Abstract
Fas/APO-1 is a transmembrane protein of the nerve growth factor/TNF alpha receptor family which signals apoptotic cell death in susceptible target cells. We have investigated the susceptibility of seven human malignant glioma cell lines to Fas/APO-1-dependent apoptosis. Sensitivity to Fas/APO-1 antibody-mediated cell killing correlated with cell surface expression of Fas/APO-1. Expression of Fas/APO-1 as well as Fas/APO-1-dependent cytotoxicity were augmented by preexposure of human malignant glioma cells to IFN gamma and TNF alpha. Further, pretreatment with TGF beta 2, IL1 and IL8 enhanced Fas/APO-1 antibody-induced glioma cell apoptosis whereas other cytokines including TNF beta, IL6, macrophage colony-stimulating factor, IL10 and IL13 had no such effect. None of the human malignant glioma cell lines was susceptible to TNF alpha-induced cytotoxicity. Fas/APO-1 antibody-sensitive glioma cell lines (n = 5), but not Fas/APO-1 antibody-resistant glioma cell lines (n = 2), became sensitive to TNF alpha when co-treated with inhibitors of RNA and protein synthesis. Resistance of human glioma cells to Fas/APO-1 antibody-mediated apoptosis was mainly related to low level expression of Fas/APO-1 and appeared not to be linked to overexpression of the anti-apoptotic protooncogene, bcl-2. Given the resistance of human malignant glioma to surgery, irradiation, chemotherapy and immunotherapy, we propose that Fas/APO-1 may be a promising target for a novel locoregionary approach to human malignant glioma. This strategy gains support from the demonstration of Fas/APO-1 expression in ex vivo human malignant glioma specimens and from the absence of Fas/APO-1 in normal human brain parenchyma.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Armitage R. J., Maraskovsky E., Tough T. W., Roux E., Schooley K., Ramsdell F., Lynch D. H. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993 Dec 1;178(6):2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Black K. L., Chen K., Becker D. P., Merrill J. E. Inflammatory leukocytes associated with increased immunosuppression by glioblastoma. J Neurosurg. 1992 Jul;77(1):120–126. doi: 10.3171/jns.1992.77.1.0120. [DOI] [PubMed] [Google Scholar]
- Bodmer S., Strommer K., Frei K., Siepl C., de Tribolet N., Heid I., Fontana A. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989 Nov 15;143(10):3222–3229. [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Constam D. B., Philipp J., Malipiero U. V., ten Dijke P., Schachner M., Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992 Mar 1;148(5):1404–1410. [PubMed] [Google Scholar]
- Debatin K. M., Süss D., Krammer P. H. Differential expression of APO-1 on human thymocytes: implications for negative selection? Eur J Immunol. 1994 Mar;24(3):753–758. doi: 10.1002/eji.1830240339. [DOI] [PubMed] [Google Scholar]
