Abstract
Flagellar motility drives propulsion of several important pathogens and is essential for human development and physiology. Motility of the eukaryotic flagellum requires coordinate regulation of thousands of dynein motors arrayed along the axoneme, but the proteins underlying dynein regulation are largely unknown. The dynein regulatory complex, DRC, is recognized as a focal point of axonemal dynein regulation, but only a single DRC subunit, trypanin/PF2, is currently known. The component of motile flagella 70 protein, CMF70, is broadly and uniquely conserved among organisms with motile flagella, suggesting a role in axonemal motility. Here we demonstrate that CMF70 is part of the DRC from Trypanosoma brucei. CMF70 is located along the flagellum, co-sediments with trypanin in sucrose gradients and co-immunoprecipitates with trypanin. RNAi knockdown of CMF70 causes motility defects in a wild-type background and suppresses flagellar paralysis in cells with central pair defects, thus meeting the functional definition of a DRC subunit. Trypanin and CMF70 are mutually conserved in at least five of six extant eukaryotic clades, indicating that the DRC was probably present in the last common eukaryotic ancestor. We have identified only the second known subunit of this ubiquitous dynein regulatory system, highlighting the utility of combined genomic and functional analyses for identifying novel subunits of axonemal sub-complexes.
Keywords: CMF70, Dynein regulatory complex, Motility, Flagellum
Introduction
Motility of flagella and cilia is required for human development and physiology, and numerous pathogenic conditions arise when motility is defective (Fliegauf et al., 2007). Flagellum and cilium are interchangeable terms for the same organelle, and refer to the eukaryotic flagellum, which is distinct from the bacterial flagellum. Flagellar motility also drives propulsion of protozoan pathogens that cause morbidity and mortality in several hundred million people worldwide (Ginger et al., 2008; Ralston et al., 2009). Therefore, an understanding of the mechanisms of flagellum motility is directly relevant to efforts to understand and treat a broad spectrum of inherited and infectious diseases in humans. Eukaryotic flagella are built on a scaffold of axonemal microtubules that are most commonly arranged in a 9+2 configuration in motile axonemes, with nine outer doublet microtubules arrayed symmetrically around a central pair of singlet microtubules. Dynein motors on the outer doublets provide the driving force for axonemal motility, and nexin links that connect adjacent doublets limit microtubule sliding and promote bending of the axoneme (Satir, 1995; Summers and Gibbons, 1971). The arrangement of dynein motors varies from cell to cell, but integral to all motile flagella is the need to coordinate motor activity along the length and around the circumference of the axoneme, to allow efficient bend initiation and propagation (Porter and Sale, 2000; Smith and Sale, 1992). Dynein regulation is considered to involve mechanical and chemical components, but the molecular mechanisms are poorly understood (Lindemann and Kanous, 1997; Lindemann and Lesich, 2010; Porter and Sale, 2000).
The dynein regulatory complex (DRC) operates in concert with the radial spokes and central pair apparatus to control axonemal dynein activity (Huang et al., 1982; Porter and Sale, 2000). The DRC was identified in Chlamydomonas reinhardtii through suppressor mutant screens to isolate extragenic suppressors of flagellar paralysis in central pair and radial spoke mutants (Huang et al., 1982). These studies led to the identification of five DRC genetic loci PF2, PF3, SUP-PF-3, SUP-PF-4 and SUP-PF-5 that are required for assembly of a large protein complex localized near the base of the second radial spoke within the axonemal repeating unit (Gardner et al., 1994; Huang et al., 1982; Mastronarde et al., 1992; Piperno et al., 1994; Piperno et al., 1992). Mutation of any one DRC gene causes loss or reduction of a subset of seven DRC polypeptides as visualized by 2D-PAGE (Huang et al., 1982; Piperno et al., 1994). Until recently, the identities of DRC genes and polypeptides were unknown. A key advance came when Rupp and Porter (Rupp and Porter, 2003) identified the PF2 gene product as a homologue of trypanin, a flagellum protein from Trypanosoma brucei previously shown to be required for propulsive motility (Hill et al., 2000; Hutchings et al., 2002). Loss of either trypanin in T. brucei or PF2 in C. reinhardtii causes defective flagellum beating in a wild-type background and suppresses flagellar paralysis in central-pair mutants (Brokaw and Kamiya, 1987; Huang et al., 1982; Hutchings et al., 2002; Ralston et al., 2006; Rupp and Porter, 2003). Interestingly, the DRC was found to be essential in the bloodstream life cycle stage of T. brucei, fueling the idea that flagellar motility might provide a novel source of drug targets in these pathogens (Ralston and Hill, 2006). A functional requirement for the DRC in vertebrates was demonstrated in Danio rerio, where morpholino knockdown of the trypanin homologue Gas8 disrupted cilium motility, leading to left–right axis defects and abnormal inner-ear development (Colantonio et al., 2009). These studies demonstrated broad conservation of DRC function across diverse taxa and revealed a novel mechanism by which cilium-driven fluid flow contributes to vertebrate development.
Recent structural studies indicate that the DRC and nexin link, which were previously considered to be separate structures, constitute a single large complex that spans the entire distance between two adjacent microtubules (Heuser et al., 2009; Mastronarde et al., 1992; Woolley, 1997; Bui et al., 2008; Bui et al., 2009; Nicastro et al., 2006). This nexin–DRC, or ‘NDRC’ (Heuser et al., 2009), complex is estimated to be ~1.5 MDa in size and is a fundamental feature of most, if not all, motile flagella, yet the only subunit identified to date is trypanin/PF2. We previously reported the identification of a set of 50 T. brucei genes that represent conserved components of motile flagella (CMF) (Baron et al., 2007b). The CMF dataset is derived of genes that exhibit the same distinctive phylogenetic distribution as trypanin, i.e. they are broadly conserved in organisms with motile flagella, but absent in organisms that lack motile flagella. Here we show through biochemical and functional analysis, that the T. brucei CMF70 protein is an NDRC subunit. Our studies double the number of known NDRC subunits and emphasize the utility of combining comparative genomic approaches with functional studies to identify components of flagellum subcomplexes.
Results
CMF70 is a DRC candidate
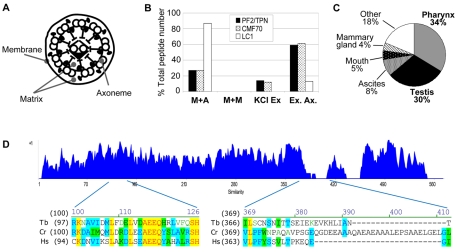
The CMF dataset is comprised of proteins with the same phylogenetic footprint as trypanin and is therefore expected to contain additional DRC subunits (Baron et al., 2007b). Pazour and colleagues (Pazour et al., 2005) conducted proteomic analyses of flagellum fractions prepared by detergent and salt extraction of intact flagella, allowing separation of proteins from the flagellum membrane, axoneme and matrix (Fig. 1A). In these analyses, protein subunits from a given flagellum subcomplex generally exhibited similar fractionation profiles, such that the relative distribution of peptides identified for each subunit was similar to others from the same complex. We therefore reasoned that DRC subunits would have fractionation profiles similar to that of trypanin. The C. reinhardtii CMF70 homologue peptide distribution paralleled that of the C. reinhardtii trypanin homologue PF2 (Fig. 1B). The human CMF70 homologue was previously identified as a sperm antigen, NYD-SP28, located along the sperm flagellum (Zheng et al., 2006). Using the Unigene database (Wheeler et al., 2003), we found that the human homologue is highly expressed in cilia-rich tissues, with 30% and 34% of total NYD-SP28 mRNAs estimated to come from testis and pharynx, respectively (Fig. 1C). The protist and human protein sequences show extensive sequence similarity throughout the proteins, with the exception of three short insertions near residues 390 and 432, and at the C-terminus of the algal protein (Fig. 1D). The phylogenetic footprint of CMF70, its trypanin-like fractionation pattern in Chlamydomonas and the expression profile of the human gene led us to consider CMF70 for further analysis as a candidate DRC subunit.
