Abstract
Regulated polyadenylation is a broadly conserved mechanism that controls key events during oogenesis. Pivotal to that mechanism is GLD-2, a catalytic subunit of cytoplasmic poly(A) polymerase (PAP). Caenorhabditis elegans GLD-2 forms an active PAP with multiple RNA-binding partners to regulate diverse aspects of germline and early embryonic development. One GLD-2 partner, RNP-8, was previously shown to influence oocyte fate specification. Here we use a genomic approach to identify transcripts selectively associated with both GLD-2 and RNP-8. Among the 335 GLD-2/RNP-8 potential targets, most were annotated as germline mRNAs and many as maternal mRNAs. These targets include gld-2 and rnp-8 themselves, suggesting autoregulation. Removal of either GLD-2 or RNP-8 resulted in shortened poly(A) tails and lowered abundance of four target mRNAs (oma-2, egg-1, pup-2, and tra-2); GLD-2 depletion also lowered the abundance of most GLD-2/RNP-8 putative target mRNAs when assayed on microarrays. Therefore, GLD-2/RNP-8 appears to polyadenylate and stabilize its target mRNAs. We also provide evidence that rnp-8 influences oocyte development; rnp-8 null mutants have more germ cell corpses and fewer oocytes than normal. Furthermore, RNP-8 appears to work synergistically with another GLD-2–binding partner, GLD-3, to ensure normal oogenesis. We propose that the GLD-2/RNP-8 enzyme is a broad-spectrum regulator of the oogenesis program that acts within an RNA regulatory network to specify and produce fully functional oocytes.
Keywords: Caenorhabditis elegans, germline, RIP-chip, maternal mRNA, GLD-3
Regulation of mRNA is essential for many aspects of metazoan development (1). One broadly used posttranscriptional mechanism relies on the regulated polyadenylation and deadenylation of mRNAs (2, 3). In the nucleus, a tract of adenosine residues is added to the 3′ end of almost every pre-mRNA; in the cytoplasm, poly(A) tail lengths are regulated to influence the use of specific mRNAs in producing a protein product (4, 5). Poly(A) tail lengthening generally stabilizes mRNA and triggers translational activation, whereas shortening destabilizes mRNA and causes translational repression (4, 6, 7). Regulated polyadenylation is extensively used in the cytoplasm of oocytes and embryos to control an array of developmental decisions (4); this posttranscriptional mechanism also regulates mRNA expression in neuronal cells, influencing long-term memory and learning (8, 9).
GLD-2 is the catalytic subunit of a major cytoplasmic poly(A) polymerase (PAP), but unlike the canonical nuclear PAP, GLD-2 does not possess an RNA-binding domain (10). Instead, a fully functional enzyme is achieved by GLD-2 binding with one or more RNA-binding proteins, which have been proposed to recruit GLD-2 PAP activity to specific RNAs (10–14). Xenopus GLD-2 binds CPEB to regulate mRNAs during oocyte maturation (14, 15); Drosophila GLD-2, known as Wispy, activates key mRNAs during oogenesis and egg activation and may do so with Orb/CPEB and Bic-C/GLD-3 as partners (16, 17).
Caenorhabditis elegans GLD-2 influences many developmental events, including the sperm/oocyte decision, entry into the meiotic cell cycle, progression through the meiotic cell cycle, progression through both spermatogenesis and oogenesis, and events in the early embryo (10, 11, 18). To accomplish these multiple roles, we proposed that GLD-2 functions combinatorially, interacting with distinct binding partners to drive distinct functions. Indeed, nematode GLD-2 forms complexes with at least two RNA-binding partners, RNP-8 and GLD-3 (10, 11). The RNP-8 protein harbors an RNA recognition motif (RRM) and binds RNA with a sequence preference for purines (11), whereas GLD-3 belongs to the Bicaudal-C family of RNA-binding proteins (19). Both GLD-2 partners stimulate GLD-2 PAP activity in vitro and coimmunoprecipitate with GLD-2 from worm extracts (10, 11). However, RNP-8 and GLD-3 do not coimmunoprecipitate with each other (11). Therefore, RNP-8 and GLD-3 appear to be bona fide GLD-2 partners that function in distinct complexes.
The target mRNAs of GLD-2/RNA-binding protein complexes are largely unknown. In C. elegans, only one target has been identified to date, the gld-1 mRNA (20), and in other organisms only a few mRNAs are known (14–17, 21). This work focuses on identification of target mRNAs of the C. elegans GLD-2/RNP-8 enzyme. Our choice of GLD-2/RNP-8 was based on its apparent simplicity. When our study began, GLD-2/RNP-8 was only known to affect one biological function. Some rnp-8 null mutants were sterile, with a masculinization of germline (Mog) phenotype, but most were fertile. Therefore, RNP-8 plays a role in oocyte fate specification but was not implicated in other germline events (Fig. 1A) (11). The importance of the RNP-8 role in oocyte fate specification was underscored by the fully Mog phenotype in rnp-8; gld-1 double mutants (11). By contrast, the other GLD-2–binding partner, GLD-3, affects multiple germline processes and also partners with GLD-4, a distinct catalytic PAP subunit (19, 22–24).
