Abstract
The surface of most protozoan parasites relies heavily upon lipid-anchored molecules, to form protective barriers and play critical functions required for infectivity. Sphingolipids (SLs) play important roles through their abundance and involvement in membrane microdomain formation, as well as serving as the lipid anchor for many of these molecules, and in some but possibly not all species, as important signaling molecules. Interactions of parasite sphingolipid metabolism with that of the host may potentially contribute to parasite survival and/or host defense. In this chapter we summarize current knowledge of SL structure, synthesis and function in several of the major parasitic protozoan groups.
Keywords: Trypanosomatid protozoa, Leishmania, Trypanosoma brucei, Trypanosoma cruzi, Plasmodium, malaria, Toxoplasma, Giardia lamblia, Trichomonas vaginalis, inositolphosphoryl ceramide (IPC), sphingolipid synthases, ethanolamine, Entameoba histolytica, palmitoylamino-3-morpholino-1-propanol (PPMP, differentiation, virulence, aureobasidin A (Aba), IPC synthase, rafts, sphingomyelin (SM), ethanolaminephosphoryl ceramide (EPC), chemotherapy, virulence
Introduction
Protozoan pathogens have tremendous negative impact on human health and prosperity. Malaria, a mosquito-borne disease caused by Plasmodium parasites, is responsible for 350–500 million cases and more than one million deaths each year. Trypanosomatid parasites including Trypanosoma cruzi, Trypanosomabrucei, and Leishmania spp infect 20–30 million people worldwide, causing a spectrum of devastating diseases from disfiguring skin lesions to lethal visceral, cardiac and cerebral infections 1–3. Other parasitic protozoans such as Toxoplasma gondii, Giardia spp., and Entameoba histolytica are widely distributed pathogens capable of causing severe diseases in humans. As yet, there are no safe vaccines for any of these parasites, leaving drug treatments as the major strategy for control. Available drugs are compromised by low efficacy, high toxicity, and wide spread resistance 2, 4. It is important, therefore, to identify parasite-specific virulence pathways and develop novel inhibitors that target them.
Sphingolipids (SLs) are ubiquitous membrane components in pathogenic protozoans (Table 1). In comparison to mammals and fungi, knowledge about metabolism, structure and function of SLs in parasitic protozoa is limited and an area of active research. Trypanosomatids such as Trypanosoma and Leishmania spp. synthesize large amounts of unglycosylated inositol phosphorylceramide (IPC), a lipid found widely among fungi and plants. Trypanosomes also synthesize sphingomyelin (SM), a lipid commonly found in mammals. Apicomplexan parasites including Plasmodium spp mostly synthesize SM and glycosyl-SLs, although a recent report suggests inositol-SLs occur in Toxoplasma gondii 5.
Table 1.
Composition of higher order SLs in parasitic protozoa
| Species | Sphingolipids identified | References |
|---|---|---|
| Leishmania major | IPC | 6, 7 |
| Trypanosoma brucei | IPC (procyclics only) | 8, 9 |
| EPC (blood stream forms only) | 10 | |
| SM | 10, 11 | |
| Glycosyl-cer | 12 | |
| Trypanosoma cruzi | IPC* | 13, 14 |
| SM | 15 | |
| Plasmodium falciparum | Glycosyl-cer, SM | 16, 17 |
| Toxoplasma gondii | Glycosyl-cer, SM, IPC? | 5, 18 |
| Trichomonas vaginalis | IPC*, SM | 19–21 |
| Giardia lamblia | SM | 22, 23 |
glycosylated and used as anchors for lipopeptidophosphoglycan (LPPG) and some surface proteins.
In this chapter, we review recent findings about the roles of SL metabolism in pathogenic protozoa.
Leishmania
Leishmania represents one of the three major lineages of trypanosomatid protozoans, which may have separated as long as 250–500 million years ago 24. Parasites within the genus Leishmania are transmitted by Phlebotomine sand flies and cause a spectrum of diseases, varying from mild self resolving cutaneous lesions, to fatal visceral infections, and disfiguring mucosal manifestations. There is a strong albeit imperfect tendency for these phenotypes to be associated with individual species, for example L. major and L. mexicana with cutaneous disease, L. donovani with visceral disease, and L. braziliensis with mucocutaneous disease. The biochemical basis underlying these different disease manifestations is largely unknown. Leishmania live extracellularly within the sand fly midgut, but within an acidified, fusogenic phagolysosome of the macrophage within the mammalian host.
Sphingolipids were reported first in L. donovani 25, 26 and thereafter in all species studied. Notably the predominant SL was IPC, more typical to those found in fungi than in the mammalian hosts. Methods for mass spectrometric analysis and quantification of IPCs were developed and have shown that the structure within both the promastigote and amastigote stage of Leishmania major are IPC-d16:1 (phosphoryl inositol N-stearoylhexadecesphing-4-enine, d16:1/18:0-PI-Cer) and IPC-d18:1 (phosphoryl inositol N-stearoylsphingosine, d18:1/18:0-PI-Cer), respectively, with IPC-d16:1 being more abundant. IPCs are present in an abundance of about 2 x 108 molecules per cell, nearly 10% of the total membrane phospholipids 6, 7, 27. Additionally, several studies have reported the presence of mammalian type sphingolipids on Leishmania amastigote residing in mammalian macrophages. Current data from diversespecies suggest that these arise in some manner by transfer from the mammalian host rather than direct synthesis by the parasite 28–30.
