Nishigori H., Tomura H., Tonooka N., Kanamori M., Yamada S., Sho K., Inoue I., Kikuchi N., Onigata K., Kojima I., Kohama T., Yamagata K., Yang Q., Matsuzawa Y., Miki T., Seino S., Kim M. Y., Choi H. S., Lee Y. K., Moore D. D., Takeda J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai K., Harnish D. C., Evans M. J. (2003) J. Biol. Chem. 278, 36418–36429 [DOI] [PubMed] [Google Scholar]
- 51.Lim W. K. (2007) Recent Pat. CNS Drug Discov. 2, 107–112 [DOI] [PubMed] [Google Scholar]
- 52.Leite C. E., Mocelin C. A., Petersen G. O., Leal M. B., Thiesen F. V. (2009) Pharmacol. Rep. 61, 217–224 [DOI] [PubMed] [Google Scholar]
- 53.Moreira F. A., Crippa J. A. (2009) Rev. Bras. Psiquiatr. 31, 145–153 [DOI] [PubMed] [Google Scholar]
- 54.Jones D. (2008) Nat. Rev. Drug Discov. 7, 961–962 [DOI] [PubMed] [Google Scholar]
- 55.Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pravettoni A., Mornati O., Martini P. G., Marino M., Colciago A., Celotti F., Motta M., Negri-Cesi P. (2007) Mol. Cell. Endocrinol. 263, 46–54 [DOI] [PubMed] [Google Scholar]
- 57.Jiang J., Chang H. L., Sugimoto Y., Lin Y. C. (2005) Anticancer Res. 25, 4081–4090 [PubMed] [Google Scholar]
- 58.Follettie M. T., Pinard M., Keith J. C., Jr., Wang L., Chelsky D., Hayward C., Kearney P., Thibault P., Paramithiotis E., Dorner A. J., Harris H. A. (2006) Endocrinology 147, 714–723 [DOI] [PubMed] [Google Scholar]
- 59.Fan X., Warner M., Gustafsson J. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19338–19343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., Spiegelman B. M. (2007) Cell Metab. 6, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paquette A., Wang D., Jankowski M., Gutkowska J., Lavoie J. M. (2008) Menopause 15, 1169–1175 [DOI] [PubMed] [Google Scholar]
- 62.Shin E. S., Lee H. H., Cho S. Y., Park H. W., Lee S. J., Lee T. R. (2007) J. Nutr. 137, 1127–1131 [DOI] [PubMed] [Google Scholar]
- 63.Liu J., Divoux A., Sun J., Zhang J., Clément K., Glickman J. N., Sukhova G. K., Wolters P. J., Du J., Gorgun C. Z., Doria A., Libby P., Blumberg R. S., Kahn B. B., Hotamisligil G. S., Shi G. P. (2009) Nat. Med. 15, 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutkowski J. M., Davis K. E., Scherer P. E. (2009) FEBS J. 276, 5738–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison C. D., Huypens P., Stewart L. K., Gettys T. W. (2009) Biochim. Biophys. Acta 1792, 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman J. M. (2002) Nutr. Rev. 60, S1–S14; discussion S68–S84, 85–87 [DOI] [PubMed] [Google Scholar]
- 67.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M. (2008) Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakano R., Kurosaki E., Yoshida S., Yokono M., Shimaya A., Maruyama T., Shibasaki M. (2006) Biochem. Pharmacol. 72, 42–52 [DOI] [PubMed] [Google Scholar]
- 69.Rieusset J., Touri F., Michalik L., Escher P., Desvergne B., Niesor E., Wahli W. (2002) Mol. Endocrinol. 16, 2628–2644 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Fang F., Wong C. W. (2010) Biochem. Pharmacol. 80, 80–85 [DOI] [PubMed] [Google Scholar]
- 71.Zhang B. B., Zhou G., Li C. (2009) Cell Metab. 9, 407–416 [DOI] [PubMed] [Google Scholar]
- 72.Maurer M., von Stebut E. (2004) Int. J. Biochem. Cell Biol. 36, 1882–1886 [DOI] [PubMed] [Google Scholar]
- 73.Shi Y., Burn P. (2004) Nat. Rev. Drug Discov. 3, 695–710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.