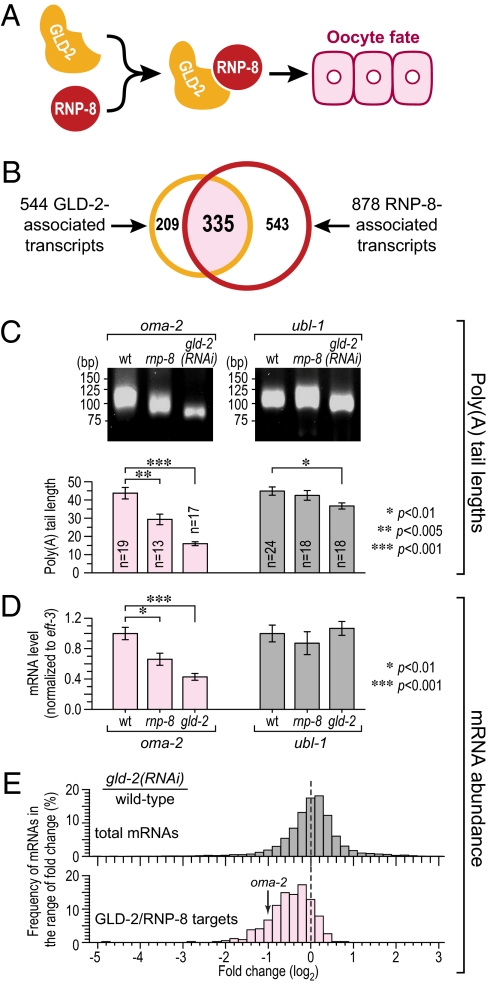
Fig. 1.
Identification of GLD-2– and RNP-8–associated mRNAs. (A) One biological function of the GLD-2/RNP-8 poly(A) polymerase is to specify the oocyte fate (11). (B) A comparison of the GLD-2– and RNP-8–associated mRNAs identifies 335 in common (pale red), which are likely GLD-2/RNP-8 target mRNAs. (C) Poly(A) tail lengths of oma-2 and ubl-1 mRNAs in wild-type, rnp-8(0), and gld-2(RNAi) adults, measured by gel analysis of PCR products (Upper) and by sequencing cloned PCR isolates (mean ± SEM) (Lower). (D) Relative abundance of oma-2 and ubl-1 mRNAs normalized to eft-3 mRNA (mean ± SEM). (E) Frequency histogram of log2-fold change in mRNA abundance in wild-type vs. gld-2(RNAi) adults, assayed on microarrays. A log2-fold change at zero means no change of mRNA level, whereas a shift toward the left means lower abundance upon GLD-2 depletion.
In this paper, we use a genomic approach to identify likely target mRNAs of the GLD-2/RNP-8 enzyme. Using the method of RNA immunoprecipitation (IP) followed by microarray analysis (RIP-chip), we found 335 mRNAs that selectively associate with both GLD-2 and RNP-8. We tested four individual mRNAs among the potential GLD-2/RNP-8 targets and demonstrated that all four rely on GLD-2 and RNP-8 for their normal poly(A) tail length and abundance. Most GLD-2/RNP-8–associated transcripts were oocyte-enriched maternal mRNAs. To understand that strong functional bias, we reexamined the rnp-8 null mutant phenotype and found that RNP-8 affects oocyte development, a role that is enhanced by GLD-3. Therefore, GLD-2/RNP-8 emerges as a broad-spectrum regulator of the oogenesis program that acts within an RNA regulatory network to specify and produce fully functional oocytes.
Results
Identification of GLD-2–Associated Transcripts.
To identify putative GLD-2 mRNA targets, we immunoprecipitated GLD-2 (Fig. S1A, Left) with associated mRNAs from wild-type adult hermaphrodite extracts, isolated RNA (WT-IP RNA), and probed microarrays. We used adults because GLD-2 and RNP-8 form a complex at this stage (11). Our strategy is outlined in Fig. S1B. Briefly, we compared the profiles of WT-IP RNAs to two other RNA profiles. We first compared them to RNAs coimmunoprecipitated from extracts depleted for GLD-2 by RNA interference (gld-2 RNAi); this comparison removed RNAs nonspecifically bound to the antibody matrix. The gld-2(RNAi) animals were sterile with similar but less severe germline defects than gld-2(0) mutants; for example, gld-2(RNAi) germlines produced recognizable oocytes, whereas gld-2(0) germlines did not (Fig. S2) (18). The differences between wild-type and gld-2(RNAi) animals might have biased our analysis toward higher-abundance mRNAs in the wild type. To avoid that bias, we next compared WT-IP RNAs to total WT RNA to remove RNAs falsely selected in the first comparison due to differences between wild-type and gld-2 RNAi extracts.
To generate each profile, mRNAs were linearly amplified, labeled, and hybridized to an Affymetrix C. elegans GeneChip; the array data were analyzed using significant analysis of microarray (SAM) (25). Briefly, SAM assigns to each probe set a score and estimates its false discovery rate (FDR). The WT-IP vs. gld-2(RNAi)-IP and WT-IP vs. total WT RNA comparisons identified 1,273 and 965 unique transcripts, respectively, as significantly enriched at an FDR of 5.5% or less. The overlap between the two sets revealed 544 transcripts (Fig. 1B; complete list in Dataset S1). Although discarding nonoverlapping genes is likely to remove some bona fide GLD-2–associated transcripts, we elected to focus on the 544 overlapping transcripts to minimize false positives. Importantly, the 50 most abundant gonadal mRNAs identified by SAGE analysis (http://elegans.bcgsc.bc.ca/) were absent from the 544 GLD-2–associated mRNAs.