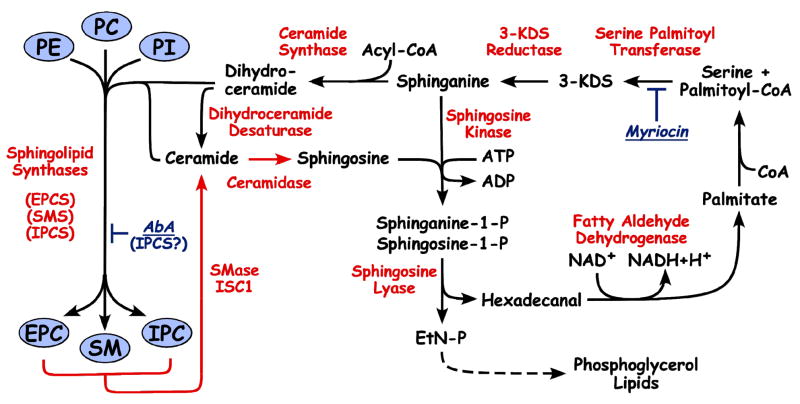
The occurrence of the parasite specific IPC suggested that Leishmania organisms encode a complete, functional SL sphingolipid pathway 31 (Figure 1). In the de novo pathway, activities and/or genes have been found for serine palmitoyl transferase (SPT1/SPT2), ceramide synthase (LAG1/CERS) and IPC synthase IPCS1 6, 27, 32, 33. On the degradative side, a sphingosine 1-P lyase SPL 33 has been functionally identified; parasites also encode a homolog of the IPC phospholipase C ISC1 (Lmj08.0200) and sphingosine kinase (LmjF26.0710). There are potential homologs encoding dihydroceramide desaturase, 3-ketosphingosine reductase, ceramidase, and sphingosine 1-P phosphatases as well, although the evolutionary distances make firm conclusions premature. Future studies will likely fill in missing steps and/or confirm the assignments above. Orthologs of all of these genes are found in the other trypanosomatid genomes.
Figure 1. Kinetoplastid Sphingolipid Pathways.
Synthetic pathways are indicated by black arrows, degradative pathways by red arrows. Names of key enzymes are indicated in red. Sites of inhibition by myriocin and aureobasidin A (AbA) are also indicated. Abbreviations: 3-KDS, 3-ketodihydrosphinganine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PI, phosphatidylinositol; EPC, ethanolaminephosphoryl ceramide; SM, sphingomyelin; IPC, inositolphosphoryl ceramide; EtN, ethanolamine; EPCS/SMS/IPCS, sphingolipid synthases as indicated; SMase, sphingomyelinase; ISC1, inositol phosphosphingolipid phospholipase-C. The attachment and role of SLs in the attachment of membrane proteins in glycoconjugates is not shown in this figure.
SL pathway genetics
Genetic studies of SL metabolism have yielded some surprises in Leishmania major. Two studies reported the generation of null mutants of one of the subunits (SPT2) required for serine palmitoyl transferase activity in L. major promastigotes, the stage normally carried by the sand fly vector 6, 27. Mass spectrometry confirmed these parasites completely lacked SLs including IPC, yet these organisms grew normally in logarithmic phase and continued to make ‘lipid rafts’ as judged by several criteria. This was remarkable as SLs are considered essential membrane components in all eukaryotes. The ability of Leishmania to survive in their absence may reflect their rather unique membrane composition, with ergosterol substituting for cholesterol, and high levels of plasmalogen ether phospholipids, both of which strongly promote raft formation.
While normal in logarithmic phase, upon entry into stationary phase spt2− parasites showed severe defects including loss of viability and abnormalities including accumulation of small vesicles reminiscent of multivesicular bodies, lipid inclusions, and defects in the formation of acidocalcisomes 34. While originally attributed to a defect in SL synthesis, subsequent studies of a null mutant (spl−) lacking the SL degradative enzyme sphingosine-1-P lyase (SPL) revealed a very similar set of phenotypes to those of spt2. Genetically this suggests that both defects lay upstream of a critical metabolite, which was predicted from metabolic pathway analysis and then shown to be ethanolamine (EtN) 33. Provision of EtN completely reversed most phenotypes of the spt2− and spl− mutants, except a modest difference in log phase cell shape. Thus, in L. major promastigotes the primary role of SL synthesis appears to be focused on provision of EtN, rather than the more typical roles in other eukaryotes critical to cell signaling and raft formation 33.
SL salvage by amastigotes
The requirement for SLs in the promastigote stage when propagated in its native sand fly vector has not been reported thus far. However, despite the complete absence of SLs, spt2− mutant parasites remain able to induce infections in the mammalian host more or less normally (when grown in EtN), consistent with the down regulation of SPT2 seen in parasite development 6, 27, 34. Remarkably, when isolated from mammalian hosts, spt2− amastigotes showed normal IPC levels 34. This mandates some form of SL salvage to occur within the amastigote-bearing phagolysosomes, either as sphingoid bases, ceramide, or complex SLs followed by some form of remodeling into parasite IPCs. The transfer of mammalian SLs to the amastigote surface mentioned earlier is one likely route for this to occur, although this has not been proven.
Given the role of SLs in mammalian cell biology and signaling, several studies have found alterations in mammalian SL metabolism in Leishmania-infected macrophages in vitro, including alterations in ceramide levels that could contribute to parasite survival 35, 36. These studies suggest the possibility of a fascinating interplay between host and parasite SL metabolism, extending beyond nutritional factors into signaling pathways relevant to parasite survival and/or host defense.
Other Leishmania species
Genetic studies of SL metabolism have concentrated thus far on L. major. One might reasonably predict from similarities in SL pathway gene content and organization amongst Leishmania species that these species might behave similarly. However the same might have been said for comparisons with trypanosomes, yet unlike L. majorde novo SL synthesis is clearly essential in these species (discussed below). There are precedents for major differences the dependency upon glycoconjugate pathways in Leishmania despite seemingly identical biochemical functions, one example being the Golgi GDP-mannose transporter which is required for amastigote virulence in L. major but not L. mexicana 37, 38.
Inhibition of SL synthetic pathways
The lack of SL dependency for promastigote growth and the reliance upon salvage rather than de novo synthesis in the amastigote stage may argue that inhibition of de novo SL synthesis, or at least that of sphingoid bases, is unlikely to be a good target for inhibition in these species. In contrast, L. major amastigotes maintain IPC levels, and in combination with the potential for interactions with host SL pathways, suggesting that inhibition of IPC synthesis may offer a valid chemotherapeutic target, as proposed in fungi.