- Deen D. F., Chiarodo A., Grimm E. A., Fike J. R., Israel M. A., Kun L. E., Levin V. A., Marton L. J., Packer R. J., Pegg A. E. Brain Tumor Working Group Report on the 9th International Conference on Brain Tumor Research and Therapy. Organ System Program, National Cancer Institute. J Neurooncol. 1993 Jun;16(3):243–272. doi: 10.1007/BF01057041. [DOI] [PubMed] [Google Scholar]
- Dhein J., Daniel P. T., Trauth B. C., Oehm A., Möller P., Krammer P. H. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol. 1992 Nov 15;149(10):3166–3173. [PubMed] [Google Scholar]
- Falk M. H., Trauth B. C., Debatin K. M., Klas C., Gregory C. D., Rickinson A. B., Calender A., Lenoir G. M., Ellwart J. W., Krammer P. H. Expression of the APO-1 antigen in Burkitt lymphoma cell lines correlates with a shift towards a lymphoblastoid phenotype. Blood. 1992 Jun 15;79(12):3300–3306. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
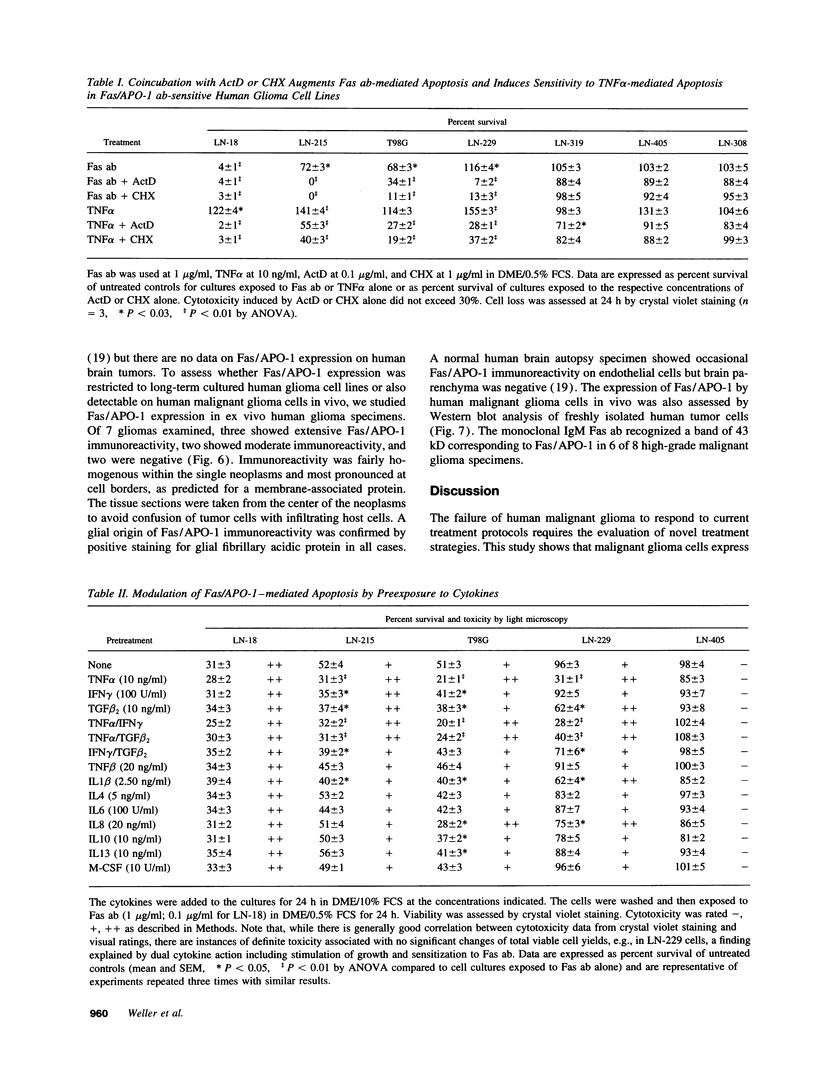
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Frei K., Piani D., Malipiero U. V., Van Meir E., de Tribolet N., Fontana A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells. Despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol. 1992 May 15;148(10):3140–3146. [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. A., Fodstad O. Immunotoxins and central nervous system neoplasia. J Neurosurg. 1992 Jan;76(1):1–12. doi: 10.3171/jns.1992.76.1.0001. [DOI] [PubMed] [Google Scholar]
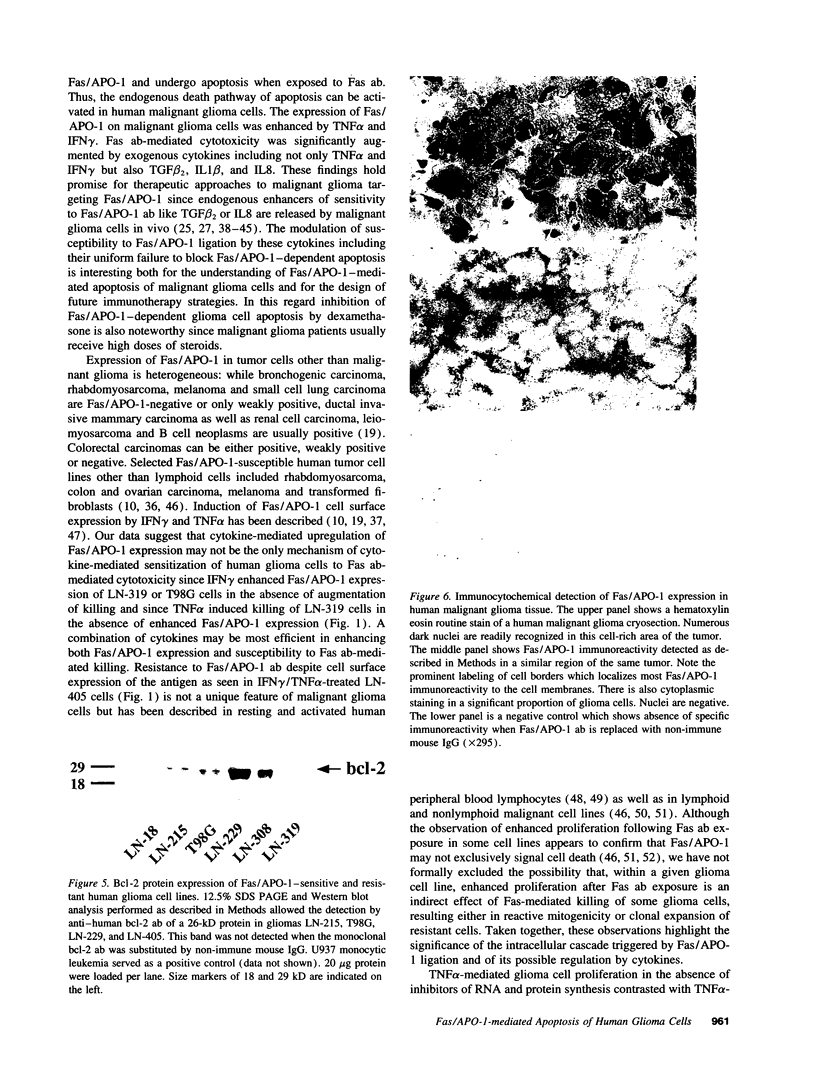
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst H. A., Scheithauer B. W., Kelly P. J., Kovach J. S. Distribution of transforming growth factor-beta 1 in human astrocytomas. Hum Pathol. 1992 Nov;23(11):1284–1288. doi: 10.1016/0046-8177(92)90297-g. [DOI] [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Itoh N., Tsujimoto Y., Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993 Jul 15;151(2):621–627. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klas C., Debatin K. M., Jonker R. R., Krammer P. H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993 Jun;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2: an antidote to programmed cell death. Cancer Surv. 1992;15:105–118. [PubMed] [Google Scholar]
- Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990 Nov;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- Leithäuser F., Dhein J., Mechtersheimer G., Koretz K., Brüderlein S., Henne C., Schmidt A., Debatin K. M., Krammer P. H., Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993 Oct;69(4):415–429. [PubMed] [Google Scholar]