We next used quantitative RT-PCR (qRT-PCR) to validate the enrichment of GLD-2 IP mRNAs with a range of SAM score rankings. Both the positive control gld-1 and six test mRNAs were highly enriched in the WT-IP compared with either gld-2-IP or total WT RNA, but negative controls gpd-1 and rps-25 were not (Fig. S3 A and B). We conclude that the 544 transcripts are likely GLD-2 target mRNAs in wild-type adults.
Identification of RNP-8–Associated Transcripts.
To identify RNP-8–associated mRNAs, we performed essentially the same procedure described above for GLD-2 except that we used an anti–RNP-8 antibody and prepared worm lysates from wild-type and rnp-8(q784) adults (Fig. S1A, Right, and C). rnp-8(q784) harbors a small C-terminal deletion, removing the epitope recognized by anti–RNP-8 antibody; rnp-8(q784) mutants exhibit no apparent defect (11). SAM analyses of the WT-IP vs. rnp-8(q784)-IP and WT-IP vs. total WT RNA comparisons deemed 1,457 and 1,251 unique transcripts, respectively, as significantly enriched at an FDR of 5.5% or less. The overlap identified 878 RNP-8–associated transcripts (Fig. 1B; complete list in Dataset S2).
To validate the enrichment of RNP-8 IP mRNAs, we performed qRT-PCR. All seven test mRNAs were enriched in the WT-IP compared with either rnp-8-IP or total WT RNA, but negative controls were not enriched (Fig. S3 C and D). We conclude that these 878 transcripts are likely RNP-8 target mRNAs in wild-type adults.
Overlap of GLD-2– and RNP-8–Associated Transcripts.
To identify mRNAs associated with both GLD-2 and RNP-8, we compared the 544 GLD-2–associated and 878 RNP-8–associated transcripts and found 335 transcripts in common (Fig. 1B; complete list in Dataset S3). We calculated a representation factor (RF) of 14 (P < E−333) for this overlap (RF is a measure of the observed number of overlapping mRNAs compared with the expected number based on random chance). Although the overlap between GLD-2– and RNP-8–associated mRNAs was significant, it was not complete. One possible reason is that GLD-2 controls polyadenylation in other complexes (e.g., GLD-2/GLD-3). Another possible reason is that differences between the two IP experiments (e.g., antibodies and controls) may impact the range of RNAs identified for the two individual proteins. Regardless, the significant overlap between GLD-2– and RNP-8–associated mRNAs strongly supports the idea that GLD-2 and RNP-8 regulate many of the same transcripts. For further analyses, we focused on these 335 putative targets, which, for simplicity, we refer to as GLD-2/RNP-8 target mRNAs.
GLD-2/RNP-8 Increases Poly(A) Tail Length and Abundance of Its Target mRNAs.
We next asked whether GLD-2 and RNP-8 affect the polyadenylation and abundance of GLD-2/RNP-8 target mRNAs. To this end, we compared lengths of poly(A) tails on endogenous mRNAs extracted from wild-type, rnp-8(tm2435), and gld-2(RNAi) adult hermaphrodites. The rnp-8(tm2435) mutant is likely to be a null mutant (11); most of these rnp-8(0) mutants are fertile with germlines similar to wild type (see below). gld-2(RNAi) animals are sterile, as described above.
For all four GLD-2/RNP-8 target mRNAs analyzed, the poly(A) tail lengths were significantly shorter in rnp-8(0) and gld-2(RNAi) backgrounds than in wild type (Fig. 1C, Left, for oma-2 and Fig. S4A for egg-1, pup-2, and tra-2), suggesting that GLD-2/RNP-8 polyadenylates its target mRNAs. We note that the poly(A) tail lengths were less affected in rnp-8 mutants than in gld-2(RNAi) animals. One plausible explanation is that GLD-2/RNP-8 target mRNAs are also polyadenylated by other factors (e.g., GLD-2/GLD-3; see below). As a control, we analyzed ubl-1 (ubiquitin-like) mRNA, which was not enriched in either GLD-2 or RNP-8 IP, and found no dramatic change in its poly(A) length (Fig. 1C, Right). We conclude that the poly(A) tails of GLD-2/RNP-8 target mRNAs are shorter upon depletion of GLD-2 or RNP-8 and that mRNAs identified by RIP-chip analysis are likely substrates for GLD-2/RNP-8 PAP.
RNAs with shortened poly(A) tails are often unstable (7). To ask whether GLD-2 or RNP-8 affects the abundance of GLD-2/RNP-8 target mRNAs, we first used qRT-PCR to compare the abundance of individual mRNAs in wild-type, rnp-8(0), and gld-2(0) adult hermaphrodites. The oma-2, egg-1, pup-2, and tra-2 mRNAs were less abundant in both rnp-8 and gld-2 mutants compared with wild type, whereas abundance of the ubl-1 control mRNA was unchanged (Fig. 1D for oma-2 and ubl-1 and Fig. S4B for egg-1, pup-2, and tra-2). To examine more mRNAs, microarrays were used to measure mRNA abundance in wild-type vs. gld-2(RNAi) animals, and data were plotted as a frequency histogram of log2-fold change. The fold change was negative for most GLD-2/RNP-8 targets after GLD-2 depletion but was more evenly distributed for total mRNAs under the same conditions (Fig. 1E). Together, these data suggest that the GLD-2/RNP-8 PAP elongates poly(A) tails of its associated mRNAs and increases their abundance.