Fig. 1.
CMF70 is a conserved component of motile flagella. (A) Cross-section cartoon of a flagellum showing compartments separated by biochemical fractionation. (B) Relative number of peptides identified by Pazour and colleagues (Pazour et al., 2005) in mass spectrometry analyses of C. reinhardtii flagellar fractions corresponding to tergitol-insoluble membrane plus axoneme (M+A), Nonidet-soluble membrane plus matrix (M+M), Nonidet-insoluble axonemes extracted with 0.6 M KCl to yield solubilized extract (KCl Ex) and insoluble extracted axonemes (Ex. Ax.). Data from Pazour and colleagues (Pazour et al., 2005) are tabulated for PF2/trypanin, CMF70 and LC1. The distribution of peptides is similar for PF2/trypanin and CMF70, but different for the outer dynein subunit LC1. (C) Expression profile of the human CMF70 homologue shows enrichment in ciliated tissues (http://www.ncbi.nlm.nih.gov/UniGene/). (D) Amino acid sequence similarity plot (Vector NTI, Invitrogen) of CMF70 homologues from T. brucei (Tb), C. reinhardtii (Cr) and H. sapiens (Hs). A value of +1 is corresponds to a stretch of identical amino acids. Representative regions of high similarity (residues 97–124) and low similarity (residues 366–408) are shown below the chart. Strictly conserved positions are highlighted in yellow, whereas residues identical in two of three sequences are highlighted in blue and conservative substitutions are green.
CMF70 is stably associated with the flagellum in T. brucei
CMF70 was detected in one proteomic analysis of detergent and salt-extracted axonemes from T. brucei (Hart et al., 2009), although not in another (Broadhead et al., 2006). To further investigate CMF70, we used in situ tagging (Oberholzer et al., 2006) to replace one CMF70 allele with an epitope-tagged copy. This results in a C-terminally HA-tagged protein that is expressed from the endogenous locus. Upon fractionation of trypanosome flagella, CMF70 fractionated quantitatively with trypanin following extraction with nonionic detergent NP-40 and 0.5 M NaCl (Fig. 2A,B), which solubilizes most outer dyneins and a large portion of the central-pair complex, but leaves the DRC stably associated with the axoneme in an insoluble ‘flagellar skeleton’ that includes the paraflagellar rod and basal body (Hill et al., 2000; Oberholzer et al., 2009; Piperno et al., 1994). Immunofluorescence showed that CMF70 was distributed along the length of the flagellum (Fig. 2C), extending from the distal tip to the proximal end of the axoneme, just anterior to the kinetoplast. This tight and uniform association with the axoneme is expected for a DRC subunit.
Fig. 2.
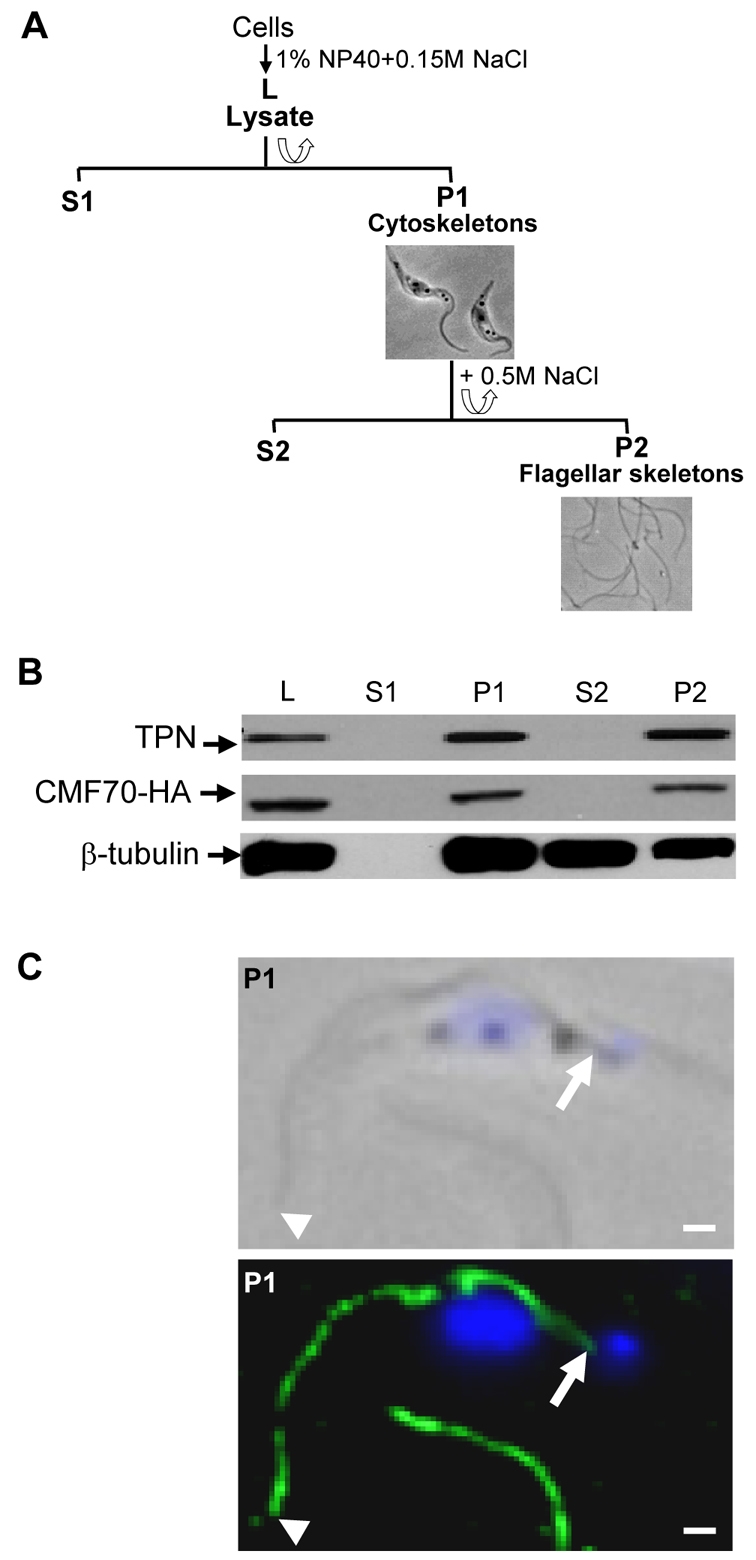
CMF70 is tightly associated with the T. brucei flagellar skeleton. (A) Flow chart depicting fractionation procedure to isolate T. brucei salt-extracted flagellar skeletons. Lysates (L) were prepared by extraction with 1% NP-40 and centrifuged for separation of detergent-soluble proteins (S1) from cytoskeletons (P1). The P1 fraction was further extracted with 0.5 M NaCl treatment to solubilize the subpellicular cytoskeleton (S2) and leave the flagellar skeletons (P2) intact. Corresponding phase-contrast images of cell (P1) and flagellar (P2) skeletons are shown. (B) Western blot analysis of whole-cell lysates and subcellular fractions prepared from HA-tagged CMF70 T. brucei cells shows a stable association of CMF70 and trypanin with the flagellar skeleton fraction (P2). The protein preparations were blotted with anti-HA (top panel), anti-trypanin (middle panel) and anti-β-tubulin (bottom panel) monoclonal antibodies. (C) Indirect immunofluorescence analysis. Cytoskeletons (P1) from CMF70-HA T. brucei cells were visualized after labeling with monoclonal anti-HA antibody (green) and DAPI (blue). Phase-contrast (top panel) and merged fluorescence (lower panel) images are shown. Arrowheads indicate the distal tip and arrows show the proximal end of the flagellum, near the kinetoplast. The flagellum of a second cell is visible at the bottom of the image. Scale bars: 1 μm.