The best studied IPC synthase (IPCS) inhibitor is aureobasidin A (AbA), which inhibits fungal growth at in the nanomolar range (~50 nM) and fungal IPC synthase in the subnanomolar range 39, 40. Leishmania species are much less sensitive, requiring 1–10 μM 32, 34, 41. While AbA inhibits L. major promastigote growth with an EC50 of 0.6 μM, MS analysis revealed no effect on IPC synthesis at this concentration, and IPC synthesis inhibition was not seen until 5.0 μM AbA 6 Consistent with these findings, studies of heterologous expressed IPCS suggests an enzymatic IC50 on the order of 100 μM 32. Lastly, AbA was similarly toxic to the spt2- parasites, which lack IPC 27, 34. These findings suggest that the major inhibitory activity of AbA against Leishmania majorin vivo may involve a target other than IPCS, while not yet excluding IPCS as a potential amastigote target for more effective inhibitors in the future.
Trypanosoma brucei (ssp) and Trypanosoma cruzi
These related trypanosome species cause two distinct human diseases: African Trypanosomiasis, or Sleeping Sickness, (T. brucei ssp), and South American Trypanosomiasis, or Chagas Disease (T. cruzi). Because of the predominance of IPC in the related kinetoplastid genus Leishmania, both groups of trypanosomes have also been generally thought to contain mostly IPC, despite early compositional studies indicating the presence of SM in each (Table 1). However, as is the case with Leishmania, there are marked differences within the genus Trypanosoma in regard to geographical distribution, life cycle, biochemistry, and disease pathology. Consequently, it is not surprising that there are significant differences in sphingolipid composition and metabolism between the trypanosomal species.
Trypanosoma brucei
African trypanosomes have a life cycle that alters between the bloodstream of the mammalian host and the insect vector, the tsetse fly (genus Glossina). There are two replicative forms that are amenable to in vitro study, the pathogenic bloodstream form and procyclic insect form, which is found in the tsetse midgut. Early compositional studies reported the presence of SM in both stages 11, 42, and the presence of IPC was suggested 11, although this was not confirmed with known standards or enzymatic treatments. More recently high-resolution mass spectroscopy has confirmed the presence of both SM and IPC, as well as the unusual phosphosphingolipid, ethanolamine phosphorylceramide (EPC) 8, 10, 43. In addition, neutral glycosphingolipids have also been indentified in bloodstream stage parasites 12. Interestingly, synthesis of phosphosphingolipids is developmentally regulated in T. brucei 10. Both compositional analyses and biosynthetic assays measuring the incorporation of fluorescent ceramide analogues indicate that SM is actively synthesized in both bloodstream and procyclic stages, but IPC is only synthesized in procyclic trypanosomes. Furthermore EPC is only found in bloodstream stage parasites. However, this finding may be a matter of sensitivity since EPC levels are very low even in the bloodstream stage. Another aspect of developmental regulation is the ceramide platform used in sphingolipid synthesis. A fairly standard mixture of dihydroceramide (d18:0/16:0, N-palmitoylsphinganine) and ceramide (d18:1/16:0, N-palmitoylsphingosine) are found in both stages, but in both the pool of ceramide precursors and mature phosphosphingolipids the ratio favors dihydroceramide in procyclic parasites and ceramide in bloodstream forms. This difference likely derives from differential expression or regulation of dihydroceramide desaturase, the enzyme responsible for conversion of dihydroceramide to ceramide. Sphingolipid synthesis is essential in T. brucei. Pharmacological 44 and genetic 43 interdiction of the first enzyme of sphingoid base synthesis, serine palmitoyl transferase, is lethal and can be rescued with the immediate downstream product, 3-ketodihydrosphingosine. Exogenous EtN cannot rescue lethality however, indicating that T. brucei relies on sphingoid base synthesis for something other than production of essential EtN, unlike L. major (Fig. 1). Presumably this dependency is synthesis of complex sphingolipids.
Trypanosoma cruzi
South American trypanosomes have a more complicated life cycle with multiple morphological forms, all of which can be maintained in vitro. Log phase cultured epimastigotes mimic the replicative form found in the insect vector, reduvid bugs. Upon entry into stationary phase, parasites differentiate to a trypomastigote form that closely mimics the infectious metacyclic trypomastigotes found in the reduvid hindgut. These can invade cultured mammalian cells where they differentiate into replicative amastigotes. Non-replicative trypomastigotes are subsequently released and go on to infect new host cells. These culture-derived trypomastigotes correspond to the circulating trypomastigotes that are responsible for maintaining the natural mammalian infection, and for transmission back to reduvids during a blood meal. Several compositional studies with epimastigotes reported the presence of SM, but failed to note the presence of IPC 45–47. However, radiolabeling with [3H]palmitate and mass chemical analyses revealed the presence of free IPC in the cellular pools of phospholipids in both epimastigotes, tissue culture derived trypomastigotes, and in vitro differentiated amastigotes 14, 48, 49. The level of IPC synthesis increases during differentiation of trypomastigotes to amastigotes, both in infected myoblasts and during in vitro differentiation 50. These studies failed to detect SM, but they did reveal the ceramide content of IPC to be a mixture of dihydroceramide (d18:0/16:0, N-palmitoylsphinganine) and ceramide (d18:1/16:0, N-palmitoylsphingosine), with lesser amounts of each containing stearate as the N-acyl group (d18:0/18:0 and d18:1/18:0). No ceramides containing longer chain fatty acids, e.g. lignoceric acid (C24:0) were detected, but it must be noted that mass chemical analyses were only performed with epimastigotes 48, and conclusions based solely on radiolabeling can be misleading. Neutral glycosphingolipids have also been identified in epimastigotes by mass chemical approaches 51. These includegluco-, galacto-, and lactosylceramides composed of sphingosine with N-acyl mixtures of saturated, unsaturated and hydroxylated fatty acids of predominantly 16 carbon (palmitate) and 24 carbon (lignocerate) lengths. Similar structures are found in neutral glycosphinolipids of heart muscle and it has been proposed that this mimicry may contribute to pathogenesis associated with chronic Chagas disease 52.