Functional Annotation and Expression Profiling of GLD-2/RNP-8 Targets.
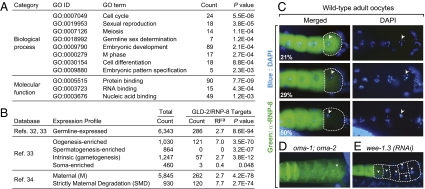
To investigate functional themes among the 335 predicted GLD-2/RNP-8 targets, we used DAVID tools (http://david.abcc.ncifcrf.gov) (26, 27) to look for enriched categories of biological processes and molecular functions, as defined in the Gene Ontology (GO) database (Fig. 2A; complete list in Dataset S4). For biological processes, the most enriched GO terms were related to cell cycle, germline development, and embryogenesis. These functions correspond well to the biological roles of GLD-2 and RNP-8 (10, 11, 18) (Discussion). For molecular functions, the most enriched GO terms were protein and nucleic acid binding, suggesting a role for GLD-2/RNP-8 targets in gene regulation or formation of macromolecular complexes.
Fig. 2.
Functional annotation and expression profiling of GLD-2/RNP-8 targets. (A) Overrepresented GO terms among GLD-2/RNP-8 targets. (B) Expression of GLD-2/RNP-8 target mRNAs was explored using published databases. Ref, reference number in bibliography. aRF is the number of overlapping genes divided by the expected number of overlapping genes drawn from the group of GLD-2/RNP-8 target mRNAs and the group corresponding to a given gene set calculated with web-based software (http://elegans.uky.edu/MA/progs/overlap_stats.html). RF above 1.0 indicates more overlap than expected between two independent groups, whereas below 1.0 indicates less overlap than expected. (C–E) Oocytes stained with anti–RNP-8 antibody (green) and DAPI (blue). Arrowheads mark oocyte nuclei. (C) RNP-8 disappears from the last oocyte undergoing maturation (dashed line) in wild-type germlines. Percentages of germlines with each staining pattern (Bottom Left) (n = 100); images arranged in likely temporal order, but each is a different germline. (D) RNP-8 fails to disappear in the oma-1; oma-2 double mutant. (E) RNP-8 protein disappears precociously in wee-1.3(RNAi) germlines.
The 335 GLD-2/RNP-8 target mRNAs were perused for genes of interest. The list includes the previously known GLD-2 target mRNA, gld-1 (20), and a key female-promoting mRNA, tra-2 (28), which fits the role of GLD-2/RNP-8 in oocyte fate specification. However, the target responsible for the role of GLD-2/RNP-8 in germline sex determination could not be deduced unambiguously because two male-promoting mRNAs were also on the list. One, fem-3, is a maternal mRNA that is critical for sex determination in the embryo (29, 30), and the other, fog-1, is likely an inactive splice variant that predominates in oocytes (31). In addition, the list contained gld-2 and rnp-8 mRNAs, suggesting positive autoregulation.
We scanned published databases (32–34) to examine the expression of GLD-2/RNP-8 target mRNAs (Fig. 2B and Dataset S3 for complete list). Most (85%; 286/335) were germline mRNAs, as might be expected. Moreover, oogenesis-enriched transcripts were more highly represented than those expressed during both oogenesis and spermatogenesis, and no spermatogenesis-enriched transcript occurred on the list. This strong oocyte bias suggests that GLD-2/RNP-8 controls a battery of mRNAs involved in oogenesis.
We next used the database of Baugh et al. (34) to ask whether the GLD-2/RNP-8 targets were maternal mRNAs (expressed in both oocytes and early embryos). Indeed, many (78%; 262/335) were maternal transcripts, and one subclass, the strictly maternal degradation (SMD) transcript, was highly enriched (46%; 120/262). SMD mRNAs are rapidly degraded in early embryos and remain low in abundance during later embryogenesis (34); they are thought to be critical for the oocyte-to-embryo transition. We therefore considered the possibility that GLD-2/RNP-8 may play some special role in the control of these SMD mRNAs.
The GLD-2 pattern of expression is not suggestive of any particular role in the oocyte-to-embryo transition: GLD-2 is present in all oocytes and early embryos (10). By contrast, RNP-8 protein was abundant in most oocytes but disappeared from the last oocyte, which had been triggered to mature just before fertilization (Fig. 2C). This RNP-8 disappearance correlated with effects on oocyte maturation: RNP-8 failed to disappear in oma-1; oma-2 germlines, which are defective in oocyte maturation (35), but RNP-8 disappeared precociously in wee-1.3 germlines, which mature precociously (36) (Fig. 2 D and E). The RNP-8 loss from oocytes that have been triggered to mature is intriguing in light of the many SMD mRNAs among GLD-2/RNP-8 targets. However, attempts to drive expression of transgenic RNP-8 in the final oocyte were not successful. We conclude that most GLD-2/RNP-8 targets are maternal mRNAs and that many are degraded in early embryos.
We attempted to identify the RNP-8–binding element with three distinct methods (in silico analysis of target 3′UTRs using MEME and cisFINDER motif search programs followed by in vitro RNA-binding assays, a yeast three-hybrid screen using random 12-mers as bait, and SELEX against random 30-mers). None of the methods identified an RNP-8–binding element. Possible explanations are that RNP-8 binds RNA with a loose consensus, that it binds an RNA structure not represented in our screens, or that its binding requires cofactors.
rnp-8 Promotes Oocyte Development.
