Abstract
Study Design
Biochemical studies aimed at optimization of protein crosslinking formulations for the treatment of degenerative disc disease and subsequent biomechanical testing of tissues treated with these formulations.
Objectives
To optimize protein crosslinking formulations for treatment of degenerating spinal discs.
Summary of Background Data
Non-surgical exogenous crosslinking therapy is a potential new, non-invasive technology for the treatment of degenerative disc disease. The technology is based upon the injection of protein crosslinking reagents into the pathological disc to restore its mechanical properties and also to potentially increase the permeability of the tissue and so facilitate the exchange of waste products and nutrients.
Methods
Diffusion of genipin was monitored following injection into spinal discs and the effects of surfactants on diffusion studied. Formulations for genipin and methylglyoxal were biochemically optimized and used to treat bovine spinal discs. Their effects on bovine annulus tissue were evaluated using a circumferential tensile test, while the genipin formulation was also tested with respect to its ability to reduce disc bulge under load.
Results
Genipin exhibited a distinct time-dependent diffusion and sodium-dodecyl-sulfate, but not Tween-20, enhanced diffusion by 30%. Two crosslinkers, genipin and methylglyoxal, were inhibited by amines but enhanced by phosphate ions. Both formulations could enhance a number of physical parameters of bovine annulus tissue, while the genipin formulation could reduce disc bulge following injections into spinal discs.
Conclusions
Formulations lacking amines and containing phosphate ions appear to be promising candidates for clinical use of the crosslinkers genipin and methylglyoxal.
Introduction
Degenerative Disc Disease (DDD) is a debilitating chronic condition1 with a US economic cost estimated at $100 billion2. The spinal disc is an avascular tissue and its cells rely on diffusion and diurnal convection for exchange of nutrients and waste products3. During aging, this process becomes gradually impaired4 as the extracellular matrix of the disc clogs and the adjacent vertebral endplates become sclerotic and calcified. Thus the oxygen content of the disc is reduced and the cells within become progressively more reliant on anaerobic glycolysis as a primary energy source. The resulting lactate production acidifies the tissue5 and this, coupled to the reduced nutrient influx, results in a decline in the tissue’s ability to repair the mechanical damage caused by daily physiological loading and unloading. Over time the tissue’s ability to support these loads lessens, leading to fissure formation, stress intensification and loss of disc height. Abnormal bulging of the weakened disc can impinge upon nerve roots, leading to the generation of pain and, in extreme cases, disc herniation.
Numerous treatment modalities are currently employed for the treatment of DDD6. In early stages these include physical therapy and pain management with analgesics and anti-inflammatory agents. While these provide immediate relief, they do not prevent disease progression which is treated with surgical procedures of escalating severity from discectomy, to spinal fusion and artificial disc/nucleus implantation. However, all these therapies are aimed at treating the symptoms of the disease and not to remedying its underlying cause – the degeneration of the disc itself.
Biological approaches aimed at preventing or reversing degeneration have been suggested, including gene, stem-cell and cytokine therapy7-9. All these approaches, however, are faced with the harsh environment of the pathological disc which is itself not conducive for optimal cellular viability.
It has also been suggested that disc repair and stabilization might be achieved, not biologically, but chemically10;11. Such non-surgical exogenous crosslinking therapy (NEXT) offers a promising non-surgical treatment for both retarding the progression and ameliorating the symptoms of DDD. It has been shown that chemical crosslinking of disc tissue leads to an increase in a number of important parameters such as proteoglycan retention, tissue strength, fatigue and tear resistance, joint stability, and a concomitant decrease in disc bulge (and therefore potentially neural compression) under load10-13. Furthermore, crosslinking of collagenous matrices can increase their permeability14;15 and, since this can occur within the intervertebral disc16, crosslinking might reverse the decline in disc cell viability and so facilitate more effective tissue repair.
Numerous chemical crosslinkers have been utilized to modify collagen matrices, such as glutaraldehyde17, proanthrocyanidins18, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide19, threose20, genipin(GP) 21 and methylglyoxal (MG)12. We have recently characterized the kinetics of these chemical crosslinkers with regard to their ability to crosslink bovine annulus fibrosus tissue (Slusarewicz, et al, submitted). We selected GP and MG as reagents for this study based on their previous use in the area of biomechanics12;22 and their relatively low toxicity23;24.
In order for NEXT to be viable, crosslinkers should be capable of diffusing within the tissue following injection and be active within the environment of the degenerating disc. In this paper, we investigate the diffusion rate of genipin in spinal discs and optimize prototype formulations containing either genipin or methylglyoxal.