As in Leishmania, the role of IPC in T. cruzi has been investigated using the fungal IPCS inhibitor AbA. High concentrations of AbA (~20 μM) inhibited in vitro differentiation of amastigotes 50 and the generation of culture-derived trypanosomatids 53. However, it is has no effect on growth of epimastigotes, even at excessive concentration (~50 μM), and likewise is completely ineffective against IPC synthase in a direct enzymatic assay with epimastigote membranes 53. These findings strongly suggest that any inhibition of trypomastigotes/amastigote differentiation is likely due to secondary off-target effects, and furthermore that there are considerable differences between fungal and kinetoplastid IPC synthases.
Unlike Leishmania and the African trypanosomes, IPC is also used as a membrane anchor for two abundant cell surface glycoconjugates: lipopeptidophosphoglycan (LPPG) and the mucin-like glycoproteins. First characterized in stationary phase epimastigotes, LPPG is a free glycosylphosphatidylinositol (GPI)-like molecule unusual in being constructed on IPC rather than phosphatidylinositol, as is typical for GPI structures 54–57. Strikingly, mucins from metacyclic trypomastigotes, derived from stationary phase epimastigote cultures, are anchored by an almost identical structure, differing primarily in the absence of two β-linked galactofuranose moieties attached to the tetramannose core of LPPG, and the presence of the ethanolamine phosphate bridge to the mucin polypeptide chain 58. In each case the ceramide glycoinositol lipid platform is a mixture of ceramide and dihydroceramide (d18:1 and d18:0), N-acylated with either palmitate (C16:0) or lignoceric acid (C24:0). Furthermore, both structures are developmentally regulated. LPPG and mucin glycoinositol lipid anchors from log phase epimastigotes are constructed on a standard phosphatidylinositol platform, but in all other regards resemble the equivalent IPC-based structures from stationary cultures 58–60. This developmental shift in glycoinositol lipid anchor structure is not universal since 1G7-antigen, a metacyclic-specific surface marker, has a glycerolipid anchor, even as the mucins are being overwhelmingly converted to a ceramide-based anchor 61. Ceramide-based glycoinositol lipid anchors are also found on the amastigote-specific Ssp4 antigen 49 and the trans-sialidase of culture derived trypomastigotes. It seems likely that these ceramide-based structures are made by remodeling of a de novo synthesized glycerolipid GPI structure, as is known to happen in yeast 62, since no evidence could be found for de novo synthesis of GPI-like structures on an IPC platform 63. A possible remodeling pathway could be transfer of the entire glycoinositol head group from the initial glycerolipid anchor to a ceramide accepter in a reaction analogous to the synthesis of phosphosphingolipids by head group exchange with phosphoglycerol lipids. Subsequent remodeling of the N-acyl group could introduce lignoceric acid, which is not found in the general pools of ceramide and IPC, into a subset of the ceramide anchors. Several of the enzymatic activities required for ceramide fatty acid remodeling have been reported in membrane extracts of T. cruzi 50.
Trypanosomatid Sphingolipid Synthases
The trypanosomatid protozoa have an orthologous gene family of documented sphingolipid synthases 10, 32. L. major has a single gene (LmjIPCS), T. brucei has four tandemly arrayed genes (TbSLS1-4), and T. cruzi has a single gene (TcSLS). The evolutionary relatedness of these genes is confirmed by their syntenic location on the chromosomes of the three species. All the gene products, have clear homology to mammalian sphingomyelin synthases 64 by reciprocal Blast searching, but no obvious sequence relationship to yeast IPC synthase 39. Nevertheless, LmjIPCS has been biochemically characterized as a true IPC synthase 32. One of the T. brucei gene products (TbSLS4) is a bifunctional SM/EPC synthase 10, and while it is possible that one or more of the other genes (TbSLS1-3) may be an IPC synthase, this seems unlikely given the high degree of sequence identity for the predicted proteins. There is currently no evidence concerning the biosynthetic specificity of the T. cruzi enzyme. The T. brucei genes are constitutively expressed and simultaneous knockdown of the entire locus by pan-specific RNAi is rapidly lethal for bloodstream parasites, likely due to toxic effects of a 4–5 fold increase in cellular levels of ceramide. No data are currently available for the essentiality of the T. cruzi ortholog. One might predict from the viability of the L. majorde novo SL synthetic mutant spt2- that Leishmania IPCS would not be essential; this is under investigation currently.
All members of the gene family contain the catalytic residues and four signature sequence motifs (D1–4) defined for mammalian SM synthases 65. Two of these motifs (D1–2) are absent from yeast IPCS, further emphasizing the lack of direct relatedness with the kinetoplastid enzymes. All of the parasite sphingolipid synthases conform to a six transmembrane topology model predicted for mammalian SM synthase 2 10. Collectively these data suggest that the kinetoplastid sphingolipid synthase family evolved from a common ancestral SM synthase gene, and that subsequently the Leishmania gene diverged to encode an IPC synthase activity. If true this implies that IPC synthetic capability has evolved at least twice in eukaryotes, once in the kinetoplastids and once in a fungal lineage.
Plasmodium falciparum
Malaria parasites of the genus Plasmodium are obligate intracellular pathogens residing in mammalian red blood cells, hepatocytes, or mosquito midgut epithelial cells. Biosynthesis of sphingomyelin (SM) and glycosphingolipids by P. falciparum parasites (intraerythrocytic stages) has been demonstrated using radiolabeled or fluorescent ceramides 16, 66. A recent report revealed the presence of an active glucosylceramide synthase, which glycosylates only dihydroceramide in vitro 67. Species of glycosphingolipids in P. falciparum include monohexosylceramides, globotriaosylceramides, globotetraosyl-ceramides, and sulfated glycosphingolipids 17, 67. No inositol SLs have been described in P. falciparum parasites.
Biosynthesis of SLs can be inhibited with low concentrations of threo-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP) or its derivative threo-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), potent inhibitors of glucosylceramide synthase and/or sphingomyelin synthase 67. Interestingly, PPMP disrupts tubovesicular membrane (TVM), an interconnected network that extends from the parasite’s vacuolar membrane to the periphery of the red cell. TVM is essential for the uptake of nutrients and the treatment of PPMP prevents the parasite from importing vital nutrients such as amino acids. Consequently, the intraerythrocytic development of P. falciparum is severely inhibited 68, 69. The link between SL biosynthesis and TVM formation is not well understood, although the traffic of nutrients appears to be dependent upon detergent resistant membrane (DRM) domains in TVM, which are SL- and cholesterol-rich 70.