CMF70 and trypanin are part of the same complex
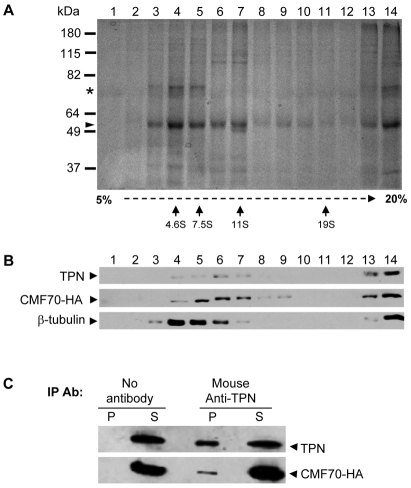
As shown above, trypanin and CMF70 are both tightly associated with the flagellar skeleton, which includes several flagellum subcomplexes (Robinson et al., 1991). To determine whether CMF70 and trypanin are in a complex, flagellar skeletons were extracted with 0.5 M KI, which has been used to solubilize intact axonemal subcomplexes in other organisms (Patel-King et al., 2004) and the soluble fraction was subjected to sucrose density gradient centrifugation and immunoprecipitation (Fig. 3). The majority of tubulin migrated near the top of the gradient, peaking in the 4.6S range. Another major doublet of approximately 70 kDa also peaked here and probably corresponds to PFR1 and PFR2 proteins of the T. brucei paraflagellar rod (PFR) (Fig. 3A), which is a paracrystalline filament that runs parallel to the axoneme and is required for normal flagellar motility (Bastin et al., 1998; Gallo and Schrevel, 1985; Santrich et al., 1997). Trypanin and CMF70 co-migrated in sucrose gradients, peaking between the 7.5S and 11S standards (Fig. 3B), which is further than anticipated for the monomeric globular proteins. These results are consistent with trypanin and CMF70 each being part of a complex, but do not assess interaction because co-sedimentation might be coincidental. To test for interaction between these two proteins, we performed co-immunoprecipitation (Fig. 3C). Immunoprecipitation was inefficient, probably as a result of sub-optimal antibody–antigen interaction in the sample caused by extraction conditions and/or limited epitope accessibility; however, anti-trypanin antibody immunoprecipitated both trypanin and CMF70. Taken together, these results indicate that CMF70 and trypanin interact directly or indirectly as part of a larger complex.
Fig. 3.
TbCMF70 is in a complex with trypanin. (A,B) Sucrose gradient fractionation of solubilized flagellar skeletons. Flagellar skeletons from CMF70-HA cells were extracted with 0.5 M KI, dialyzed against immunoprecipitation buffer and centrifuged to remove insoluble material. The soluble fraction was subjected to 5–20% sucrose gradient centrifugation and fractions were analyzed by SDS–PAGE and Coomassie Blue staining for total protein (A) or immunoblotting using anti-trypanin (TPN), anti-HA or anti-β-tubulin antibodies as indicated (B). In A, arrows indicate the position of migration in sucrose gradients for calibration standards. Tubulin (arrowhead) and the PFR1 and PFR2 (asterisk) proteins are indicated. (C) Solubilized flagellar skeletons from CMF70-HA T. brucei cells were immunoprecipitated with or without the mouse monoclonal anti-TPN antibody and analyzed by immunoblotting of the supernatants (S) and precipitated beads (P) using rabbit polyclonal anti-trypanin (TPN) or mouse monoclonal anti-HA antibodies (CMF70-HA).
Loss of trypanin weakens the association of CMF70 with the axoneme
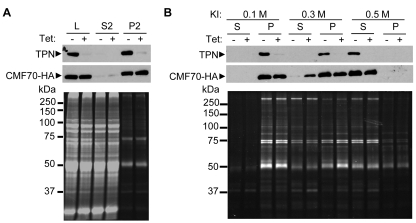
Biochemical data suggested that trypanin and CMF70 might be part of a larger complex and this was explored further by testing for genetic interactions in trypanosomes in which trypanin and CMF70 were knocked down. In Chlamydomonas, disruption of individual DRC subunits compromises the complex, and results in reduced levels of a subset of other DRC subunits in the flagellum (Huang et al., 1982; Piperno et al., 1994). In the pf2 mutant, which lacks trypanin/PF2, five of seven DRC polypeptides are lost or reduced in the flagellum (Huang et al., 1982; Piperno et al., 1994). We therefore hypothesized that if CMF70 is a DRC subunit, loss of trypanin might alter CMF70 abundance or interaction with the axoneme. To test this, we introduced a construct for Tet-inducible trypanin RNAi into the cell line expressing HA-tagged CMF70 and then examined CMF70 in the presence and absence of trypanin. Trypanin knockdown had no significant effect on CMF70-HA abundance in whole-cell extracts or flagellar skeletons (Fig. 4A). To investigate the stability of axoneme association, flagellar skeletons from trypanin-knockdown and control cells were extracted with increasing concentrations of KI (Fig. 4B). In control cells, neither trypanin nor CMF70 were significantly solubilized by 0.1–0.3 M KI, whereas 0.5 M KI yielded complete solubilization. In trypanin-knockdown cells, however, 0.3 M KI solubilized a significant fraction of CMF70. Therefore, loss of trypanin compromises the association of CMF70 with the axoneme, as predicted for a DRC subunit. In the reciprocal experiment, CMF70 knockdown similarly weakened the trypanin axoneme interaction, but did not impact trypanin total abundance (data not shown).
Fig. 4.
Loss of trypanin weakens the association of CMF70 with the axoneme. (A) Protein fractions, as defined in Fig. 2, were prepared from CMF70-HA cells harboring a Tet-inducible trypanin RNAi knockdown construct grown with (+) or without (−) 1 μg/ml tetracycline for 72 hours. Samples were analyzed by SDS-PAGE and immunoblotting with antibodies against trypanin (TPN, top panel) or HA (CMF70-HA, middle panel), or stained for total protein with Sypro-ruby (lower panel). (B) P2 flagellar skeletons from uninduced (−) and Tet-induced (+) trypanin RNAi CMF70-HA cells were extracted with the indicated concentration of KI and separated by centrifugation into supernatants (S) and pellets (P). Samples were analyzed as in A. Treatment with 0.3 M KI partially solubilized CMF70-HA from the axoneme when trypanin was ablated by RNAi but not in control cells. Results are representative of three independent experiments.
CMF70 is required for normal motility
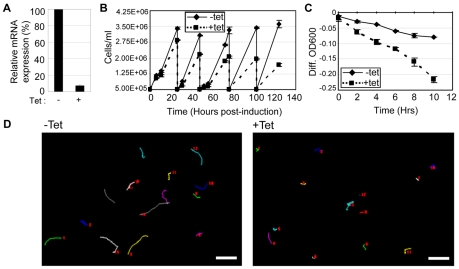
The phylogenetic distribution, expression profile and biochemical properties of CMF70 support a role for this protein in the DRC. To directly assess function, we used Tet-inducible RNAi. Although we did not observe a motility defect in a previously described CMF70-knockdown cell line (Baron et al., 2007b), subsequent analysis using qRT-PCR showed that this line did not exhibit significant knockdown of cmf70 mRNA (not shown). We therefore generated a new Tet-inducible RNAi knockdown line. Within 48 hours of induction, cmf70 transcript levels were reduced to 7±1% of uninduced controls (Fig. 5A). In CMF70-knockdown cultures, cells accumulated in small clusters of 5–10 cells, which were visible by the third day after induction (data not shown), similar to cell division defects observed in other motility mutants (Baron et al., 2007b; Branche et al., 2006; Ralston et al., 2006). This phenotype might explain the slowed growth of CMF70-knockdown cells that was apparent at 2 days after induction and became more pronounced over time (Fig. 5B). Within 24 hours of induction, and before the appearance of clusters in the culture, CMF70-knockdown cells exhibited significant motility defects, as demonstrated by sedimentation (Fig. 5C). By 48 hours after induction, cell propulsion was severely compromised, as demonstrated by motility trace analysis (Fig. 5D). High-resolution video microscopy of single cells revealed that the flagellar beat of CMF70 knockdowns (supplementary material Movie 2) is erratic and less vigorous than in control cells (supplementary material Movie 1). The wave did not efficiently transmit to the cell body and cell movements were planar in most cases, in contrast to the rapid, three-dimensional, bihelical beating and cellular rotation exhibited by wild-type T. brucei (Rodriguez et al., 2009) (supplementary material Movie 1). Transmission electron microscopy (TEM) analysis did not reveal obvious differences in axoneme ultrastructure between CMF70-knockdown cells and controls (supplementary material Fig. S1), although this is not unexpected, because TEM analysis of trypanin knockdowns likewise did not reveal any differences from controls (Hutchings et al., 2002) and minor differences might not be revealed.