Materials and Methods
Genipin was obtained from Challenge Bioproducts Co., Ltd. (Taiwan). All other reagents were from Sigma. Bovine 4-6 month old lumbar intervertebral disc tissue was obtained frozen and thawed at room temperature prior to use. While having similar cross-sectional area as human discs, calf discs have less disc height and are notably non-degenerated. The relative uniformity of these discs leads to minimization of variability in properties.
Diffusion Studies
GP as selected as a representative chemical crosslinker for these studies because its crosslinked adducts turn blue in the presence of oxygen25;26, and can be readily visualized. GP (15mM) was dissolved in phosphate-buffered saline (PBS) and 200μl injected into the discs of individual calf lumbar motion segments, with a 22-gauge needle at a depth of 1cm in the left and right lateral regions, and incubated at 37°C for 1, 3 or 6 hours. The discs were then transected and both halves frozen at -20°C overnight to facilitate the color development of the genipin crosslinked products. Samples were thawed and photographed with a reference scale and the photographs imported into ImageJ software (NIH). Following normalization to the scale, the areas were measured manually by drawing around each zone. In most cases both surfaces of the transected disc were measured, though in a few samples where the cut was too close to the endplate this was not possible. The average of three measurements for each face was used to determine the area of the diffusion zone in mm2. Differences between injectates were analyzed using a T-test.
In experiments using surfactants 15mM GP was formulated in either PBS alone or PBS containing 0.1% (w/v) of either SDS or Tween-20. Solutions were injected and analyzed as described above.
Reagent Optimization
GP and MG were selected for further development since they are both relatively small molecules and substantially less toxic than glutaraldehyde23;24;27, a commonly used crosslinking reagent in the field of tissue engineering.
Approximately 3-5g of frozen bovine annulus was cut into 1-2mm2 pieces and homogenized in a 50ml Falcon tube in 4°C distilled water using a homogenizer fitted with a 10mm stainless steel, saw toothed generator probe. The temperature of the suspension did not exceed 25°C. After 1-2 minutes large particles were allowed to settle and the fine suspension was decanted into a fresh tube. The process was repeated to completion and the tissue harvested by centrifugation at 4500rpm for 5 minutes. Tissue pellets were stored at -20°C until needed.
Crosslinking extent was assessed by determining the loss in sensitivity of the tissue to digestion by collagenase. Approximately 20-30mg aliquots of homogenized tissue were weighed accurately and 0.5ml of crosslinker added while one sample was treated with buffer alone. Samples were shaken at 1500rpm and 37°C for 1 hour. Following centrifugation at 10000rpm for 2 minutes, 0.5ml of a 1mg/ml solution of type I collagenase from Clostridium histolyticum in Collagenase Buffer (100mM Tris pH7.5, 10mM CaCl2) was added to the tissue pellets which were incubated as above, but for 20 minutes. Following a second centrifugation, proteolysis was monitored using a colorimetric hydroxyproline assay28-32 to quantify the release of peptides from the tissue and into the final supernatant. A solution of collagenase alone was used as a spectrophotometer blank and the data were normalized to the results obtained from buffer-treated tissue. Statistical significances were determined using the T-test.
Using this assay, we confirmed previous reports33;34 that both these crosslinkers are most active at elevated pH (Slusarewicz, et al, submitted). We therefore decided to conduct our experiments under alkaline conditions (pH 9). However, the degenerating human disc is an acidic environment5;35 and thus is not conducive for the optimal crosslinking activity of these reagents. We therefore buffered our formulations in order to maintain this pH and to counteract the ongoing production of lactate within the degenerating disc.
Tris (2-Amino-2-hydroxymethyl-propane-1,3-diol) buffer has been previously used clinically for the treatment of blood acidosis36 and therefore represents a potentially useful buffer for this purpose. However, due to concerns that the amine group in Tris might react with both GP and MG and lower the efficiency of crosslinking, we decided to also test a second buffer, EPPS (3-[4-(2-Hydroxyethyl)-1-piperazinyl] propanesulfonic acid), which lacks amines. We used subsaturating concentrations of both crosslinkers, which we determined using the assays described here (data not shown), in order to discern any positive or negative effects of the buffer on crosslinking. Tissue was crosslinked using 0.1mM GP or 0.4mM MG in 100mM Tris or EPPS buffer at pH 9.
Phosphate ions stimulate the reaction of glucose with proteins, possibly by binding to the target at basic residues adjacent to the crosslinking site and catalyzing the conversion of Schiff base intermediates to stable ketoamine products37;38. Since glucose and MG participate in advanced glycation endproduct crosslink formation via Maillard-type reactions37;39, we decided to investigate whether phosphate could stimulate crosslinking in our system. Samples were incubated at pH 8 with 1mM GP or 2mM MG at pH 9 or with 0.1mM GP or 0.4mM MG in either 100mM EPPS buffer or 100mM EPPS buffer containing 100mM tri-sodium phosphate.
