Abstract
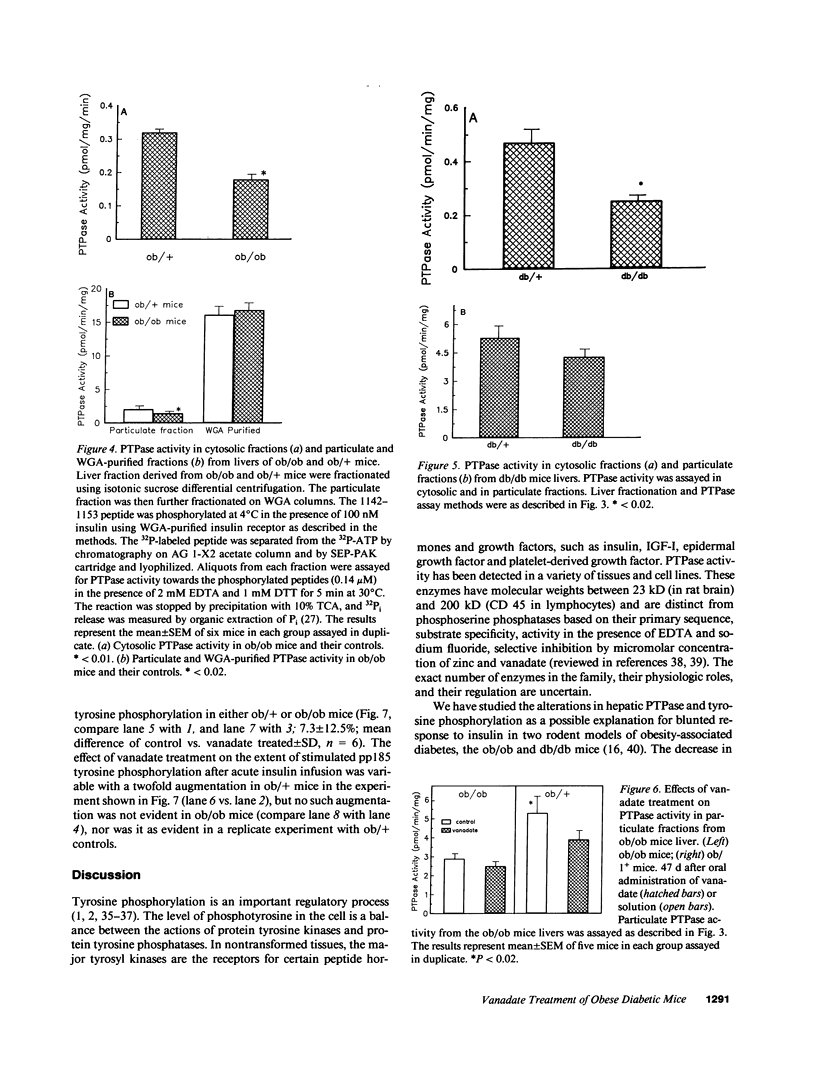
We have studied the effects of oral administration of vanadate, an insulinometic agent and a potent inhibitor of phosphotyrosyl protein phosphatase (PTPase) in vitro, on blood glucose and PTPase action, in two hyperinsulinemic rodent models of non-insulin-dependent diabetes mellitus (NIDDM). Oral administration of vanadate (0.25 mg/ml in the drinking water) to ob/ob mice for 3 wk lowered blood glucose level from 236 +/- 4 to 143 +/- 2 mg/dl without effect on body weight. Administration of vanadate to db/db mice produced a similar effect. Electron microscopic examination revealed no signs of hepatotoxicity after 47 d of treatment. There was a slight reduction in insulin receptor autophosphorylation when tested by immunoblotting with antiphosphotyrosine antibody after in vivo stimulation, and the phosphorylation of the endogenous substrate of the insulin receptor, pp185, was markedly decreased in the ob/ob mice. Both cytosolic and particulate PTPase activities in liver of ob/ob mice measured by dephosphorylation of a 32P-labeled peptide corresponding to the major site of insulin receptor autophosphorylation were decreased by approximately 50% (P less than 0.01). In db/db diabetic mice, PTPase activity in the cytosolic fraction was decreased to 53% of control values (P less than 0.02) with no significant difference in the particulate PTPase activity. Treatment with vanadate did not alter hepatic PTPase activity as assayed in vitro, or receptor and substrate phosphorylation as assayed in vivo, in ob/ob mice despite its substantial effect on blood glucose. These data indicate that vanadate is an effective oral hypoglycemic treatment in NIDDM states and suggest that its major effects occurs distal to the insulin receptor tyrosine kinase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfiore F., Iannello S., Rabuazzo A. M., Campione R. Metabolic effects of short-term fasting in obese hyperglycaemic humans and mice. Int J Obes. 1987;11(6):631–640. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang A. Y., Schneider D. I. Abnormalities in hepatic enzyme activities during development of diabetes in db mice. Diabetologia. 1970 Jun;6(3):274–278. doi: 10.1007/BF01212238. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967 Apr;3(2):238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- Cooper D. R., Konda T. S., Standaert M. L., Davis J. S., Pollet R. J., Farese R. V. Insulin increases membrane and cytosolic protein kinase C activity in BC3H-1 myocytes. J Biol Chem. 1987 Mar 15;262(8):3633–3639. [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Gil J., Miralpeix M., Carreras J., Bartrons R. Insulin-like effects of vanadate on glucokinase activity and fructose 2,6-bisphosphate levels in the liver of diabetic rats. J Biol Chem. 1988 Feb 5;263(4):1868–1871. [PubMed] [Google Scholar]