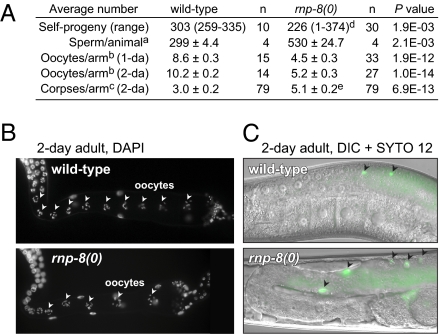
Many GLD-2/RNP-8 target mRNAs are expressed during oogenesis, and GLD-2 is required for normal oogenesis (18). By contrast, most rnp-8 null mutants are self-fertile and appear normal (11). To ask whether RNP-8 might affect oogenesis, we counted the self-progeny of wild-type and fertile rnp-8(0) hermaphrodites as a gross measure of sperm number and oocyte success. The wild-type brood size was ~300, whereas rnp-8 brood size was smaller (Fig. 3A). We next examined gamete morphology and number. The rnp-8 sperm appeared normal and produced self- or cross-progeny. However, sperm number was higher than normal in rnp-8 hermaphrodites (Fig. 3A), and mature oocytes formed ~4 h later than normal. Therefore, the hermaphrodite switch from spermatogenesis to oogenesis is compromised in rnp-8 mutants. One explanation of the low rnp-8 brood size might have been a defect in sperm function, but rnp-8 null mutant hermaphrodites mated with wild-type males still had a small brood size (average = 224, n = 7), suggesting an oocyte defect. Normally, oogenic germ cells either differentiate or undergo physiological cell death as they move proximally in the ovary (37). We found two oogenesis defects in adult rnp-8 germlines: there were fewer oocytes in rnp-8 mutants than in wild type and more germ cell corpses (Fig. 3 A–C). Therefore, RNP-8 affects several aspects of oogenesis: oocyte fate specification (11), the hermaphrodite sperm/oocyte switch, formation of the normal number of oocytes, and germ cell deaths.
Fig. 3.
rnp-8 promotes oocyte development. (A) The numbers of self-progeny, sperm, oocytes, and germ cell corpses were counted in wild-type and rnp-8(tm2435) null mutants. 1-da and 2-da indicate 1 d and 2 d past L4, respectively. Mean ± SEM; P value by two-tailed, unpaired t test. aSperm were counted in only one arm, and the number was doubled to estimate the total number of sperm per animal. bThe number of diakinesis nuclei from DAPI-stained adult germlines. cThe number of germ cell corpses in adult germlines, using the vital dye SYTO 12. dNine percent of rnp-8(0) animals were sterile due to masculinization of the germline and were excluded to count broods. eMasculinized germlines were excluded to count germ cell corpses. (B and C) Adult hermaphrodite germlines (2 d past L4). Wild-type (Upper) and rnp-8(0) mutants (Lower). (B) DAPI-stained germlines. Arrowheads mark oocyte nuclei in diakinesis throughout the proximal arm of the gonad. (C) Merged differential interference contrast (DIC) micrographs of SYTO 12-stained germlines. Arrowheads mark apoptotic cells.
rnp-8 and gld-3 Work Synergistically to Promote Oocyte Development.
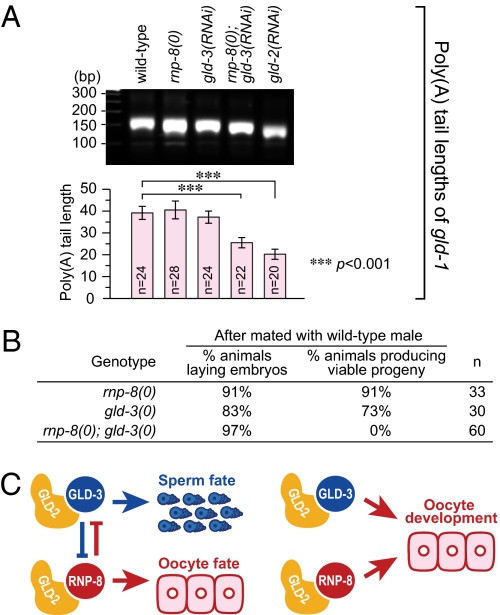
RNP-8 and GLD-3 antagonize each other in their control of germline sex (11, 19), but both also promote oocyte development (19). To explore their relationship, we assayed the effect of RNP-8 and GLD-3 on polyadenylation of gld-1 mRNA, the one target they have in common, and also examined rnp-8; gld-3 double null mutants. We assayed gld-1 polyadenylation in adults because both RNP-8 and GLD-3 are present then; removal of either RNP-8 or GLD-3 had little effect on gld-1 polyadenylation, but removal of both resulted in a significant reduction in the length of the poly(A) tail (Fig. 4A). In addition, we saw synergistic effects on oogenesis. Appearance of the first oocyte and the RME-2 oocyte marker was later in the rnp-8; gld-3 double mutant than in either the rnp-8 or gld-3 single mutant. Finally, each single mutant produced viable progeny when wild-type sperm was provided (11, 19), but the double mutant produced only dead embryos (Fig. 4B). We conclude that rnp-8 and gld-3 work synergistically to promote oocyte development.