Mechanical Testing
For circumferential tensile testing, 58 circumferential specimens of annulus were cut from 29 bovine lumbar discs, further cut to narrow a central region. The tensile tests of circumferential annulus specimens were conducted before our observations that Tris exhibited an inhibitory effect on crosslinking and we therefore soaked the tissue at pH 9 in either 10mM GP in 100mM Tris/tri-sodium phosphate or 20mM MG in 100mM EPPS/tri-sodium phosphate at 37°C for 4-hours, or in either buffer alone.
The smallest cross-section in the necked-down region of each sample was measured using a rotating laser micrometer. The specimen was positioned in custom clamps and a tensile test was run at a constant displacement rate of 0.3mm/sec. Peak modulus, ultimate tensile strength, yield stress (using a 0.1% offset method), yield strain, and resilience (total energy absorbed at the yield point) were calculated from the stress-strain data. Data were expressed as the percentage change in the parameter in a treated specimen compared to the mean of the untreated samples. A Mann-Whitney non-parametric test was used to determine significance of mean differences between groups (α≤0.05).
Disc bulge measurements were conducted on twelve calf lumbar motion segments that were potted in polyurethane and randomly assigned to receive either an injection of 10mM Genipin in 100mM EPPS/100mM tri-sodium phosphate, pH 9 or an injection of buffer alone. Discs were injected with 0.75ml of solution at both antero-lateral positions (i.e. 1.5ml per disc) using a 1ml syringe and a 22 gauge needle, and then incubated for 4-hours at 37°C under an 89N axial load. After incubation, specimens were clamped into place on a materials test system (TestResources 100R) such that the load axis was parallel to the specimen’s anatomical superior-inferior axis and centered on the disc’s center of mass. Cyclic compressive load from 0N to 400N was applied at 0.05 mm/sec for 40 cycles to condition the specimen. A rotating LK-081 laser micrometer (Keyence) was used to measure the anterior surface of the disc at three heights (midline and 2mm above and below) at static loads of 50 and 400N. Disc surface position was analyzed for each applied load and at each height using custom Octave code. Bulge was calculated as the difference in disc surface position at each height between the 50 and 400N loads. One specimen from the treatment group was 2 standard deviations away from the mean of the remaining samples and was discarded as an outlier. Statistical significance was determined using the Mann-Whitney-test.
Results
The efficacy of NEXT will depend on a number of factors, including the ability to deliver the crosslinker over a large portion of the annulus and the efficiency of the crosslinker once introduced into the milieu of the degenerating disc. In the case of delivery, we first assessed the diffusion of GP, a possible therapeutic crosslinker, following injection into the annulus of bovine spinal discs.
GP diffusion following injection formed clear “zones” (Fig.1) whose area increased time-dependently (Fig.2). We also examined whether two surfactants (SDS and Tween-20) could enhance diffusion of these markers (Fig.3), but only SDS did so significantly (by approximately 30%, p=0.021). We therefore decided to not pursue surfactants, since the effect we observed with SDS was modest and did not justify the added complications of its inclusion in a formulation.
Figure 1.
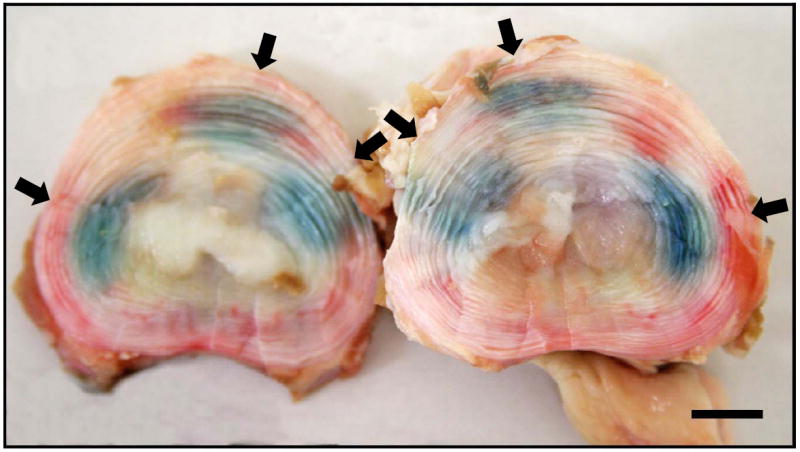
Example of genipin diffusion in a calf lumbar spinal disc. GP was injected into the disc of a single motion segment and incubated for 1 hour at 37°C. Arrows indicate the injection points. Bars = 10mm.