Fig. 5.
CMF70 is required for normal flagellar motility in T. brucei. (A) qRT-PCR analysis shows a dramatic decrease in cmf70 mRNA in CMF70-knockdown cells (+tet) compared with control cells (−tet). (B) Growth curves of uninduced (−Tet) and induced (+Tet) CMF70-knockdown cells show slowed growth within 24 hours of RNAi induction. Each curve represents an average of two independent cell counts and the error bars indicate s.d. (C) Sedimentation assays (Bastin et al., 1999; Ralston et al., 2006) on uninduced (−Tet) versus induced (+Tet) CMF70 RNAi cells show that CMF70-knockdown cells sediment, indicative of a motility defect. The curves represent an average of two independent measurements and the error bars indicate s.d. (D) Motility traces of uninduced (−tet) and induced (+tet) CMF70 RNAi cells. Lines trace the movement of individual cells over a 35 second time interval. Numbers in each panel represent individual cells. Scale bars: 50 μm.
CMF70 knockdown suppresses flagellar paralysis of PF16 knockdown
The functional definition of a DRC gene is that loss of gene function suppresses flagellar beat defects of central-pair mutants (Huang et al., 1982). We previously showed that trypanin meets this criterion in T. brucei, because trypanin knockdown suppresses beat defects that occur upon PF16 knockdown (Ralston et al., 2006). We generated dual knockdown of CMF70 and PF16 to determine whether loss of CMF70 suppressed the beat defect of PF16 single knockdowns. By 24 hours after induction, PF16 single and PF16–CMF70 double knockdowns showed a decrease in PF16 expression to about 40% of that seen in uninduced controls (Fig. 6A). In the double knockdown, CMF70 expression was reduced to approximately 38% of the control value (Fig. 6A). As expected (Ralston et al., 2006), PF16 knockdown caused nearly complete paralysis, with the vast majority of cells having only rudimentary beating at the distal tip of the flagellum and most cells showing a characteristic curvature of the anterior cell body (supplementary material Movie 3). Therefore, a 60% reduction in PF16 expression is sufficient to induce severe and penetrant flagellum beat defects. In the PF16–CMF70 double knockdown, the cell curvature and the near-paralysis of flagella observed in the PF16 single knockdown was replaced by more sustained flagellum beating (Fig. 6B and supplementary material Movie 4). Occasionally, double-knockdown cells with improved flagellar beat showed a small net forward movement, although this was different from movement observed in wild type cells (data not shown). These data demonstrate that loss of CMF70 suppresses flagellar paralysis of central-pair knockdown, thus providing functional evidence that CMF70 is a DRC component.
Fig. 6.
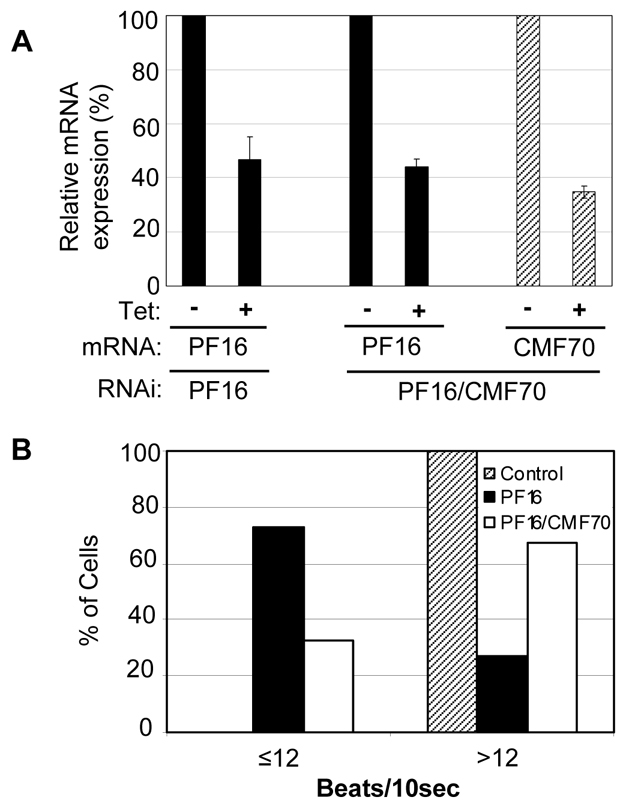
Loss of CMF70 suppresses flagellar paralysis of central pair knockdowns. (A) qRT-PCR analysis of pf16 and cmf70 transcripts in PF16 single-knockdown or PF16–CMF70 double-knockdown cells. Expression of RNAi-targeted transcripts in tetracycline-induced (+ Tet) cultures is shown relative to uninduced controls (−Tet). Averages of two independent experiments are shown with error bars representing s.d. (B) Analysis of flagellar beating in PF16 single-knockdown (n=55), PF16–CMF70 double-knockdown (n=50) and control cells (n=57). Beats at the tip of the flagellum were quantified over a 10 second interval, as described (Ralston et al., 2006).
Discussion
CMF70 and the deep branching origins of the dynein regulatory complex
Using phylogenetic, biochemical and functional analyses, we have identified a subunit of the DRC from T. brucei. Recent structural analyses indicate that the DRC and nexin link, previously considered to be separate entities, constitute a single, massive structure that connects adjacent outer-doublet microtubules (Heuser et al., 2009; Mastronarde et al., 1992; Woolley, 1997; Bui et al., 2008; Bui et al., 2009; Nicastro et al., 2006). This NDRC (Heuser et al., 2009) complex is a fundamental feature of the axoneme and participates in the basic mechanism of axonemal motility and regulation of axonemal dyneins. Nexin links were observed nearly 50 years ago (Gibbons, 1963; Warner, 1976) and the DRC was described nearly 30 years ago (Huang et al., 1982), yet before the current study, only a single NDRC subunit had been identified. Our identification of a second NDRC subunit through a combination of phylogenetic and functional genomics approaches therefore represents a major step toward the identification of the remaining NDRC subunits.
