Abstract
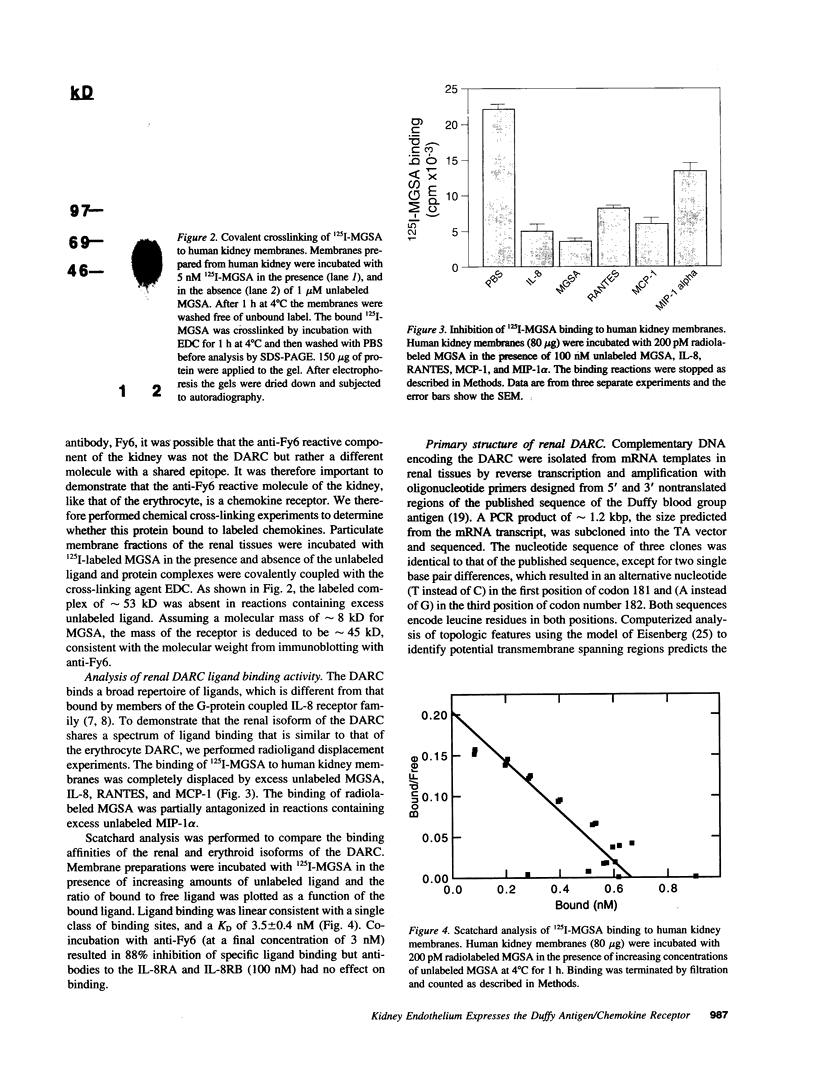
The human erythrocyte chemokine receptor has recently been shown to be identical to the Duffy blood group antigen and is expressed in multiple organs, including kidney. Here we have examined the molecular properties of the renal isoform. Immunoblot analysis of erythrocyte and kidney detergent lysates, with a monoclonal antibody (Fy6) to the Duffy antigen, revealed that the renal isoform had a molecular mass of 43-45 kD, which could be distinguished from that observed in erythroid cells (38-47 kD). Chemical cross-linking of kidney membranes to 125I-melanoma growth stimulatory activity (MGSA) indicated that the renal chemokine receptor had a molecular mass of 38-45 kD. Binding of 125I-labeled MGSA to kidney membranes was competitively inhibited by the addition of unlabeled MGSA, IL-8, regulated on activation, normal T expressed and secrted, and monocyte chemotactic protein-1. Scatchard analysis of MGSA binding showed that the chemokine receptor from renal tissues had a binding affinity of 3.5 nM similar to that observed for the erythroid isoform (5-10 nM). The primary structure of the renal chemokine receptor predicted from the nucleotide sequence of cDNA from renal tissues is identical to that reported for the erythroid isoform. Immunocytochemical staining of kidney with Fy6 localized expression to endothelial cells present in postcapillary venules. These studies implicate the Duffy antigen/chemokine receptor in the complex interactions between postcapillary endothelial cells and granulocytes, which are modulated by pro-inflammatory chemokines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W., Nichols M. E., Rubinstein P. In vitro evaluation of the role of the Duffy blood group in erythrocyte invasion by Plasmodium vivax. J Exp Med. 1989 May 1;169(5):1795–1802. doi: 10.1084/jem.169.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron J. P., Le Van Kim C., Colin Y. Glycophorin C and related glycoproteins: structure, function, and regulation. Semin Hematol. 1993 Apr;30(2):152–168. [PubMed] [Google Scholar]
- Chaudhuri A., Polyakova J., Zbrzezna V., Williams K., Gulati S., Pogo A. O. Cloning of glycoprotein D cDNA, which encodes the major subunit of the Duffy blood group system and the receptor for the Plasmodium vivax malaria parasite. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10793–10797. doi: 10.1073/pnas.90.22.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A., Zbrzezna V., Johnson C., Nichols M., Rubinstein P., Marsh W. L., Pogo A. O. Purification and characterization of an erythrocyte membrane protein complex carrying Duffy blood group antigenicity. Possible receptor for Plasmodium vivax and Plasmodium knowlesi malaria parasite. J Biol Chem. 1989 Aug 15;264(23):13770–13774. [PubMed] [Google Scholar]
- Chaudhuri A., Zbrzezna V., Polyakova J., Pogo A. O., Hesselgesser J., Horuk R. Expression of the Duffy antigen in K562 cells. Evidence that it is the human erythrocyte chemokine receptor. J Biol Chem. 1994 Mar 18;269(11):7835–7838. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Chan J. Y., Chasis J. A., Kan Y. W., Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J Biol Chem. 1991 May 5;266(13):8273–8280. [PubMed] [Google Scholar]
- Conboy J. G., Chasis J. A., Winardi R., Tchernia G., Kan Y. W., Mohandas N. An isoform-specific mutation in the protein 4.1 gene results in hereditary elliptocytosis and complete deficiency of protein 4.1 in erythrocytes but not in nonerythroid cells. J Clin Invest. 1993 Jan;91(1):77–82. doi: 10.1172/JCI116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbonne W. C., Rice G. C., Mohler M. A., Apple T., Hébert C. A., Valente A. J., Baker J. B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991 Oct;88(4):1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Fina L., Molgaard H. V., Robertson D., Bradley N. J., Monaghan P., Delia D., Sutherland D. R., Baker M. A., Greaves M. F. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990 Jun 15;75(12):2417–2426. [PubMed] [Google Scholar]