Figure 2.
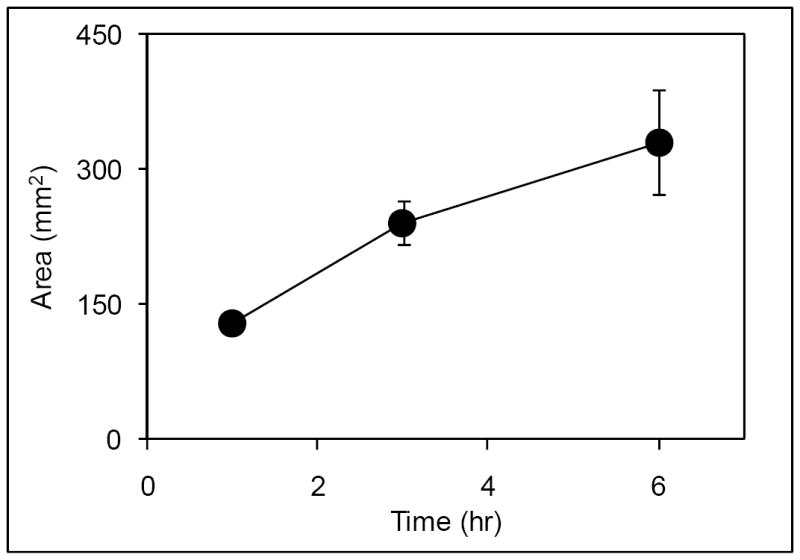
Diffusion kinetics of genipin. GP was injected into bovine lumbar discs and incubated for 1 (n=7), 3 (n=4) or 6 hours (n=4) at 37°C. Data are presented ± SD.
Figure 3.
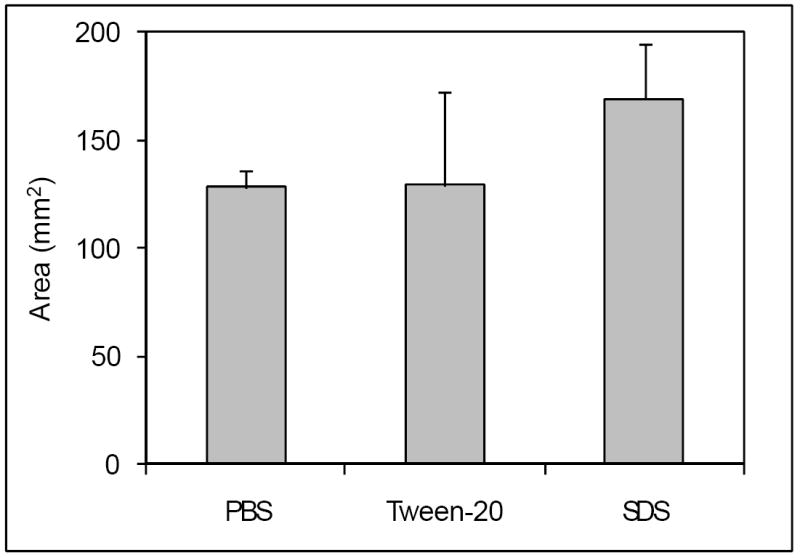
Effect of surfactants on diffusion of genipin. GP in PBS (n=7) or in PBS containing Tween-20 (n=3) or SDS (n=5) was injected and incubated for 1-hour. Data are presented ± SD.
When testing the effects of buffers on crosslinking we found that it was less efficient in the presence of Tris buffer when compared to EPPS (Fig.4) for both GP (p=0.0013) and MG (p=0.0021). Under these conditions GP crosslinking was 47% more efficient in EPPS while the enhancement for MG was 44%. In addition, phosphate ions increased crosslinking by GP by 72% at pH 8 (p<0.001) and by 44% at pH 9 (p<0.01), while MG was 50% more efficient at pH 8 (p<0.001) but unaffected at pH 9 (Fig.5). Such effects were not due to the added sodium in the mixture since tri-potassium phosphate caused similar effects (data not shown).
Figure 4.