Another interesting finding is that de novo ceramide synthesis is not required for TVM homeostasis; instead, ceramide derived from the turnover of host SLs are likely to be the substrate for SL biosynthesis in P. falciparum 16. A recently cloned PfSMase, the enzyme responsible for SM hydrolysis to ceramide and phosphocholine, was found to be essential for the parasite, and its inhibition impaired the maturation of P. falciparum trophozoites into schizonts 71. Together, these findings suggest SL metabolism could provide targets for new antimalarial drugs. Indeed, ceramide analogs including PPMP and PDMP have shown potent anti- P. falciparum activity in vitro, although their mechanism of action is still under investigation 72, 73.
Toxoplasma gondii
Toxoplasma gondii is the most versatile and widespread member of the Apicomplexan family. It is capable of infecting a wide array of host cells, causing a disease (toxoplasmosis) of major medical and veterinary importance worldwide. De novo synthesis of ceramide, glycosylated ceramide and SM in T. gondii has been demonstrated by tritiated serine and galactose labeling, followed by thin layer chromatography analysis 18. Two well-documented SL synthesis inhibitors, L-cycloserine (which blocks the activity of SPT) and threo-PPMP, strongly inhibit the synthesis of these SLs. AbA treatment inhibits T. gondii replication irreversibly and induces morphological changes in the parasite cell shape and integrity, with increased cell vacuolization 5. Importantly, analyses of parasite lipids did reveal an inositol-containing species that was resistant to alkaline treatment and whose synthesis was inhibited by AbA treatment – all properties of IPC. It is not yet clear whether IPC synthesis is required for parasite replication and more definitive characterizations of T. gondii SLs and enzymes are underway.
Trichomonas vaginalisand Giardia lamblia
Trichomonas vaginalis is an extracellular aerotolerant flagellated protozoan responsible for trichomoniasis, a common sexually transmitted disease in industrialized countries. Characterized SLs in T. vaginalis include SM and IPC 19, 21. Importantly, IPC anchors a group of lipophosphoglycan (LPG)-like glycoconjugates to the cell surface of T. vaginalis 74. The detailed polysaccharide structure of T. vaginalis LPG is still unknown, but it contains 50 to 54 monosaccharide residues with galactose and glucosamine being the most common species. To study the role of LPG in T. vaginalis, two LPG mutants were recently generated by chemical mutagenesis 75. Both mutants had truncated form of LPG and failed to bind Ricinnus comunis agglutinin I (RCA120) and wheat germ agglutinin, indicating alterations in surface galactose and glucosamine residues. Although mutants grew normally in culture, they were less adherent and less toxic to human vaginal ectocervical cells than the parental strain. Together these results suggest that T. vaginalis adherence to host cells is mediated by LPG.
To further explore the contribution of IPC-anchored LPG to T. vaginalis pathogenesis, the effect of purified LPG on human reproductive tractl epithelial cells was tested 76. Briefly, T. vaginalis LPG triggered an increased expression of interleukin 8 (IL-8) by human endocervical, ectocervical, and vaginal epithelial cells. IL-8 promotes the transmigration of neutrophils across the endothelium and the production of macrophage inflammatory protein 3α, a chemoattractant for immune cells. Such effects were dose dependent and sustained in the absence of cytotoxicity, suggesting that early involvement of chemokine production by epithelial cells induced by nontoxic doses of LPG precedes cytotoxic effects.
Similar to T. vaginalis, Giardia lamblia is a flagellated protozoan parasite. This aerotolerant anaerobe colonizes and reproduces in human small intestine, causing giardiasis, a common cause of severe diarrhea infecting approximately 200 million people worldwide. It is believed that G. lamblia has only limited ability to synthesize phospholipids, long-chain fatty acids, and sterols de novo; therefore, this protozoan has developed a special process to acquire lipids from the lipid-rich environment of the human small intestine, i.e. via deacylation/reacylation reactions (the Lands cycle) 77, 78. It is known that G. lamblia synthesizes SM 22, 79, although more detailed structure characterization has yet to be performed.
A recent report indicates that PPMP blocks Giardia replication in vitro and causes a cytokinesis arrest 80. In addition, PPMP induced a significant (~90%) reduction in G. lamblia differentiation into cysts, the parasite stage responsible for the transmission of the disease. In another study, the giardial ceramide glucosyltransferase 1 gene (gglct-1) was transcribed only in encysting cells and the treatment of PPMP altered the expression of cyst wall protein transcripts in encysting cells 23. These combined data suggest that SL synthesis genes are involved in key events in giardial pathobiology. However, effect of PPMP and other SL-synthesis inhibitors on the lipid metabolism of G. lamblia has yet to be elucidated.
Concluding Remarks
Our understanding of SL structure and function in pathogenic protozoans is still in its infancy.
Current data suggest SL metabolites play important roles in multiple aspects of cellular life including differentiation, replication, trafficking, and the synthesis of virulence factors.
SLs can be synthesized de novo and via salvage pathways.
Future directions include: 1) to determine the composition of SL species in pathogenic protozoans; 2) to identify and characterize key enzymes involved in SL metabolism; 3) to define the molecular basis by which SLs contribute to infections; and 4) to develop specific inhibitors of SL metabolism and evaluate their potential as novel drugs against parasites.
Acknowledgments
Supported by United States Public Health Service Grants R01AI031078 (S.M.B.), R01-AI35739 (J.D.B.), R15AI076909 (K.Z.), and the Texas Tech University Office of Vice President for Research (K.Z.). We thank Drs. Fong-Fu Hsu and John Turk of the Dept of Medicine at Washington University School of Medicine for their invaluable assistance in the mass spectrometric analysis of trypanosomatid sphingolipids, which were supported by USPHS grants P41-RR00954, P60-DK20579 and P30-DK56341 to their laboratories.
Contributor Information
Kai Zhang, Email: kai.zhang@ttu.edu.
James D. Bangs, Email: jdbangs@wisc.edu.
Stephen M. Beverley, Email: beverley@borcim.wustl.edu.