CMF70 was first identified as a candidate DRC component based on genome comparisons to identify genes with a phylogenetic distribution that is similar to that of trypanin (Baron et al., 2007b). This analysis used the sequence of a single protein, trypanin, to probe genomes from a limited number of organisms. The availability of two NDRC protein sequences, trypanin and CMF70, together with additional completed genome sequences now allows us to probe more deeply into the origins of the NDRC. There is disagreement over the root of the eukaryotic tree, but consensus for six major clades, Opisthokonts, Ameobozoa, Plantae, Chromalveolates, JEH (Jakobids, Euglenozoa, Heterolobosea) and POD (Parabasalids, Oxymonads and Diplomonads) (Fritz-Laylin et al., 2010). Trypanin and CMF70 homologues are both conserved in the five major eukaryotic clades that have a flagellated representative with a completed genome sequence (Fig. 7). These groups encompass much of the known diversity in eukaryotic organisms (Fritz-Laylin et al., 2010). We did not identify homologous sequences in the genome database of Physarum, a flagellated member of the Amoebozoans, but this is difficult to interpret, particularly because the genome database is incomplete. The mutual conservation of trypanin and CMF70 in at least five out of the six major extant eukaryotic groups indicates that the NDRC or an NDRC-like complex was present in the last common eukaryotic ancestor. This finding is consistent with the idea that the ancestral eukaryote had a motile cilium (Mitchell, 2004). Functional analysis of trypanin and CMF70, and identification of additional NDRC subunits, will therefore provide insight into the fundamental mechanisms of axonemal motility.
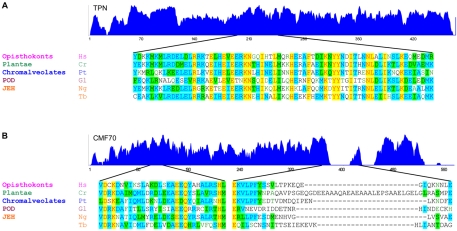
Fig. 7.
Deep-branching origins of the dynein regulatory complex. Similarity plots (top) with representative sequence alignments (bottom) are shown for trypanin (A) and CMF70 (B) from organisms representing the indicated eukaryotic clades (Fritz-Laylin et al., 2010). JEH encompasses Jakobids, Euglenozoa, Heterolobosea, whereas POD encompasses Parabasalids, Oxymonads and Diplomonads (Fritz-Laylin et al., 2010). Non-trypanosome sequences are from Homo sapiens (Hs), C. reinhardtii (Cr), Paramecium tetraurelia (Pt), Giardia lamblia (Gl) and Naegleria gruberi (Ng). The T. brucei sequence (Tb) is shown for reference. Trypanin proteins show high similarity along their lengths, including several strictly conserved residues. Sequence alignment for the C-terminal portion of the GMAD microtubule-binding domain in the human trypanin homologue (residues 189–260) (Bekker et al., 2007) is shown for reference. CMF70 proteins show high similarity along their lengths. Sequence alignment is shown from conserved and non-conserved regions for reference. Dissimilarity at the N and C-termini of CMF70 derives from unique insertions in the human, Paramecium and trypanosome sequences, suggestive of species-specific functions. Strictly conserved positions are highlighted in yellow, whereas residues identical in three or more sequences are highlighted in blue and conservative substitutions are green.
CMF70 and DRC polypeptides
The DRC has been most extensively studied in C. reinhardtii and although a one-to-one correlation of DRC composition between C. reinhardtii and T. brucei cannot be assumed, it is nonetheless informative to consider our findings in the context of data on the C. reinhardtii complex. The C. reinhardtii DRC is estimated to contain at least seven subunits, DRC1–DRC7, based on 2D-PAGE, which identified proteins reduced or missing in axonemes of drc mutants (Huang et al., 1982; Piperno et al., 1994). Except for DRC4, which corresponds to trypanin/PF2 (Rupp and Porter, 2003), the identities of DRC1–DRC7 are not known. DRC1 and DRC2 are retained in pf-2 axonemes, whereas the other DRC polypeptides are lost (Huang et al., 1982; Piperno et al., 1994). The predicted molecular mass of the C. reinhardtii CMF70 homologue FAP250 is 65 kDa (Merchant et al., 2007; Pazour et al., 2005), which is intermediate between the reported size of DRC3 (62 kDa) and DRC2 (70 kDa) (Huang et al., 1982). Five drc genetic loci have been identified and mutation at a given locus causes loss or reduction of a subset of the DRC1–DRC7 polypeptides (Huang et al., 1982; Piperno et al., 1992). Of these mutants, sup-pf-4 and pf-3 retain trypanin/PF2 in the axoneme, as is the case for the CMF70 knockdown in T. brucei. On this basis, it is possible that C. reinhardtii CMF70/FAP250 corresponds to the gene product of SUP-PF-4 or PF-3, although other scenarios are certainly possible and assignment of CMF70 to a given DRC protein or DRC locus is premature at this point. The C. reinhardtii CMF70 ortholog is located on genome scaffold 44, which is encompassed by linkage group XIV (Merchant et al., 2007). The only mapped mutation overlapping with scaffold 44 is the ida5 locus (Merchant et al., 2007), which encodes actin (Ohara et al., 1998).
It is quite possible that CMF70 represents a protein that was not identified in the earlier Chlamydomonas studies, because additional proteins are probably part of the complex. For example, Heuser and colleagues (Heuser et al., 2009) estimate the size of the NDRC to be in the order of 1.5 MDa, which is much larger than the combined molecular masses of DRC1–DRC7. Cross-correlation of DRC structures lost or remaining in electron tomograms of drc mutant axonemes, versus axonemal polypeptides lost or remaining in these mutants, allowed tentative assignments for DRC1–DRC6 within the native complex (Heuser et al., 2009). Several large domains of the complex were not accounted for by DRC1–DRC6, indicating that several additional polypeptides remain to be uncovered. Therefore, seven is a minimal estimate for DRC subunit number and CMF70 might correspond to one of the subunits not encompassed by DRC1–DRC7.
CMF70 and trypanin function
Knockdown of trypanin or CMF70 causes motility defects, but the phenotypes differ. Trypanin knockdowns have a vigorously beating flagellum, but are unable to generate a propulsive beat, so the cell tumbles and spins in place (Hutchings et al., 2002). CMF70-knockdown cells exhibit flagellum beating that is not so vigorous and is sometimes restricted to the tip. Moreover, the CMF70 knockdown does not exhibit the tumbling motion that characterizes the trypanin knockdown. Thus, the two proteins have distinct functional and/or structural roles within the complex. Motility in T. brucei is driven by compound, bi-helical waves in the flagellum (Rodriguez et al., 2009) and further analyses to compare waveforms of trypanin and CMF70 knockdowns will be informative for delineating the specific beat properties attributed to each subunit.
Both trypanin and CMF70 are predicted to contain several coiled-coil domains, which suggests that there is an interaction with other proteins and is consistent with an NDRC that is extensively interconnected with neighboring axonemal structures (Heuser et al., 2009; Nicastro et al., 2006; Piperno et al., 1992). High connectivity to other axonemal structures might explain the ability of each protein to remain associated with the axoneme in the absence of the other, albeit with reduced stability. The human trypanin homologue Gas8 contains a microtubule-binding domain that is conserved in trypanin (Fig. 7) (Bekker et al., 2007; Hill et al., 2000), so it is anticipated that trypanin interacts with axonemal microtubules, although the electron tomogram structure places DRC4, the trypanin/PF2 gene product, away from the axoneme in the preparations used for cryoelectron tomography (Heuser et al., 2009). Specific CMF70 interactions remain to be determined. Perhaps of interest, the Giardia CMF70 homologue is thought to contain a BAR (Bin–Amphiphysin–Rvs) domain, which functions as a curvature-sensing module and transmits information on membrane morphology to the actin cytoskeleton (Itoh et al., 2005; Peter et al., 2004). There is no evidence to indicate an interaction of the NDRC with membranes, but sensing curvature or flexing of the axoneme is an integral feature of mechanical models for axonemal motility and is a role attributed to nexin links (Lindemann and Kanous, 1997).
Despite the crucial importance of coordination of dynein activity in flagellar motility, very little is known about the mechanisms and proteins that regulate axonemal dynein. Our work identifies CMF70 as a subunit of the NDRC, which functions as a focal point of regulatory interactions underlying axonemal motility. Continued analysis of NDRC structure and function is expected to illuminate novel routes for therapeutic intervention in ciliary and infectious diseases, as well as advance our understanding of fundamental mechanisms of axonemal motility.