- Green A. The insulin-like effect of sodium vanadate on adipocyte glucose transport is mediated at a post-insulin-receptor level. Biochem J. 1986 Sep 15;238(3):663–669. doi: 10.1042/bj2380663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy G. R., Gordon J., Walker L., Michell R. H., Brown G. Redistribution of protein kinase C during mitogenesis of human B lymphocytes. Biochem Biophys Res Commun. 1986 Feb 26;135(1):146–153. doi: 10.1016/0006-291x(86)90954-x. [DOI] [PubMed] [Google Scholar]
- Heffetz D., Zick Y. H2O2 potentiates phosphorylation of novel putative substrates for the insulin receptor kinase in intact Fao cells. J Biol Chem. 1989 Jun 15;264(17):10126–10132. [PubMed] [Google Scholar]
- Heyliger C. E., Tahiliani A. G., McNeill J. H. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985 Mar 22;227(4693):1474–1477. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S. Phosphotyrosyl-protein phosphatases. II. Identification and characterization of two heat-stable protein inhibitors. J Biol Chem. 1989 May 5;264(13):7754–7759. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., White M. F., Kahn C. R. Phosphorylation of the insulin receptor in cultured hepatoma cells and a solubilized system. Methods Enzymol. 1985;109:609–621. doi: 10.1016/0076-6879(85)09118-2. [DOI] [PubMed] [Google Scholar]
- Lau K. H., Farley J. R., Baylink D. J. Phosphotyrosyl protein phosphatases. Biochem J. 1989 Jan 1;257(1):23–36. doi: 10.1042/bj2570023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Machicao F., Urumow T., Wieland O. H. Evidence for phosphorylation of actin by the insulin receptor-associated protein kinase from human placenta. FEBS Lett. 1983 Oct 31;163(1):76–80. doi: 10.1016/0014-5793(83)81167-3. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J Clin Invest. 1989 Sep;84(3):976–983. doi: 10.1172/JCI114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerovitch J., Farfel Z., Sack J., Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J Biol Chem. 1987 May 15;262(14):6658–6662. [PubMed] [Google Scholar]
- Meyerovitch J., Shechter Y., Amir S. Vanadate stimulates in vivo glucose uptake in brain and arrests food intake and body weight gain in rats. Physiol Behav. 1989 Jun;45(6):1113–1116. doi: 10.1016/0031-9384(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S. A., Brautigan D. L. Membrane protein phosphotyrosine phosphatase in rabbit kidney. Proteolysis activates the enzyme and generates soluble catalytic fragments. Biochem J. 1987 May 1;243(3):747–754. doi: 10.1042/bj2430747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman I., Horland A. A., Teebor G. W. Glycolytic and gluconeogenic enzyme activities in the hereditary obese-hyperglycemic syndrome and in acquired obesity. Diabetologia. 1970 Jun;6(3):313–316. doi: 10.1007/BF01212244. [DOI] [PubMed] [Google Scholar]