- Gallagher P. G., Forget B. G. Spectrin genes in health and disease. Semin Hematol. 1993 Jan;30(1):4–20. [PubMed] [Google Scholar]
- Hadley T. J., David P. H., McGinniss M. H., Miller L. H. Identification of an erythrocyte component carrying the Duffy blood group Fya antigen. Science. 1984 Feb 10;223(4636):597–599. doi: 10.1126/science.6695171. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Horuk R., Chitnis C. E., Darbonne W. C., Colby T. J., Rybicki A., Hadley T. J., Miller L. H. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993 Aug 27;261(5125):1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- Horuk R., Colby T. J., Darbonne W. C., Schall T. J., Neote K. The human erythrocyte inflammatory peptide (chemokine) receptor. Biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry. 1993 Jun 8;32(22):5733–5738. doi: 10.1021/bi00073a002. [DOI] [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Luscinskas F. W., Kiely J. M., Luis E. A., Darbonne W. C., Bennett G. L., Liu C. C., Obin M. S., Gimbrone M. A., Jr, Baker J. B. Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils. J Immunol. 1990 Nov 1;145(9):3033–3040. [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Andersson M. A., Lodish H. F. Multiple tissue-specific sites of transcriptional initiation of the mouse anion antiport gene in erythroid and renal cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7149–7153. doi: 10.1073/pnas.84.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Le Van Kim C., Colin Y., Mitjavila M. T., Clerget M., Dubart A., Nakazawa M., Vainchenker W., Cartron J. P. Structure of the promoter region and tissue specificity of the human glycophorin C gene. J Biol Chem. 1989 Dec 5;264(34):20407–20414. [PubMed] [Google Scholar]
- McPherson G. A. A practical computer-based approach to the analysis of radioligand binding experiments. Comput Programs Biomed. 1983 Aug-Oct;17(1-2):107–113. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Mason S. J., Clyde D. F., McGinniss M. H. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976 Aug 5;295(6):302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Mason S. J., Dvorak J. A., McGinniss M. H., Rothman I. K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975 Aug 15;189(4202):561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Neote K., Darbonne W., Ogez J., Horuk R., Schall T. J. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem. 1993 Jun 15;268(17):12247–12249. [PubMed] [Google Scholar]
- Neote K., DiGregorio D., Mak J. Y., Horuk R., Schall T. J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993 Feb 12;72(3):415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- Nichols M. E., Rubinstein P., Barnwell J., Rodriguez de Cordoba S., Rosenfield R. E. A new human Duffy blood group specificity defined by a murine monoclonal antibody. Immunogenetics and association with susceptibility to Plasmodium vivax. J Exp Med. 1987 Sep 1;166(3):776–785. doi: 10.1084/jem.166.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- SANGER R., RACE R. R., JACK J. The Duffy blood groups of New York negroes: the phenotype Fy (a-b-). Br J Haematol. 1955 Oct;1(4):370–374. doi: 10.1111/j.1365-2141.1955.tb05523.x. [DOI] [PubMed] [Google Scholar]
- Schall T. J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Tanner M. J. Molecular and cellular biology of the erythrocyte anion exchanger (AE1). Semin Hematol. 1993 Jan;30(1):34–57. [PubMed] [Google Scholar]
- Tilg H., Pape D., Trehu E., Shapiro L., Atkins M. B., Dinarello C. A., Mier J. W. A method for the detection of erythrocyte-bound interleukin-8 in humans during interleukin-1 immunotherapy. J Immunol Methods. 1993 Aug 9;163(2):253–258. doi: 10.1016/0022-1759(93)90129-u. [DOI] [PubMed] [Google Scholar]
- Wasniowska K., Eichenberger P., Kugele F., Hadley T. J. Purification of a 28 kD non-aggregating tryptic peptide of the Duffy blood group protein. Biochem Biophys Res Commun. 1993 Apr 30;192(2):366–372. doi: 10.1006/bbrc.1993.1424. [DOI] [PubMed] [Google Scholar]