Materials and Methods
Trypanosome cell culture and transfection
Procyclic-form 29-13 trypanosomes (Wirtz et al., 1999) were used for all experiments and were maintained and transfected as described previously (Hutchings et al., 2002). Clonal lines were obtained by limiting dilution. Tetracycline induction for RNAi knockdown was done using 1 μg/ml tetracycline.
Database searches and sequence alignments
T. brucei CMF70 (NCBI accession number: XP_828802) and trypanin were as reported previously (Baron et al., 2007b; Hutchings et al., 2002). Trypanin and CMF70 homologues were identified in NCBI databases as reciprocal best BLAST hits using the T. brucei sequences as the original query. Accession numbers for the resulting trypanin proteins are NP_001472 (human), Q7XJ96 (C. reinhardtii), XP_01445638 (Paramecium tetraurelia), XM_001704250.1 (Giardia lamblia) and XP_002683213.1 (Naegleria gruberi). Accession numbers for the resulting CMF70 proteins are NP_149115.2 (human), XP001449564.1 (P. tetraurelia), EES99144.1 (G. lamblia) and XP_002683154.1 (N. gruberi). The NCBI entry for the C. reinhardtii CMF70 ortholog is incomplete, but the complete sequence is available from the C. reinhardtii genome database (www.jgi.doe.gov/chlamy; protein ID: 536221). Protein sequence alignments and similarity plots were performed using the multiple amino acid sequence alignment algorithm of Vector NTI Advance Suite 9 (Invitrogen). Analysis of coiled-coil secondary structures was carried out using the COILS algorithm (Lupas et al., 1991). For estimating the human CMF70 expression profile, the unigene expression profile database (http://www.ncbi.nlm.nih.gov/UniGene/) was used. The database estimates gene expression patterns by calculating EST counts as a percentage of total ESTs in the cDNA library sources using data reported by submitters of the sequence information (Wheeler et al., 2003).
DNA constructs for in situ tagging and RNAi
In situ tagging (Oberholzer et al., 2009; Oberholzer et al., 2006) of CMF70 was carried out using pYS53, which was kindly provided by Dan Ray (UCLA, Los Angeles, CA) and is derived from the pMOTag3H vector (Oberholzer et al., 2006) by replacing the G418 antibiotic resistance gene in pMOTag3H with the puromycin-resistance gene from pN-PURO-PTP (Schimanski et al., 2005). For directing integration into the cmf70 locus, a 368 bp fragment immediately upstream of the CMF70 stop codon was amplified from 29-13 T. brucei genomic DNA with forward (5′-GGGGTACCTCTAGACAGGAAGGCCAAGTGAAG-3′) and reverse (5′-GGGGTACCCCTCGTCATGTCGGAGACGAACTT-3′) primers containing KpnI sites, then ligated into pYS53 immediately upstream of the HA cassette. Subsequently, a 390 bp fragment flanked with BamHI sites was amplified from the 3′UTR immediately downstream of the CMF70 stop codon using forward (5′-CGGGATCCGCAATTCCTGTTGGGGTATGAG-3′) and reverse (5′-CGGGATCCGAATTCCCTTCCGCACAGATAG-3′) primers carrying BamHI sites. The resulting product was cloned into the BamHI site downstream of the puromycin-resistance cassette. The entire tagging cassette (CMF70ORF-HA-igr-PuroR-CMF703′UTR) was excised from the resulting plasmid and stably transfected into 29-13 cells.
RNAi constructs were generated in p2T7-Ti-B (LaCount et al., 2002) to drive transcription of double-stranded RNA using opposing, tetracycline-inducible promoters. The p2T7-Ti-B/trypanin vector (Ralston et al., 2006) was introduced into the cell line harboring HA-tagged CMF70. For CMF70 knockdown, a 444 bp fragment corresponding to nucleotides 201–644 of the cmf70 ORF, identified using the RNAit algorithm (Redmond et al., 2003), was amplified from 29-13 genomic DNA using forward primer, 5′-ATGGATTTCTTTCCTGCGTG-3′ and reverse primer, 5′-TCCATCTGTTTTCGGTCCTC-3′. The resulting product was then cloned into the HindIII site of p2T7-Ti-B. For dual knockdown of PF16 and CMF70, the 444 bp cmf70 ORF fragment was cloned into the HindIII site of p2T7-Ti-B/PF16 (Ralston et al., 2006) to generate p2T7-Ti-B/PF16/CMF70. The p2T7-Ti-B/PF16 plasmid was used as described (Ralston et al., 2006) for PF16 knockdown. All constructs were sequenced to verify accuracy.
Quantitative, real-time RT-PCR analysis
For quantitative real-time PCR (qRT-PCR) analysis, cells were induced for 24 hours. Total RNA was extracted with an RNeasy kit (Qiagen) and treated with amplification grade DNase (Invitrogen). cDNA was synthesized from 2 μg total RNA using SuperScript II Reverse Transcriptase kit with oligo(dT) primers (Invitrogen) according to the manufacturer's instructions. qRT-PCR was conducted on the DNA Engine Opticon 2 (MJ Research, Bio-Rad) according to the manufacturer's instructions. Gene-specific primer sets were calibrated against a standard curve of T. brucei genomic DNA and PCR specificity was verified by melt curve analysis of the PCR products. For CMF70, the forward and reverse primer sequences were respectively 5′-AATGCGAAGGGATGGGAACA-3′ and 5′-CCTCCTTGCTCCTCCAACCA-3′. qRT-PCR was performed in duplicate on two independent RNA preparations and values were normalized against two housekeeping genes, GAPDH (Tb927.6.4280/Tb927.6.4300) and ribosomal protein RPS6 (Tb10.70.7020/Tb10.707030), as internal loading controls. The forward and reverse primers for housekeeping genes were respectively 5′-GGCTGATGTCTCTGTGGTGGA-3′ and 5′-GGCTGTCGCTGATGAAGTCG-3′ for GAPDH, and 5′-AGATTGGCGTTGGAGCGAAA-3′ and 5′-GACCGAAACCAGAGACCAGCA-3′ for RPS6. Relative gene expression was determined using the 2ΔΔCT method. Percentage expression of induced targeted genes was evaluated relative to non-induced targeted genes.
Cell growth and motility analyses
Cell densities were monitored by using a Z1 Coulter Counter (Beckman) and plotted using the averages of two counts. Sedimentation assays were performed as previously described (Bastin et al., 1999; Ralston et al., 2006). Motility trace analysis was carried out as described (Baron et al., 2007b). Quantitative analysis of flagellum beating was carried out as described (Ralston et al., 2006).
Trypanosome cell fractionation and immunoblotting
Trypanosome fractionation was adapted from published methods (Oberholzer et al., 2009; Robinson et al., 1991). In summary, procyclic mid-log phase cells were pelleted (2000 g, 5 minutes), washed twice with PBS, resuspended in PEME (100 mM PIPES, 2 mM EGTA, 1 mM MgSO4, 0.1 mM EDTA) supplemented with 1% Nonidet-P40 (NP-40), protease inhibitors Aprotinin (5 μg/ml) and Leupeptin (5 μg/ml), plus 0.5 mg/ml DNAse I, and incubated at room temperature for 10 minutes. The resulting whole-cell lysate (L) was centrifuged (2000 g, 10 minutes) to separate detergent-solubilized proteins (S1) and pelleted cytoskeletons (P1), which were washed in PEME containing 1% NP-40 and protease inhibitors. The cytoskeletons (P1) were then washed in PMN (10 mM NaPO4 pH 7.4, 1 mM MgCl2, 150 mM NaCl) plus 1% NP-40 and protease inhibitors, resuspended in the same buffer containing 500 mM NaCl and incubated on ice for 30 minutes to depolymerize sub-pellicular microtubules. The sample was then centrifuged at 16,000 g for 10 minutes, the supernatant (S2) was removed and the flagellar skeleton pellet (P2) was washed in PMN/NP40 extraction buffer. Where indicated, flagellar skeletons (P2) were further extracted with 0.1, 0.3 or 0.5 M KI for 5 minutes at room temperature and centrifuged at 3000 g for 10 minutes to separate solubilized material (S3) and insoluble (P3) axonemal components.