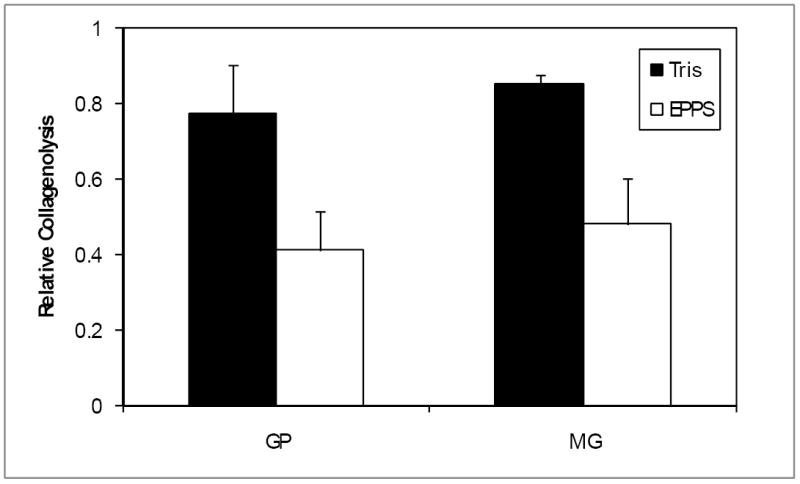
Buffers effects on genipin and methylglyoxal crosslinking. Tissue was incubated with GP or MG at 37°C for 1 hour in either Tris or EPPS buffers at pH 9. Crosslinking was quantified and normalized to untreated tissue. Data are presented ± SD (n=5).
Figure 5.
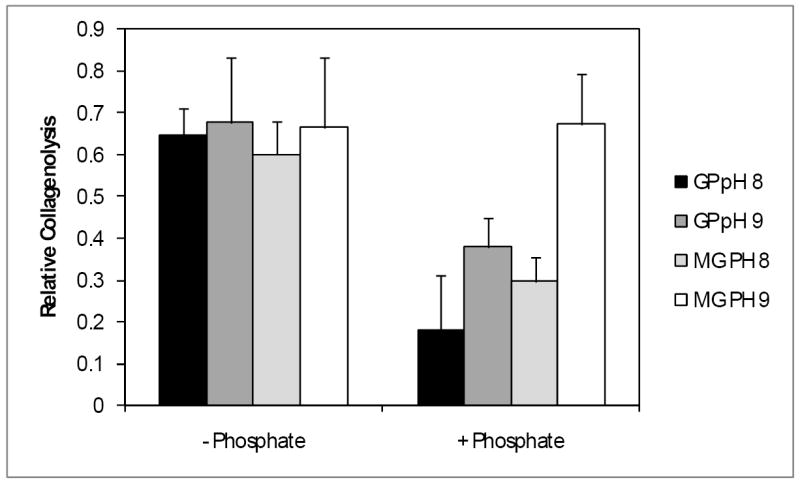
Effect of phosphate on genipin and methylglyoxal mediated crosslinking. Tissue was incubated with GP or MG at pH 8 or 9 at 37°C for 1 hour, in the presence or absence of phosphate. Crosslinking was quantified and normalized to untreated tissue. Data are presented ± SD (n=5).
The mechanical properties of tissues treated with crosslinker were compared to those treated with buffer alone using a circumferential tensile test (Fig.6). GP elicited increases of 66, 36 and 57% in yield stress, yield strain and resilience or energy required to generate non-recoverable deformation (p=0.012, 0.048 and 0.019, respectively), to the annulus tissue. Peak modulus trended 23% higher with GP treatment, but this difference was not statistically significant. In contrast, MG treatment resulted in a 69% increase in modulus (p=0.009), and trended higher in yield strain (46%) and ultimate tensile strength (27%), although these increases were not statistically significant. A summary of all our circumferential testing data is shown in Table 1.
Figure 6.
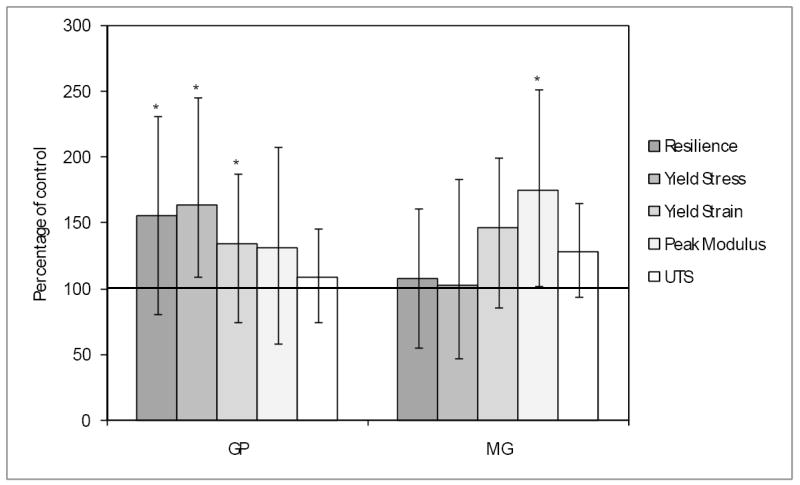
Circumferential tensile testing of crosslinked annulus tissue. Tissue was crosslinked with GP (n=17) or MG (n=13) or in GP buffer (n=16) or MG buffer (n=12) alone and subjected to testing. Data are presented ± SD. Results marked with an asterisk denote statistical significant differences between the treated and buffer samples.