- Mapara M. Y., Bargou R., Zugck C., Döhner H., Ustaoglu F., Jonker R. R., Krammer P. H., Dörken B. APO-1 mediated apoptosis or proliferation in human chronic B lymphocytic leukemia: correlation with bcl-2 oncogene expression. Eur J Immunol. 1993 Mar;23(3):702–708. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- Maxwell M., Galanopoulos T., Neville-Golden J., Antoniades H. N. Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. J Neurosurg. 1992 May;76(5):799–804. doi: 10.3171/jns.1992.76.5.0799. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Uehara T., Nibu R., Tsuji T., Yachie A., Yonehara S., Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992 Dec 1;149(11):3753–3758. [PubMed] [Google Scholar]
- Morimoto H., Yonehara S., Bonavida B. Overcoming tumor necrosis factor and drug resistance of human tumor cell lines by combination treatment with anti-Fas antibody and drugs or toxins. Cancer Res. 1993 Jun 1;53(11):2591–2596. [PubMed] [Google Scholar]
- Möller P., Henne C., Leithäuser F., Eichelmann A., Schmidt A., Brüderlein S., Dhein J., Krammer P. H. Coregulation of the APO-1 antigen with intercellular adhesion molecule-1 (CD54) in tonsillar B cells and coordinate expression in follicular center B cells and in follicle center and mediastinal B-cell lymphomas. Blood. 1993 Apr 15;81(8):2067–2075. [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 Aug 26;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Meterissian S., Ford R. J. Fas/APO-1 expression and function on malignant cells of hematologic and nonhematologic origin. J Immunother Emphasis Tumor Immunol. 1993 Oct;14(3):234–241. doi: 10.1097/00002371-199310000-00011. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini P. A., Rivoltini L., Silvani A., Boiardi A., Parmiani G. Factors, including transforming growth factor beta, released in the glioblastoma residual cavity, impair activity of adherent lymphokine-activated killer cells. Cancer Immunol Immunother. 1993 Jun;36(6):409–416. doi: 10.1007/BF01742258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Hofman F. M., Apuzzo M. L., Hinton D. R. Cytokines and immunoregulatory molecules in malignant glial neoplasms. J Neurosurg. 1992 Aug;77(2):265–273. doi: 10.3171/jns.1992.77.2.0265. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Osborne B. A. Programmed cell death, apoptosis and killer genes. Immunol Today. 1993 Dec;14(12):582–590. doi: 10.1016/0167-5699(93)90197-S. [DOI] [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993 Apr;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Sen S., D'Incalci M. Apoptosis. Biochemical events and relevance to cancer chemotherapy. FEBS Lett. 1992 Jul 27;307(1):122–127. doi: 10.1016/0014-5793(92)80914-3. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Szalay M. G., Paskar L., Sahai B. M., Boyer M., Singh B., Green D. R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990 May 1;144(9):3326–3333. [PubMed] [Google Scholar]