Protein samples were analyzed by immunoblotting as previously described (Hill et al., 1999; Ralston et al., 2006). Anti-HA monoclonal antibody HA.11 (Covance, Emeryville, CA) was used at 1:2500. Anti-trypanin monoclonal antibody (Ralston et al., 2006) was used at 1:5000. β-tubulin was used as a loading control and was detected by the monoclonal antibody E7 (Chu and Klymkowsky, 1989) obtained from the Developmental Studies Hybridoma Bank maintained by University of Iowa Department of Biological Sciences.
Immunoprecipitation and sucrose gradients
KI-solubilized flagellar substructures (S3) from 5×107 cells were dialyzed into immunoprecipitation (IP) buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% NP-40, 1 mM DTT). The dialysate was incubated with or without 1.44 μg anti-trypanin mouse monoclonal antibody for 18 hours at 4°C on a rotator. Samples were incubated with 100 μl Protein-G-Sepharose 4 Fast Flow beads (GE Healthcare) for 3 hours at 4°C on a rotator. Beads were collected by centrifugation at 1000 g in a Beckman Coulter Microfuge 18 centrifuge. Supernatant was removed and beads were washed three times with IP buffer. Supernatant and bead pellet samples were analyzed by immunoblotting.
For sucrose gradient centrifugation, 0.5 M KI-solubilized substructures (S3) from CMF70-HA axonemes were dialyzed against PMN buffer (10 mM NaPO4, pH 7.4, 1 mM MgCl2, 500 mM NaCl) to remove KI, then layered on top of a 13 ml linear 5–20% density gradient of sucrose in PMN buffer containing protease inhibitors Aprotinin (5 μg/ml) and Leupeptin (5 μg/ml). The sample was centrifuged at 245,000 g for 16 hours at 4°C in a Beckman Optima L-90K ultracentrifuge using a SW 41 rotor and 14 fractions of ~1 ml each were collected from the top of the gradient and analyzed by SDS–PAGE and immunoblotting. Standards, BSA (4.6S), lactate dehydrogenase (7.5S), catalase (11S) and thyroglobulin (19S) (Amersham) were run in parallel to calibrate the sucrose gradients.
Immunofluorescence and electron microscopy
Immunofluorescence was carried out on cytoskeletons as described (Hutchings et al., 2002; Ralston et al., 2006) by using monoclonal anti-HA antibody HA.11 at 1:400. Anti-mouse secondary antibody conjugated to Alexa Fluor 488 (Molecular Probes, Carlsbad, CA) was used at 1:400. Samples were mounted in Vectashield H-1200 (Vector Laboratories, Burlingame, CA) containing DAPI (4′,6-diamidino-2-phenylindole) for the visualization of nuclear and kinetoplast DNA and imaged on a Zeiss Axioskop II compound microscope with a 63× oil-immersion objective. For transmission electron microscopy, flagellar skeletons were prepared using a single-step fractionation procedure that preserves axoneme ultrastructure (Oberholzer et al., 2009). Cells were washed twice in PBS, resuspended in PMN with 1% Nonidet-P40 (NP-40), protease inhibitors Aprotinin (5 μg/ml) and Leupeptin (5 μg/ml), plus 0.5 mg/ml DNAse I, then incubated for 10 minutes at room temperature followed by 30 minutes on ice. Insoluble flagellar skeletons were pelleted at 16,000 g, washed twice in PMN buffer plus 1% NP40 and subjected to transmission electron microscopy as described previously (Baron et al., 2007a).
Supplementary Material
Acknowledgments
We are grateful to Rachelle Crosbie (UCLA) for providing access to her sucrose gradient sedimentation equipment and to Randy Nessler (University of Iowa) for assistance with electron microscopy. We thank Steven Karpowicz and Sabeeha Merchant (UCLA) for assistance with C. reinhardtii genome information. We thank George Cross (Rockefeller University) for the 29-13 cell line and Dan Ray (UCLA) for the pYS53 plasmid. J.H.M. was supported by a MARC U*STAR traineeship from the NIH/NIGMS T34GM08563-15. M.T. is the recipient of an NIH-NRSA (GM07185) fellowship. Funding for the work was provided by grants from the NIH (R01AI052348), the Beckman Young Investigator program and the Burroughs Wellcome Trust to K.L.H. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/20/3587/DC1
References
- Baron D. M., Kabututu Z. P., Hill K. L. (2007a). Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J. Cell Sci. 120, 1513-1520 [DOI] [PubMed] [Google Scholar]
- Baron D. M., Ralston K. S., Kabututu Z. P., Hill K. L. (2007b). Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J. Cell Sci. 120, 478-491 [DOI] [PubMed] [Google Scholar]
- Bastin P., Sherwin T., Gull K. (1998). Paraflagellar rod is vital for trypanosome motility. Nature 391, 548 [DOI] [PubMed] [Google Scholar]
- Bastin P., Pullen T. J., Sherwin T., Gull K. (1999). Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J. Cell Sci. 112, 3769-3777 [DOI] [PubMed] [Google Scholar]
- Bekker J. M., Colantonio J. R., Stephens A. D., Clarke W. T., King S. J., Hill K. L., Crosbie R. H. (2007). Direct interaction of Gas11 with microtubules: implications for the dynein regulatory complex. Cell Motil. Cytoskeleton 64, 461-473 [DOI] [PubMed] [Google Scholar]
- Branche C., Kohl L., Toutirais G., Buisson J., Cosson J., Bastin P. (2006). Conserved and specific functions of axoneme components in trypanosome motility. J. Cell Sci. 119, 3443-3455 [DOI] [PubMed] [Google Scholar]
- Broadhead R., Dawe H. R., Farr H., Griffiths S., Hart S. R., Portman N., Shaw M. K., Ginger M. L., Gaskell S. J., McKean P. G., et al. (2006). Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440, 224-227 [DOI] [PubMed] [Google Scholar]
- Brokaw C. J., Kamiya R. (1987). Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskeleton 8, 68-75 [DOI] [PubMed] [Google Scholar]
- Bui K. H., Sakakibara H., Movassagh T., Oiwa K., Ishikawa T. (2008). Molecular architecture of inner dynein arms in situ in Chlamydomonas reinhardtii flagella. J. Cell Biol. 183, 923-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui K. H., Sakakibara H., Movassagh T., Oiwa K., Ishikawa T. (2009). Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J. Cell Biol. 186, 437-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. T., Klymkowsky M. W. (1989). The appearance of acetylated alpha-tubulin during early development and cellular differentiation in Xenopus. Dev. Biol. 136, 104-117 [DOI] [PubMed] [Google Scholar]
- Colantonio J. R., Vermot J., Wu D., Langenbacher A. D., Fraser S., Chen J. N., Hill K. L. (2009). The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature 457, 205-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M., Benzing T., Omran H. (2007). When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880-893 [DOI] [PubMed] [Google Scholar]
- Fritz-Laylin L. K., Prochnik S. E., Ginger M. L., Dacks J. B., Carpenter M. L., Field M. C., Kuo A., Paredez A., Chapman J., Pham J., et al. (2010). The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 140, 631-642 [DOI] [PubMed] [Google Scholar]
- Gallo J., Schrevel J. (1985). Homologies between paraflagellar rod proteins from trypanosomes and euglenoids revealed by a monoclonal antibody. Eur. J. Cell Biol. 36, 163-168 [PubMed] [Google Scholar]
- Gardner L. C., O'Toole E., Perrone C. A., Giddings T., Porter M. E. (1994). Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J. Cell Biol. 127, 1311-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R. (1963). Studies on the protein components of cilia from tetrahymena pyriformis. Proc. Natl. Acad. Sci. USA 50, 1002-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginger M. L., Portman N., McKean P. G. (2008). Swimming with protists: perception, motility and flagellum assembly. Nat. Rev. Microbiol. 6, 838-850 [DOI] [PubMed] [Google Scholar]
- Hart S. R., Lau K. W., Hao Z., Broadhead R., Portman N. (2009). Analysis of the trypanosome flagellar proteome using a combined electron transfer/collisionally activated dissociation strategy. J. Am. Soc. Mass Spectrom. 20, 167-175 [DOI] [PubMed] [Google Scholar]