Table 1.
Summary of circumferential tensile testing data. Annulus specimens were treated with either GP or MG, or sham-treated with the respective buffer, and then subjected to tensile testing. Five parameters were measured and the results are reported as the mean and standard deviation of each group. Units for the parameters are MPa (resilience, yield stress, UTS – ultimate tensile strength), mm/mm (yield strain) and GPa (peak modulus). Treated results marked with an asterisk denote a significant difference relative to the corresponding sham.
| Resilience | Yield Stress | Yield Strain | Peak Modulus | UTS | |
|---|---|---|---|---|---|
| GP Sham | 1.09±0.59 | 0.96±0.59 | 5.08±2.37 | 14.32±5.58 | 8.08±2.56 |
| GP Treatment | 1.71±0.80* | 1.59±0.76* | 6.90±2.64* | 19.15±10.74 | 8.81±2.87 |
| MG Sham | 1.83±1.50 | 1.72±1.49 | 5.08±2.61 | 9.40±3.10 | 6.61±3.07 |
| MG Treatment | 1.97±0.97 | 1.76±0.94 | 7.42±3.03 | 16.40±6.81* | 8.49±2.29 |
We supplemented these data by measuring the effect of GP in optimized buffer with respect to its ability to reduce disc bulge under load following injection (a therapeutically more meaningful method of delivery). Specimens treated with buffer demonstrated a mean disc bulge of 0.28mm (±0.07) while GP reduced the value to 0.17mm (±0.07). This 37.5% reduction was statistically significant (p=0.04).
Discussion
NEXT holds the promise of an effective and nonsurgical treatment for DDD and would be accomplished by the injection of chemical crosslinking agents into the affected spinal disc. NEXT would act to stabilize and strengthen the disc, and promote maintenance of hydration and the diffusion of nutrients and oxygen into, and waste products out of, the tissue.
We have shown GP is capable of substantial diffusion following injection into a disc (Fig.2) and that SDS, but not Tween-20 can enhance its diffusion (Fig. 3). SDS is a harsh anionic surfactant and its ability to denature proteins may have “loosened” the annulus matrix more than the milder, non-ionic Tween-20, thus leading to faster GP diffusion. The harshness of SDS coupled to its relatively modest effect led us to exclude it from further consideration.
Formulation optimization showed that GP and methylglyoxal (MG) crosslinking was less efficient in Tris than in EPPS buffer (Fig.4), suggesting that the use of amines should be avoided. We also demonstrated that phosphate ions can enhance the reaction of both GP and MG (Fig.5). The inclusion of phosphate into the formulation can also broaden the buffering capacity of the solution (data not shown), further counteracting the acidity of the degenerating disc. The effect of phosphate appeared to be pH dependent. Since both GP and MG activities increase with pH this may have been due to the elevated activity masking the phosphate effect. MG activity is more sensitive to pH than GP (Slusarewicz, et al, submitted), and was also unaffected by phosphate at pH 9. MG’s reactivity at this pH may have been so high that it could not be further enhanced by phosphate. Nevertheless, we decided to retain phosphate in the MG formulation, since we would expect the pH to drop following injection into the acidic environment of the disc.
The enhancement of GP-mediated crosslinking by phosphate was surprising and may be due to the involvement of aldehyde groups in the GP crosslinking reaction. It has been postulated that the initial step in the reaction involves a nucleophilic attack on the C-3 olefinic carbon atom in GP by an amine, resulting in the opening of its dihydropyran ring, followed by a second attack of the attached amine on the resulting aldehyde group25. This second attack closes the ring with the substitution of the amine nitrogen (and attached moieties) for the original oxygen in the GP structure. Crosslinking then occurs through polymerization of the GP derivatives and the molecules to which they are coupled25;26. Alternatively, the hemiacetal within the dihydropyran ring could undergo consecutive steps of hydration, opening the ring to produce two aldehyde groups40 which could either react with different protein-bound amines to form a crosslink directly or react with a single amine and then follow the reaction described above. In either pathway Schiff bases form via reaction of amines with aldehydes and their rearrangements could be catalyzed by phosphate ions in a manner similar to those formed during protein glycation.