Fig. 4.
RNP-8 and GLD-3 work synergistically to promote oocyte development. (A) Poly(A) tail lengths of gld-1 mRNA in wild-type, rnp-8(0), gld-3(RNAi), rnp-8(0); gld-3(RNAi), and gld-2(RNAi) adults, measured by gel analysis of PCR products (Upper) and by sequencing cloned PCR isolates (mean ± SEM) (Lower). (B) Removal of RNP-8 enhances gld-3 oogenesis defects. (C) Model for combinatorial control of germ cell fate specification and development by GLD-2/GLD-3 and GLD-2/RNP-8.
Discussion
GLD-2/RNP-8 Is a Broad-Spectrum Regulator of Oogenesis mRNAs.
The catalytic subunit of cytoplasmic PAP, GLD-2, influences multiple aspects of C. elegans germline development and early embryogenesis. Here, we focus on the GLD-2/RNP-8 enzyme and identify a set of 335 putative target mRNAs, which include the only previously known GLD-2 target in C. elegans, gld-1 mRNA (20). Poly(A) tail lengths of four putative target mRNAs were shortened after depletion of either GLD-2 or RNP-8, whereas a control mRNA was not dramatically affected. Moreover, the abundance of putative targets was lowered after depletion of either GLD-2 or RNP-8. Therefore, these 335 mRNAs are likely targets of the GLD-2/RNP-8 complex.
The GLD-2/RNP-8 enzyme was already known for its role in oocyte fate specification (11), and now we find that GLD-2/RNP-8 also influences oocyte development. Consistent with a role in oogenesis, maternal mRNAs stand out as the major GLD-2/RNP-8 targets (78%). GLD-2 and its homologs were already known to regulate individual maternal mRNAs in frogs (cyclin B, gld-2), flies (cortex, bicoid, Toll, torso), and worms (gld-1) (14–17, 20, 21). This study takes this theme to a genomic level and provides evidence that the GLD-2/RNP-8 enzyme regulates a battery of maternal mRNAs.
How does GLD-2/RNP-8 affect those mRNAs? As mentioned above, GLD-2/RNP-8 is likely to polyadenylate and stabilize its target mRNAs. That activity is consistent with effects of cytoplasmic polyadenylation previously seen in frogs, flies, and worms (10, 11, 14–17, 20, 21). We do not yet know if GLD-2/RNP-8 target mRNAs are activated to produce protein or are maintained in a silenced state for later translational activation. Nonetheless, we suggest that GLD-2 polyadenylates and stabilizes a battery of maternal mRNAs during oogenesis.
GLD-2/RNP-8 Influences Normal Oogenesis.
In addition to its previously known role in oocyte fate specification (11), we now find that RNP-8 also affects oogenesis. Specifically, RNP-8 is required for formation of the normal number of oocytes and for control of the number of germ cell corpses. The mechanism by which GLD-2/RNP-8 exerts its effect on oogenesis remains an issue for future work. However, we suggest two ideas based on the current study. One is that GLD-2/RNP-8 may affect germ cell death via its control of the ced-5 and ced-6 mRNAs, which are both on the target list; both ced-5 and ced-6 promote engulfment of apoptotic cells (38), and an engulfment defect could explain the increased number of cell corpses in rnp-8 mutants. Another possibility is that GLD-2/RNP-8 has its effects via control of a different target, the cpb-3 mRNA, which encodes a C. elegans homolog of CPEB (39). The cpb-3 and rnp-8 null phenotypes are similar—both mutants have reduced brood sizes, more germ cell corpses, and fewer oocytes (40).
GLD-2/RNP-8 is one of several cytoplasmic PAPs that promote oogenesis. Another GLD-2 partner, GLD-3, also promotes oogenesis (19), and we now find that the two GLD-2 partners, RNP-8 and GLD-3, act synergistically. In addition, the catalytic subunit of a different cytoplasmic PAP, GLD-4, also promotes oogenesis (24). A simple model is that oocyte formation demands the combined action of multiple PAPs all working together to ensure a rapid and massive accumulation of maternal mRNAs in developing oocytes.
The common role of RNP-8 and GLD-3 in oocyte formation is intriguing in light of the antagonism between RNP-8 and GLD-3 during germline sex determination (Fig. 4C) (11). One likely explanation of their antagonistic functions relies on differences in complex formation during development. In larvae, as sperm are being specified, GLD-2 forms a complex with GLD-3 but not with RNP-8; however, in adults, as oocytes are being made, both GLD-2/GLD-3 and GLD-2/RNP-8 complexes are formed (11). Moreover, GLD-2, GLD-3, and RNP-8 are all present in developing oocytes (10, 11, 19). An understanding of the specific roles of these complexes must await the identification of GLD-3 targets in both GLD-2 and GLD-4 PAP complexes. The only clue to date is that GLD-2, RNP-8, GLD-3, and GLD-4 all converge on activation of gld-1 mRNA (20, 24).
Regulation of Cytoplasmic Polyadenylation and the Oogenesis Program.