References
- 1.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Simarro PP, Jannin J, Cattand P. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 2008;5:e55. doi: 10.1371/journal.pmed.0050055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham AC. Parasitic adaptive mechanisms in infection by Leishmania. Exp Mol Pathol. 2002;72:132–141. doi: 10.1006/exmp.2002.2418. [DOI] [PubMed] [Google Scholar]
- 4.Croft SL, Coombs GH. Leishmaniasis--current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Sonda S, Sala G, Ghidoni R, Hemphill A, Pieters J. Inhibitory effect of aureobasidin A on Toxoplasma gondii. Antimicrob Agents Chemother. 2005;49:1794–1801. doi: 10.1128/AAC.49.5.1794-1801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K, Showalter M, Revollo J, Hsu FF, Turk J, Beverley SM. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 2003;22:6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu FF, Turk J, Zhang K, Beverley SM. Characterization of inositol phosphorylceramides from Leishmania major by tandem mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2007;18:1591–1604. doi: 10.1016/j.jasms.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guther ML, Lee S, Tetley L, Acosta-Serrano A, Ferguson MA. GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, and are not the only components of the surface coat. Mol Biol Cell. 2006;17:5265–5274. doi: 10.1091/mbc.E06-08-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridberg A, Olson CL, Nakayasu ES, Tyler KM, Almeida IC, Engman DM. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J Cell Sci. 2008;121:522–535. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 10.Sutterwala SS, Hsu FF, Sevova ES, et al. Developmentally regulated sphingolipid synthesis in African trypanosomes. Mol Microbiol. 2008;70:281–296. doi: 10.1111/j.1365-2958.2008.06393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik PK, Field MC, Menon AK, Cross GA, Yee MC, Butikofer P. Molecular species analysis of phospholipids from Trypanosoma brucei bloodstream and procyclic forms. Mol Biochem Parasitol. 1993;58:97–105. doi: 10.1016/0166-6851(93)90094-e. [DOI] [PubMed] [Google Scholar]
- 12.Uemura A, Watarai S, Kushi Y, Kasama T, Ohnishi Y, Kodama H. Analysis of neutral glycosphingolipids from Trypanosoma brucei. Vet Parasitol. 2006;140:264–272. doi: 10.1016/j.vetpar.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Bertello LE, Goncalvez MF, Colli W, de Lederkremer RM. Structural analysis of inositol phospholipids from Trypanosoma cruzi epimastigote forms. Biochem J. 1995;310 (Pt 1):255–261. doi: 10.1042/bj3100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhrig ML, Couto AS, Colli W, de Lederkremer RM. Characterization of inositolphospholipids in Trypanosoma cruzi trypomastigote forms. Biochim Biophys Acta. 1996;1300:233–239. doi: 10.1016/0005-2760(96)00021-5. [DOI] [PubMed] [Google Scholar]
- 15.Quinones W, Urbina JA, Dubourdieu M, Luis Concepcion J. The glycosome membrane of Trypanosoma cruzi epimastigotes: protein and lipid composition. Exp Parasitol. 2004;106:135–149. doi: 10.1016/j.exppara.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Gerold P, Schwarz RT. Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 2001;112:29–37. doi: 10.1016/s0166-6851(00)00336-4. [DOI] [PubMed] [Google Scholar]
- 17.Landoni M, Duschak VG, Peres VJ, et al. Plasmodium falciparum biosynthesizes sulfoglycosphingolipids. Mol Biochem Parasitol. 2007;154:22–29. doi: 10.1016/j.molbiopara.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Azzouz N, Rauscher B, Gerold P, Cesbron-Delauw MF, Dubremetz JF, Schwarz RT. Evidence for de novo sphingolipid biosynthesis in Toxoplasma gondii. Int J Parasitol. 2002;32:677–684. doi: 10.1016/s0020-7519(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 19.Costello CE, Glushka J, van Halbeek H, Singh BN. Structural characterization of novel inositol phosphosphingolipids of Tritrichomonas foetus and Trichomonas vaginalis. Glycobiology. 1993;3:261–269. doi: 10.1093/glycob/3.3.261. [DOI] [PubMed] [Google Scholar]
- 20.Singh BN, Costello CE, Beach DH. Structures of glycophosphosphingolipids of Tritrichomonas foetus: a novel glycophosphosphingolipid. Arch Biochem Biophys. 1991;286:409–418. doi: 10.1016/0003-9861(91)90059-r. [DOI] [PubMed] [Google Scholar]
- 21.Beach DH, Holz GG, Jr, Singh BN, Lindmark DG. Phospholipid metabolism of cultured Trichomonas vaginalis and Tritrichomonas foetus. Mol Biochem Parasitol. 1991;44:97–108. doi: 10.1016/0166-6851(91)90225-u. [DOI] [PubMed] [Google Scholar]
- 22.Kaneda Y, Goutsu T. Lipid analysis of Giardia lamblia and its culture medium. Ann Trop Med Parasitol. 1988;82:83–90. doi: 10.1080/00034983.1988.11812213. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez Y, Shpak M, Duarte TT, et al. Novel role of sphingolipid synthesis genes in regulating giardial encystation. Infect Immun. 2008;76:2939–2949. doi: 10.1128/IAI.00116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes AP, Nelson K, Beverley SM. Evolution of nuclear ribosomal RNAs in kinetoplastid protozoa: perspectives on the age and origins of parasitism. Proc Natl Acad Sci USA. 1993;90:11608–11612. doi: 10.1073/pnas.90.24.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- 26.Kaneshiro ES, Jayasimhulu K, Lester RL. Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J Lipid Res. 1986;27:1294–1303. [PubMed] [Google Scholar]
- 27.Denny PW, Goulding D, Ferguson MA, Smith DF. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol. 2004;52:313–327. doi: 10.1111/j.1365-2958.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- 28.McConville MJ, Blackwell JM. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 29.Winter G, Fuchs M, McConville MJ, Stierhof YD, Overath P. Surface antigens of Leishmania mexicana amastigotes: characterization of glycoinositol phospholipids and a macrophage-derived glycosphingolipid. J Cell Sci. 1994;107 (Pt 9):2471–2482. doi: 10.1242/jcs.107.9.2471. [DOI] [PubMed] [Google Scholar]