Using a circumferential tensile test, we examined the effect of crosslinking of annulus tissue by formulations containing GP or MG. Both were effective in enhancing at least one relevant mechanical parameter of tissue that had been treated in vitro by soaking (Fig.6). Interestingly, GP appeared to exert an equal if not more pronounced effect than MG, even though the formulation was sub-optimal due to the use of Tris buffer and the MG concentration was twice that of GP. One possible explanation may lie in the ability of GP to polymerize as part of the crosslinking reaction25;26. Two protein bound amines can only be crosslinked if the distance between them can be spanned by the reagent, and so GP polymers may be able to access more amine pairs than MG. Additionally, MG crosslinks may be less common between adjacent collagen fibrils whereas GP may be able to span inter-fibrillar distances more readily. This combination of increased number of potential crosslinking sites and types due to the longer potential spanning distance of GP polymers may result in very different mechanical effects.
We also tested GP in a more clinically relevant manner by injecting the crosslinker directly into bovine lumbar discs using an EPPS/phosphate buffer. The GP treatment significantly reduced the bulge of the treated discs under a 400N axial load by 37.5% compared to discs injected with buffer alone and demonstrated the potential of NEXT when applied by injection. The relevance towards decompression of adjacent neural structures (radiculopathy) and nociceptor excitation thresholds (discogenic pain) due to this degree of disc bulge reduction should be further explored in future studies using human tissues. Increases in disc bulge has been associated with increasing disc degeneration in the clinic41. It is expected that increased levels of degeneration combined with an increased disc height should amplify both the amount of disc bulge and the NEXT reduction of bulge in human discs.
In order for NEXT to be therapeutically viable, it needs to exhibit an acceptable biocompatibility profile. Two areas of concern could be the effect of the formulation on the viability of the cells of the disc and the possibility of neurotoxicity from leakage of crosslinkers from the disc and into the spinal canal or onto nerve roots. We are in the process of conducting studies to address these issues, but our preliminary data indicate that both formulations do not affect cell viability when injected subcutaneously (data not shown).
Having developed suitable, optimized formulations, we next plan to determine their effects on a broad range of mechanical parameters of human spinal motion segments following injection, as a prelude to clinical testing of NEXT technology in the clinical setting.
Some surfactants may be able to enhance the diffusion of crosslinkers within the disc.
Excipients (including buffers) containing amine moieties reduce the reaction efficiency of the crosslinkers genipin and methylglyoxal.
Both genipin and methylglyoxal crosslinking is enhanced by phosphate ions.
Buffered, alkaline formulations of genipin and methylglyoxal containing phosphate ions are able to alter the physical properties of disc tissue following soaking and such a genipin formulation reduces disc bulge under load when injected into spinal discs.
Acknowledgments
This work was supported by the National Institutes of Health (1R43 AR055014-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 4.Gu WY, Mao XG, Foster RJ, et al. The anisotropic hydraulic permeability of human lumbar anulus fibrosus. Influence of age, degeneration, direction, and water content. Spine. 1999;24:2449–55. doi: 10.1097/00007632-199912010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kitano T, Zerwekh JE, Usui Y, et al. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop Relat Res. 1993:372–7. [PubMed] [Google Scholar]
- 6.Schnake KJ, Putzier M, Haas NP, et al. Mechanical concepts for disc regeneration. Eur Spine J. 2006;15(Suppl 3):S354–S360. doi: 10.1007/s00586-006-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobajima S, Kim JS, Gilbertson LG, et al. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 9.Acosta FL, Jr, Lotz J, Ames CP. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: a critical overview. Neurosurg Focus. 2005;19:E4. doi: 10.3171/foc.2005.19.3.5. [DOI] [PubMed] [Google Scholar]
- 10.Chuang SY, Odono RM, Hedman TP. Effects of exogenous crosslinking on in vitro tensile and compressive moduli of lumbar intervertebral discs. Clin Biomech. 2007;22:14–20. doi: 10.1016/j.clinbiomech.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Hedman TP, Saito H, Vo C, et al. Exogenous cross-linking increases the stability of spinal motion segments. Spine. 2006;31:480–5. doi: 10.1097/01.brs.0000224531.49174.ea. [DOI] [PubMed] [Google Scholar]