Analysis of the GLD-2/RNP-8 enzyme and its battery of target mRNAs has provided a revealing glimpse into how cytoplasmic polyadenylation affects the oogenesis program—from oocyte specification through oocyte formation. Two additional findings suggest possible mechanisms for the regulation of GLD-2/RNP-8 activity. First, the gld-2 and rnp-8 mRNAs themselves were found among GLD-2/RNP-8 targets. Therefore, both gld-2 and rnp-8 are likely subject to positive autoregulation. In addition, gld-3 mRNA was found among GLD-2/RNP-8 targets, which might further amplify the signal for GLD-2-mediated polyadenylation. We suggest that positive auto- and cross-regulation of GLD-2 enzymes helps to meet the demand for rapid accumulation of maternal mRNAs and to ensure the normally rapid rate of oocyte formation. Previously, Xenopus GLD-2 was also found to activate its own mRNA (21). Therefore, GLD-2 autoregulation may be a conserved feature of oogenesis.
The RNP-8 protein disappears during oocyte maturation and that disappearance likely eliminates GLD-2/RNP-8 activity. The molecular mechanism of RNP-8 loss is not known, but its correlation with oocyte maturation implicates regulators of maturation (e.g., OMA-1 and OMA-2 zinc finger proteins) (35) as potential regulators of RNP-8 protein stability. GLD-2/RNP-8 targets include several maternal mRNAs critical for the oocyte-to-embryo transition (e.g., oma-2, egg-4/5, mbk-2) (41). Furthermore, GLD-2/RNP-8 targets are enriched in the SMD subclass of maternal mRNAs, which are rapidly degraded in early embryos (34). An appealing hypothesis is that GLD-2/RNP-8 polyadenylation of SMD mRNAs stabilizes them during oogenesis and therefore contributes to their activation but that RNP-8 loss during oocyte maturation breaks the positive feedback loop driving the oocyte-to-embryo transition. The predicted consequence of RNP-8 loss is a decrease in the poly(A) tail length, which may contribute to degradation of SMD maternal mRNAs in early embryos. A similar phenomenon may occur in both Drosophila and Xenopus. Drosophila GLD-2 (Wispy) is required for destabilization of maternal mRNAs during egg activation (42), and the Xenopus GLD-2 binding partner CPEB must be degraded during oocyte maturation for proper entry into meiosis II (43). Regardless of specific mechanisms operating in each organism, we emphasize that GLD-2/RNP-8 appears to share a number of common features with other GLD-2 enzymes (e.g., regulation of oogenesis, positive autoregulation, a role in the oocyte-to-embryo transition). Therefore, our analysis of GLD-2/RNP-8 may be representative of cytoplasmic PAPs more broadly.
Materials and Methods
Immunoprecipitations and Microarrays.
Immunoprecipitations were performed as described (11). Microarrays were performed by the University of Wisconsin Gene Expression Center as described (44). Each RNA sample was linearly amplified, labeled, and used to probe C. elegans Affymetrix GeneChips; array data were extracted, normalized, and analyzed by standard procedures (SI Materials and Methods).
PCR-Based Poly(A) Tail Length Assay.
This technique was performed as described (45) with minor modifications (SI Materials and Methods).
qRT-PCR.
qRT-PCR was carried out by standard procedures (SI Materials and Methods).
SYTO 12 Staining.
This technique was performed as described (46).
Supplementary Material
Acknowledgments
We thank M. Wickens, S. Crittenden, J. Friend, and A. Kershner for critical reading of the manuscript; other J.K. laboratory members for helpful comments and insights; A. Helsley-Marchbanks and L. Vanderploeg for manuscript and figure preparation; and N. Davis for experimental assistance. This work was supported by National Institutes of Health Grant GM069454. J.K. is an investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE23843).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012611107/-/DCSupplemental.
References
- 1.Thompson B, Wickens M, Kimble J. In: Translational control in development. Translational Control in Biology and Medicine, Cold Spring Harbor Monograph Series. Mathews MB, Sonenberg N, Hershey JWB, editors. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 507–544. [Google Scholar]
- 2.Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–229. doi: 10.1016/j.bbagrm.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol. 2008;9:337–344. doi: 10.1038/nrm2370. [DOI] [PubMed] [Google Scholar]
- 4.Wickens M, Goodwin EB, Kimble J, Strickland S, Hentze MW. Translational control of developmental decisions. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 295–370. [Google Scholar]
- 5.Edmonds M. A history of poly A sequences: From formation to factors to function. Prog Nucleic Acid Res Mol Biol. 2002;71:285–389. doi: 10.1016/s0079-6603(02)71046-5. [DOI] [PubMed] [Google Scholar]
- 6.Richter JD. Influence of polyadenylation-induced translation on metazoan development and neuronal synaptic function. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 785–805. [Google Scholar]
- 7.Jacobson A, Peltz SW. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 8.Sossin WS, Lacaille JC. Mechanisms of translational regulation in synaptic plasticity. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.03.011. 10.1016/j.conb.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter JD, Klann E. Making synaptic plasticity and memory last: Mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Eckmann CR, Kadyk LC, Wickens M, Kimble J. A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature. 2002;419:312–316. doi: 10.1038/nature01039. [DOI] [PubMed] [Google Scholar]