- 30.Schneider P, Rosat JP, Ransijn A, Ferguson MA, McConville MJ. Characterization of glycoinositol phospholipids in the amastigote stage of the protozoan parasite Leishmania major. Biochem J. 1993;295:555–564. doi: 10.1042/bj2950555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivens AC, Peacock CS, Worthey EA, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denny PW, Shams-Eldin H, Price HP, Smith DF, Schwarz RT. The protozoan inositol phosphorylceramide synthase: a novel drug target that defines a new class of sphingolipid synthase. J Biol Chem. 2006;281:28200–28209. doi: 10.1074/jbc.M600796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, Pompey JM, Hsu FF, et al. Redirection of sphingolipid metabolism toward de novo synthesis of ethanolamine in Leishmania. EMBO J. 2007;26:1094–1104. doi: 10.1038/sj.emboj.7601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, Hsu FF, Scott DA, Docampo R, Turk J, Beverley SM. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005;55:1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh S, Bhattacharyya S, Das S, et al. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasitic survival. Mol Cell Biochem. 2001;223:47–60. doi: 10.1023/a:1017996609928. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh S, Bhattacharyya S, Sirkar M, et al. Leishmania donovani suppresses activated protein 1 and NF-kappaB activation in host macrophages via ceramide generation: involvement of extracellular signal-regulated kinase. Infect Immun. 2002;70:6828–6838. doi: 10.1128/IAI.70.12.6828-6838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003;301:1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- 38.Ilg T, Demar M, Harbecke D. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J Biol Chem. 2001;276:4988–4997. doi: 10.1074/jbc.M008030200. [DOI] [PubMed] [Google Scholar]
- 39.Nagiec MM, Nagiec EE, Baltisberger JA, Wells GB, Lester RL, Dickson RC. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J Biol Chem. 1997;272:9809–9817. doi: 10.1074/jbc.272.15.9809. [DOI] [PubMed] [Google Scholar]
- 40.Takesako K, Ikai K, Haruna F, et al. Aureobasidins, new antifungal antibiotics: taxonomy, fermentation, isolation, and properties. Journal of Antibiotics. 1991;44:919–924. doi: 10.7164/antibiotics.44.919. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka AK, Valero VB, Takahashi HK, Straus AH. Inhibition of Leishmania (Leishmania) amazonensis growth and infectivity by aureobasidin A. J Antimicrob Chemother. 2007;59:487–492. doi: 10.1093/jac/dkl518. [DOI] [PubMed] [Google Scholar]
- 42.Godfrey DG. Phospholipids of Trypanosoma lewisi, T. vivax, T. congolense, and T. brucei. Experimental Parasitology. 1967;20:106–118. doi: 10.1016/0014-4894(67)90028-8. [DOI] [PubMed] [Google Scholar]
- 43.Fridberg A, Olsen CL, Nakayasu ES, Tyler KM, Almeida IC, Engman DM. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. Journal of Cell Science. 2008;121:522–535. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 44.Sutterwala SS, Creswell CH, Sanyal S, Menon AK, Bangs JD. De novo sphingolipid synthesis is essential for viability, but not transport of glycosylphosphatidylinositol-anchored proteins in African trypanosomes. Eukaryotic Cell. 2007;6:454–464. doi: 10.1128/EC.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Da Silveira JF, Colli W. Chemical composition of the plasma membrane from epimastigote forms of Trypanosoma cruzi. Biochimica et Biophysica Acta. 1981;644:341–350. doi: 10.1016/0005-2736(81)90392-8. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira MM, Timm SL, Costa SCG. Lipid composition of Trypanosoma cruzi. Comp Biochem Physiol. 1977;58B:195–199. doi: 10.1016/0305-0491(77)90109-2. [DOI] [PubMed] [Google Scholar]
- 47.Quiñones W, Urbina JA, Dubourdieu M, Concepción JL. The glycosome membrane of Trypansoma cruzi epimastigotes: protein and lipid composition. Experimental Parasitology. 2004;106:135–149. doi: 10.1016/j.exppara.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Bertello L, Goncalvez MF, Colli W, de Lederkremer RM. Structural analysis of inositol phospholipids fromTrypanosoma cruzi epimastigote forms. Biochemical Journal. 1995;310:255–261. doi: 10.1042/bj3100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertello LE, Andrews NW, Lederkremer RM. Developmentally regulated expression of ceramide in Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1996:143–151. doi: 10.1016/0166-6851(96)02645-x. [DOI] [PubMed] [Google Scholar]
- 50.Salto ML, Bertello LE, Vieira M, Docampo R, Moreno SN, de Lederkremer RM. Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryot Cell. 2003;2:756–768. doi: 10.1128/EC.2.4.756-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barreto-Bergter E, Vermelho AB, Hartmann R, Pohlentz G, Klein RA, Egge H. Structural characterization of neutral glycosphingolipids from Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1992;51:263–270. doi: 10.1016/0166-6851(92)90076-v. [DOI] [PubMed] [Google Scholar]
- 52.Vermelho AB, Meirelles MNL, Pereira MC, Pohlentz G, Barreto-Bergter E. Heart muscle cells share common neutral glycosphingolipids with Trypanosoma cruzi. Acta Tropica. 1997;64:131–143. doi: 10.1016/s0001-706x(96)00627-4. [DOI] [PubMed] [Google Scholar]
- 53.Figueiredo JM, Dias WB, Mendonca-Previato L, Previato JO, Heise N. Characterization of the inositol phosphorylceramide synthase activity from Trypanosoma cruzi. Biochem J. 2005;387:519–529. doi: 10.1042/BJ20041842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lederkremer RM, Casal OL, Tanaka CT, Colli W. Ceramide and inositol content of the lipopeptidophosphoglycan from Trypanosoma cruzi. Biochemical Biophysical Research Communications. 1978;85:1268–1274. doi: 10.1016/0006-291x(78)91140-3. [DOI] [PubMed] [Google Scholar]