- 12.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39:1021–9. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Yerramalli CS, Chou AI, Miller GJ, et al. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6:13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane SM, Robinson GB. In vitro glycation of glomerular basement membrane alters its permeability: a possible mechanism in diabetic complications. FEBS Lett. 1995;375:41–4. doi: 10.1016/0014-5793(95)01171-a. [DOI] [PubMed] [Google Scholar]
- 15.Boyd-White J, Williams JC., Jr Effect of cross-linking on matrix permeability. A model for AGE-modified basement membranes. Diabetes. 1996;45:348–53. doi: 10.2337/diab.45.3.348. [DOI] [PubMed] [Google Scholar]
- 16.Hedman T, Saito H, Chuang SY. Matrix modification increases hydraulic permeability of the annulus fibrosus. Orthop Trans. 2006;31:1241. [Google Scholar]
- 17.Hoffmann B, Seitz D, Mencke A, et al. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2009;20:1495–503. doi: 10.1007/s10856-009-3707-3. [DOI] [PubMed] [Google Scholar]
- 18.Han B, Jaurequi J, Tang BW, et al. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65:118–24. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 19.Gratzer PF, Lee JM. Control of pH alters the type of cross-linking produced by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) treatment of acellular matrix vascular grafts. J Biomed Mater Res. 2001;58:172–9. doi: 10.1002/1097-4636(2001)58:2<172::aid-jbm1004>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Verzijl N, De Groot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–23. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Sung HW, Liang IL, Chen CN, et al. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin) J Biomed Mater Res. 2001;55:538–46. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Sung HW, Chang Y, Chiu CT, et al. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res. 1999;47:116–26. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Peters MA, Hudson PM, Jurgelske W., Jr The acute toxicity of methylglyoxal in rats: the influence of age, sex, and pregnancy. Ecotoxicol Environ Saf. 1978;2:369–74. doi: 10.1016/s0147-6513(78)80009-8. [DOI] [PubMed] [Google Scholar]
- 24.Sung HW, Huang RN, Huang LL, et al. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10:63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- 25.Touyama R, Inoue K, Takeda Y, et al. Studies on the blue pigments produced from genipin and methylamine. II. On the formation mechanisms of brownish-red intermediates leading to the blue pigment formation. Chem Pharm Bull. 1994;42:1571–8. [Google Scholar]
- 26.Touyama R, Takeda Y, Inoue K, et al. Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem Pharm Bull. 1994;42:668–73. [Google Scholar]
- 27.Ohno K, Yasuhara K, Kawasaki Y, et al. Comparative studies on acute toxicity of glutaraldehyde using young and old rats. Eisei Shikenjo Hokoku. 1991:92–7. [PubMed] [Google Scholar]
- 28.Bannister DW, Burns AB. Adaptation of the Bergman and Loxley technique for hydroxyproline determination to the autoanalyzer and its use in determining plasma hydroxyproline in the domestic fowl. Analyst. 1970;95:596–600. doi: 10.1039/an9709500596. [DOI] [PubMed] [Google Scholar]
- 29.Berg RA. Determination of 3- and 4-hydroxyproline. Methods Enzymol. 1982;82 Pt A:372–98. doi: 10.1016/0076-6879(82)82074-0. [DOI] [PubMed] [Google Scholar]
- 30.Prockop DJ, Udenfriend S, Lindstedt S. A simple technique for measuring the specific activity of labeled hydroxyproline in biological materials. J Biol Chem. 1961;236:1395–8. [PubMed] [Google Scholar]
- 31.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 32.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 33.Murata-Kamiya N, Kamiya H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Res. 2001;29:3433–8. doi: 10.1093/nar/29.16.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung HW, Chang Y, Liang IL, et al. Fixation of biological tissues with a naturally occurring crosslinking agent: fixation rate and effects of pH, temperature, and initial fixative concentration. J Biomed Mater Res. 2000;52:77–87. doi: 10.1002/1097-4636(200010)52:1<77::aid-jbm10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24:1195–6. doi: 10.1007/BF02146615. [DOI] [PubMed] [Google Scholar]
- 36.Kallet RH, Jasmer RM, Luce JM, et al. The treatment of acidosis in acute lung injury with tris-hydroxymethyl aminomethane (THAM) Am J Respir Crit Care Med. 2000;161:1149–53. doi: 10.1164/ajrccm.161.4.9906031. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Watkins NG, Neglia-Fisher CI, Dyer DG, et al. Effect of phosphate on the kinetics and specificity of glycation of protein. J Biol Chem. 1987;262:7207–12. [PubMed] [Google Scholar]
- 39.De Groot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004;4:301–5. doi: 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Yamano T, Tsujimoto Y, Noda T, et al. Hepatotoxicity of gardenia yellow color in rats. Toxicol Lett. 1988;44:177–82. doi: 10.1016/0378-4274(88)90144-0. [DOI] [PubMed] [Google Scholar]
- 41.Cheung KM, Chan D, Karppinen J, et al. Association of the Taq I allele in vitamin D receptor with degenerative disc disease and disc bulge in a Chinese population. Spine. 2006;31:1143–8. doi: 10.1097/01.brs.0000216530.41838.d3. [DOI] [PubMed] [Google Scholar]