- Shacter E. Organic extraction of Pi with isobutanol/toluene. Anal Biochem. 1984 May 1;138(2):416–420. doi: 10.1016/0003-2697(84)90831-5. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Meyerovitch J., Amir S. The use of post-binding agents in studying insulin action and its relation to experimental diabetes. Biochem Pharmacol. 1988 May 15;37(10):1891–1896. doi: 10.1016/0006-2952(88)90533-3. [DOI] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J. W., Brautigan D. L. Specificity of protein phosphotyrosine phosphatases. Comparison with mammalian alkaline phosphatase using polypeptide substrates. J Biol Chem. 1985 Feb 25;260(4):2042–2045. [PubMed] [Google Scholar]
- Stengard J. H., Saarni H. U., Karvonen R. I., Lahtela J. T., Kärki N. T., Stenbäck F., Sotaniemi E. A. Hepatic drug metabolism and the activities of NADPH generating enzymes and glucose-6-phosphatase in phenobarbital treated genetically obese (ob/ob) mice. Biomed Pharmacother. 1987;41(7):389–396. [PubMed] [Google Scholar]
- Stroop S. D., Helinek G., Greene H. L. More sensitive flameless atomic absorption analysis of vanadium in tissue and serum. Clin Chem. 1982 Jan;28(1):79–82. [PubMed] [Google Scholar]
- Strout H. V., Vicario P. P., Saperstein R., Slater E. E. The insulin-mimetic effect of vanadate is not correlated with insulin receptor tyrosine kinase activity nor phosphorylation in mouse diaphragm in vivo. Endocrinology. 1989 Apr;124(4):1918–1924. doi: 10.1210/endo-124-4-1918. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Speeg K. V., Jr, Cohen S., Garbers D. L. Phosphotyrosyl-protein phosphatase of TCRC-2 cells. J Biol Chem. 1982 Jul 10;257(13):7298–7301. [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Whipple J. H., Fujita-Yamaguchi Y., Dubler R. E., Cheng K., Larner J. A novel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. J Biol Chem. 1984 May 25;259(10):6650–6658. [PubMed] [Google Scholar]
- Tanti J. F., Grémeaux T., Brandenburg D., Van Obberghen E., Le Marchand-Brustel Y. Brown adipose tissue in lean and obese mice. Insulin-receptor binding and tyrosine kinase activity. Diabetes. 1986 Nov;35(11):1243–1248. doi: 10.2337/diab.35.11.1243. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
- Tornqvist H. E., Pierce M. W., Frackelton A. R., Nemenoff R. A., Avruch J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J Biol Chem. 1987 Jul 25;262(21):10212–10219. [PubMed] [Google Scholar]
- Vicario P., Brady E. J., Slater E. E., Saperstein R. Insulin receptor tyrosine kinase activity is unaltered in ob/ob and db/db mouse skeletal muscle membranes. Life Sci. 1987 Sep 7;41(10):1233–1241. doi: 10.1016/0024-3205(87)90201-3. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Yang S. D., Yu J. S., Liu J. S., Tzen T. C., Wang J. K. The type-1 protein phosphatase activating factor FA is a membrane-associated protein kinase in brain, liver, heart and muscles. Biochem Biophys Res Commun. 1987 Jan 15;142(1):38–46. doi: 10.1016/0006-291x(87)90448-7. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]