- 11.Kim KW, et al. Antagonism between GLD-2 binding partners controls gamete sex. Dev Cell. 2009;16:723–733. doi: 10.1016/j.devcel.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Read RL, Martinho RG, Wang S-W, Carr AM, Norbury CJ. Cytoplasmic poly(A) polymerases mediate cellular responses to S phase arrest. Proc Natl Acad Sci USA. 2002;99:12079–12084. doi: 10.1073/pnas.192467799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saitoh S, et al. Cid13 is a cytoplasmic poly(A) polymerase that regulates ribonucleotide reductase mRNA. Cell. 2002;109:563–573. doi: 10.1016/s0092-8674(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 14.Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–651. doi: 10.1016/j.cell.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Rouhana L, et al. Vertebrate GLD2 poly(A) polymerases in the germline and the brain. RNA. 2005;11:1117–1130. doi: 10.1261/rna.2630205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui J, Sackton KL, Horner VL, Kumar KE, Wolfner MF. Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation. Genetics. 2008;178:2017–2029. doi: 10.1534/genetics.107.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benoit P, Papin C, Kwak JE, Wickens M, Simonelig M. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development. 2008;135:1969–1979. doi: 10.1242/dev.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadyk LC, Kimble J. Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development. 1998;125:1803–1813. doi: 10.1242/dev.125.10.1803. [DOI] [PubMed] [Google Scholar]
- 19.Eckmann CR, Kraemer B, Wickens M, Kimble J. GLD-3, a Bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev Cell. 2002;3:697–710. doi: 10.1016/s1534-5807(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 20.Suh N, Jedamzik B, Eckmann CR, Wickens M, Kimble J. The GLD-2 poly(A) polymerase activates gld-1 mRNA in the Caenorhabditis elegans germ line. Proc Natl Acad Sci USA. 2006;103:15108–15112. doi: 10.1073/pnas.0607050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouhana L, Wickens M. Autoregulation of GLD-2 cytoplasmic poly(A) polymerase. RNA. 2007;13:188–199. doi: 10.1261/rna.333507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckmann CR, Crittenden SL, Suh N, Kimble J. GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Genetics. 2004;168:147–160. doi: 10.1534/genetics.104.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen D, Hubbard EJA, Schedl T. Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev Biol. 2004;268:342–357. doi: 10.1016/j.ydbio.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Schmid M, Küchler B, Eckmann CR. Two conserved regulatory cytoplasmic poly(A) polymerases, GLD-4 and GLD-2, regulate meiotic progression in C. elegans. Genes Dev. 2009;23:824–836. doi: 10.1101/gad.494009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennis G, Jr., et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- 27.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 28.Hodgkin JA, Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977;86:275–287. [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgkin J. Sex determination in the nematode C. elegans: Analysis of tra-3 suppressors and characterization of fem genes. Genetics. 1986;114:15–52. doi: 10.1093/genetics/114.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenquist TA, Kimble J. Molecular cloning and transcript analysis of fem-3, a sex-determination gene in Caenorhabditis elegans. Genes Dev. 1988;2:606–616. doi: 10.1101/gad.2.5.606. [DOI] [PubMed] [Google Scholar]
- 31.Jin S-W, Kimble J, Ellis RE. Regulation of cell fate in Caenorhabditis elegans by a novel cytoplasmic polyadenylation element binding protein. Dev Biol. 2001;229:537–553. doi: 10.1006/dbio.2000.9993. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, et al. Identification of genes expressed in the hermaphrodite germ line of C. elegans using SAGE. BMC Genomics. 2009;10:213. doi: 10.1186/1471-2164-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 34.Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. doi: 10.1242/dev.00302. [DOI] [PubMed] [Google Scholar]
- 35.Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two zinc finger proteins, OMA-1 and OMA-2, are redundantly required for oocyte maturation in C. elegans. Dev Cell. 2001;1:187–199. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 36.Burrows AE, et al. The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation. Development. 2006;133:697–709. doi: 10.1242/dev.02241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gartner A, Boag PR, Blackwell TK. Germline survival and apoptosis. WormBook. 2008 doi: 10.1895/wormbook.1.145.1. ed. The C. elegans Research Community. Available at: http:/www.wormbook.org, 10.1895/wormbook.1.145.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conradt B, Xue D. Programmed cell death. WormBook. 2005 doi: 10.1895/wormbook.1.32.1. ed. The C. elegans Research Community. Available at: http:/www.wormbook.org, 10.1895/wormbook.1.32.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luitjens C, Gallegos M, Kraemer B, Kimble J, Wickens M. CPEB proteins control two key steps in spermatogenesis in C. elegans. Genes Dev. 2000;14:2596–2609. doi: 10.1101/gad.831700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa E, Karashima T, Sumiyoshi E, Yamamoto M. C. elegans CPB-3 interacts with DAZ-1 and functions in multiple steps of germline development. Dev Biol. 2006;295:689–699. doi: 10.1016/j.ydbio.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Marcello MR, Singson A. Fertilization and the oocyte-to-embryo transition in C. elegans. BMB Rep. 2010;43:389–399. doi: 10.5483/bmbrep.2010.43.6.389. [DOI] [PubMed] [Google Scholar]
- 42.Tadros W, et al. Regulation of maternal transcript destabilization during egg activation in Drosophila. Genetics. 2003;164:989–1001. doi: 10.1093/genetics/164.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez R, Barnard D, Richter JD. Differential mRNA translation and meiotic progression require Cdc2-mediated CPEB destruction. EMBO J. 2002;21:1833–1844. doi: 10.1093/emboj/21.7.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kershner AM, Kimble J. Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc Natl Acad Sci USA. 2010;107:3936–3941. doi: 10.1073/pnas.1000495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlesworth A, Cox LL, MacNicol AM. Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J Biol Chem. 2004;279:17650–17659. doi: 10.1074/jbc.M313837200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.