- Suda T., Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994 Mar 1;179(3):873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Tada M., Sawamura Y., Sakuma S., Suzuki K., Ohta H., Aida T., Abe H. Cellular and cytokine responses of the human central nervous system to intracranial administration of tumor necrosis factor alpha for the treatment of malignant gliomas. Cancer Immunol Immunother. 1993;36(4):251–259. doi: 10.1007/BF01740907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Yabu K., Kobayashi S. Detection of active form of transforming growth factor-beta in cerebrospinal fluid of patients with glioma. Jpn J Cancer Res. 1993 May;84(5):544–548. doi: 10.1111/j.1349-7006.1993.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia L. A., Ayres T. M., Wong G. H., Goeddel D. V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993 Sep 10;74(5):845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Van Meir E., Ceska M., Effenberger F., Walz A., Grouzmann E., Desbaillets I., Frei K., Fontana A., de Tribolet N. Interleukin-8 is produced in neoplastic and infectious diseases of the human central nervous system. Cancer Res. 1992 Aug 15;52(16):4297–4305. [PubMed] [Google Scholar]
- Vanhaesebroeck B., Reed J. C., De Valck D., Grooten J., Miyashita T., Tanaka S., Beyaert R., Van Roy F., Fiers W. Effect of bcl-2 proto-oncogene expression on cellular sensitivity to tumor necrosis factor-mediated cytotoxicity. Oncogene. 1993 Apr;8(4):1075–1081. [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Aguila H. L., Weissman I. L. Bcl-2 prevents death of factor-deprived cells but fails to prevent apoptosis in targets of cell mediated killing. Int Immunol. 1992 Jul;4(7):821–824. doi: 10.1093/intimm/4.7.821. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Weller M., Constam D. B., Malipiero U., Fontana A. Transforming growth factor-beta 2 induces apoptosis of murine T cell clones without down-regulating bcl-2 mRNA expression. Eur J Immunol. 1994 Jun;24(6):1293–1300. doi: 10.1002/eji.1830240608. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida J., Wakabayashi T., Mizuno M., Sugita K., Yoshida T., Hori S., Mori T., Sato T., Karashima A., Kurisu K. Clinical effect of intra-arterial tumor necrosis factor-alpha for malignant glioma. J Neurosurg. 1992 Jul;77(1):78–83. doi: 10.3171/jns.1992.77.1.0078. [DOI] [PubMed] [Google Scholar]
- Zuber P., Accolla R. S., Carrel S., Diserens A. C., de Tribolet N. Effects of recombinant human tumor necrosis factor-alpha on the surface phenotype and the growth of human malignant glioma cell lines. Int J Cancer. 1988 Nov 15;42(5):780–786. doi: 10.1002/ijc.2910420525. [DOI] [PubMed] [Google Scholar]