- 55.Lederkremer RM, Lima C, Ramirez MI, Casal OL. Structural features of the lipopeptidophosphoglycan from Trypanosoma cruzi common with the glycophosphatidylinositol anchors. European Journal of Biochemistry. 1990;192:337–345. doi: 10.1111/j.1432-1033.1990.tb19232.x. [DOI] [PubMed] [Google Scholar]
- 56.Lederkremer RM, Lima C, Ramirez MI, Ferguson MAJ, Homans SW, Thomas-Oates J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi epimastigotes. Journal of Biological Chemistry. 1991;266:23670–23675. [PubMed] [Google Scholar]
- 57.Previato JO, Gorin PAJ, Mazurek M, et al. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. Journal of Biological Chemistry. 1990;265:2518–2526. [PubMed] [Google Scholar]
- 58.Acosta-Serrano A, Schenkman S, NY, Mehlert A, Richardson JM, Ferguson MAJ. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. Journal of Biological Chemistry. 1995;270:27244–27253. doi: 10.1074/jbc.270.45.27244. [DOI] [PubMed] [Google Scholar]
- 59.Lederkremer RM, Lima CE, Ramirez MI, Goncalvez MF, Colli W. Hexadecylpalmitoylglycerol or ceramide is linked to similar glycophosphoinositol anchor-like structures in Trypanosoma cruzi. European Journal of Biochemistry. 1993;218:929–936. doi: 10.1111/j.1432-1033.1993.tb18449.x. [DOI] [PubMed] [Google Scholar]
- 60.Previato JO, Jones C, Xavier MT, et al. Structural characterization of the major glycosylphosphatidylinositol membrane-anchored glycoprotein form epimastigote forms of Trypanosoma cruzi Y-strain. Journal of Biological Chemistry. 1995;270:7241–7250. doi: 10.1074/jbc.270.13.7241. [DOI] [PubMed] [Google Scholar]
- 61.Heise N, Cardoso de Almeida ML, Ferguson MAJ. Characterization of the lipid moiety of the glycosylphosphatidyl inositol anchor of Trypanosoma cruzi 1G7-antigen. Molecular and Biochemical Parasitology. 1995;70:71–84. doi: 10.1016/0166-6851(95)00009-p. [DOI] [PubMed] [Google Scholar]
- 62.Pittet M, Conzelman A. Biosynthesis and function of GPI proteins in the yeast Saccharomyces cerevisiae. Biochimica et Biophysica Acta. 2007;1771:405–420. doi: 10.1016/j.bbalip.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Bertello LE, Alves MJ, Colli W, Lederkremer RM. Inositolphosphoceramide is not a substrate for the first steps in the biosynthesis of glycoinositolphospholipids in Trypanosoma cruzi. Molecular and Biochemical Parasitology. 2004;133:71–80. doi: 10.1016/j.molbiopara.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. Journal of Biological Chemistry. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 65.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. European Molecular Biology Organization Journal. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haldar K, Uyetake L, Ghori N, Elmendorf HG, Li WL. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1991;49:143–156. doi: 10.1016/0166-6851(91)90137-u. [DOI] [PubMed] [Google Scholar]
- 67.Couto AS, Caffaro C, Uhrig ML, et al. Glycosphingolipids in Plasmodium falciparum. Presence of an active glucosylceramide synthase. Eur J Biochem. 2004;271:2204–2214. doi: 10.1111/j.1432-1033.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 68.Lauer SA, Ghori N, Haldar K. Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proc Natl Acad Sci U S A. 1995;92:9181–9185. doi: 10.1073/pnas.92.20.9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lauer SA, Rathod PK, Ghori N, Haldar K. A membrane network for nutrient import in red cells infected with the malaria parasite. Science. 1997;276:1122–1125. doi: 10.1126/science.276.5315.1122. [DOI] [PubMed] [Google Scholar]
- 70.Lauer S, VanWye J, Harrison T, et al. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. Embo J. 2000;19:3556–3564. doi: 10.1093/emboj/19.14.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanada K, Palacpac NM, Magistrado PA, et al. Plasmodium falciparum phospholipase C hydrolyzing sphingomyelin and lysocholinephospholipids is a possible target for malaria chemotherapy. J Exp Med. 2002;195:23–34. doi: 10.1084/jem.20010724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pankova-Kholmyansky I, Flescher E. Potential new antimalarial chemotherapeutics based on sphingolipid metabolism. Chemotherapy. 2006;52:205–209. doi: 10.1159/000093037. [DOI] [PubMed] [Google Scholar]
- 73.Labaied M, Dagan A, Dellinger M, et al. Anti-Plasmodium activity of ceramide analogs. Malar J. 2004;3:49. doi: 10.1186/1475-2875-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh BN, Beach DH, Lindmark DG, Costello CE. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch Biochem Biophys. 1994;309:273–280. doi: 10.1006/abbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- 75.Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot Cell. 2005;4:1951–1958. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fichorova RN, Trifonova RT, Gilbert RO, et al. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun. 2006;74:5773–5779. doi: 10.1128/IAI.00631-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson GR, Ramirez D, Maier J, Castillo C, Das S. Giardia lamblia: incorporation of free and conjugated fatty acids into glycerol-based phospholipids. Exp Parasitol. 1999;92:1–11. doi: 10.1006/expr.1999.4389. [DOI] [PubMed] [Google Scholar]
- 78.Das S, Castillo C, Stevens T. Phospholipid remodeling/generation in Giardia: the role of the Lands cycle. Trends Parasitol. 2001;17:316–319. doi: 10.1016/s1471-4922(01)01901-8. [DOI] [PubMed] [Google Scholar]
- 79.Mohareb EW, Rogers EJ, Weiner EJ, Bruce JI. Giardia lamblia: phospholipid analysis of human isolates. Ann Trop Med Parasitol. 1991;85:591–597. doi: 10.1080/00034983.1991.11812614. [DOI] [PubMed] [Google Scholar]
- 80.Sonda S, Stefanic S, Hehl AB. A sphingolipid inhibitor induces a cytokinesis arrest and blocks stage differentiation in Giardia lamblia. Antimicrob Agents Chemother. 2008;52:563–569. doi: 10.1128/AAC.01105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]