- Heuser T., Raytchev M., Krell J., Porter M. E., Nicastro D. (2009). The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 187, 921-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K. L., Hutchings N. R., Russell D. G., Donelson J. E. (1999). A novel protein targeting domain directs proteins to the anterior cytoplasmic face of the flagellar pocket in African trypanosomes. J. Cell Sci. 112, 3091-3101 [DOI] [PubMed] [Google Scholar]
- Hill K. L., Hutchings N. R., Grandgenett P. M., Donelson J. E. (2000). T lymphocyte-triggering factor of african trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J. Biol. Chem. 275, 39369-39378 [DOI] [PubMed] [Google Scholar]
- Huang B., Ramanis Z., Luck D. J. (1982). Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell 28, 115-124 [DOI] [PubMed] [Google Scholar]
- Hutchings N. R., Donelson J. E., Hill K. L. (2002). Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J. Cell Biol. 156, 867-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Erdmann K. S., Roux A., Habermann B., Werner H., De Camilli P. (2005). Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 9, 791-804 [DOI] [PubMed] [Google Scholar]
- LaCount D. J., Barrett B., Donelson J. E. (2002). Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J. Biol. Chem. 277, 17580-17588 [DOI] [PubMed] [Google Scholar]
- Lindemann C. B., Kanous K. S. (1997). A model for flagellar motility. Int. Rev. Cytol. 173, 1-72 [DOI] [PubMed] [Google Scholar]
- Lindemann C. B., Lesich K. A. (2010). Flagellar and ciliary beating: the proven and the possible. J. Cell Sci. 123, 519-528 [DOI] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. (1991). Predicting coiled coils from protein sequences. Science 252, 1162-1164 [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N., O'Toole E. T., McDonald K. L., McIntosh J. R., Porter M. E. (1992). Arrangement of inner dynein arms in wild-type and mutant flagella of Chlamydomonas. J. Cell Biol. 118, 1145-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S. S., Prochnik S. E., Vallon O., Harris E. H., Karpowicz S. J., Witman G. B., Terry A., Salamov A., Fritz-Laylin L. K., Maréchal-Drouard L., et al. (2007). The chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. R. (2004). Speculations on the evolution of 9+2 organelles and the role of central pair microtubules. Biol. Cell 96, 691-696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro D., Schwartz C., Pierson J., Gaudette R., Porter M. E., McIntosh J. R. (2006). The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944-948 [DOI] [PubMed] [Google Scholar]
- Oberholzer M., Morand S., Kunz S., Seebeck T. (2006). A vector series for rapid PCR-mediated C-terminal in situ tagging of Trypanosoma brucei genes. Mol. Biochem. Parasitol. 145, 117-120 [DOI] [PubMed] [Google Scholar]
- Oberholzer M., Lopez M. A., Ralston K. S., Hill K. L. (2009). Approaches for functional analysis of flagellar proteins in african trypanosomes. In Cilia: Model Organisms and Intraflagellar Transport (ed. King S. M., Pazour G. J.), pp. 21-57 Elsevier Academic Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara A., Kato-Minoura T., Kamiya R., Hirono M. (1998). Recovery of flagellar inner-arm dynein and the fertilization tubule in chlamydomonas ida5 mutant by transformation with actin genes. Cell Struct. Funct. 23, 273-281 [DOI] [PubMed] [Google Scholar]
- Patel-King R. S., Gorbatyuk O., Takebe S., King S. M. (2004). Flagellar radial spokes contain a Ca2+-stimulated nucleoside diphosphate kinase. Mol. Biol. Cell 15, 3891-3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Agrin N., Leszyk J., Witman G. B. (2005). Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495-499 [DOI] [PubMed] [Google Scholar]
- Piperno G., Mead K., Shestak W. (1992). The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 118, 1455-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Mead K., LeDizet M., Moscatelli A. (1994). Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J. Cell Biol. 125, 1109-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. E., Sale W. S. (2000). The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151, F37-F42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston K. S., Hill K. L. (2006). Trypanin, a component of the flagellar Dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathog. 2, e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston K. S., Lerner A. G., Diener D. R., Hill K. L. (2006). Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryotic Cell 5, 696-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston K. S., Kabututu Z. P., Melehani J. H., Oberholzer M., Hill K. L. (2009). The Trypanosoma brucei flagellum: moving parasites in new directions. Annu. Rev. Microbiol. 63, 335-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S., Vadivelu J., Field M. C. (2003). RNAit: an automated web-based tool for the selection of RNAi targets in Trypanosoma brucei. Mol. Biochem. Parasitol. 128, 115-118 [DOI] [PubMed] [Google Scholar]
- Robinson D., Beattie P., Sherwin T., Gull K. (1991). Microtubules, tubulin, and microtubule-associated proteins of trypanosomes. Methods Enzymol. 196, 285-299 [DOI] [PubMed] [Google Scholar]
- Rodriguez J. A., Lopez M. A., Thayer M. C., Zhao Y., Oberholzer M., Chang D. D., Kisalu N. K., Penichet M. L., Helguera G., Bruinsma R., et al. (2009). Propulsion of African trypanosomes is driven by bihelical waves with alternating chirality separated by kinks. Proc. Natl. Acad. Sci. USA 106, 19322-19327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp G., Porter M. E. (2003). A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J. Cell Biol. 162, 47-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santrich C., Moore L., Sherwin T., Bastin P., Brokaw C., Gull K., LeBowitz J. H. (1997). A motility function for the paraflagellar rod of Leishmania parasites revealed by PFR-2 gene knockouts. Mol. Biochem. Parasitol. 90, 95-109 [DOI] [PubMed] [Google Scholar]
- Satir P. (1995). Landmarks in cilia research from Leeuwenhoek to us. Cell Motil. Cytoskeleton 32, 90-94 [DOI] [PubMed] [Google Scholar]
- Schimanski B., Nguyen T. N., Gunzl A. (2005). Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryotic Cell 4, 1942-1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. F., Sale W. S. (1992). Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science 257, 1557-1559 [DOI] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. (1971). Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc. Natl. Acad. Sci. USA 68, 3092-3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F. D. (1976). Ciliary inter-microtubule bridges. J. Cell Sci. 20, 101-114 [DOI] [PubMed] [Google Scholar]
- Wheeler D. L., Church D. M., Federhen S., Lash A. E., Madden T. L., Pontius J. U., Schuler G. D., Schriml L. M., Sequeira E., Tatusova T. A., et al. (2003). Database resources of the National Center for Biotechnology. Nucleic Acids Res. 31, 28-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E., Leal S., Ochatt C., Cross G. A. (1999). A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99, 89-101 [DOI] [PubMed] [Google Scholar]
- Woolley D. M. (1997). Studies on the eel sperm flagellum. I. The structure of the inner dynein arm complex. J. Cell Sci. 110, 85-94 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Zhang J., Wang L., Zhou Z., Xu M., Li J., Sha J. H. (2006). Cloning and characterization of a novel sperm tail protein, NYD-SP28. Int. J. Mol. Med. 18, 1119-1125 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.