Abstract
Purpose
To evaluate quantitative cerebral blood flow (qCBF) with traditional time-based measurements or metrics of cerebral perfusion: time to peak (Tmax) and mean transit time (MTT) in stroke patients.
Materials and Methods
Nine ischemic stroke patients (4 Male, 5 Female, 63±16 years old) were included in the study which was HIPAA compliant and institutional review board approved. Cerebral perfusion was quantified using the Bookend method. Mean values of qCBF, Tmax, and MTT were determined in regions of interest (ROIs). ROIs were drawn on diffusion weighted images in diffusion positive, critically ischemic (CI), in ipsilateral normal region immediately surrounding the critically ischemic region, the presumed penumbra (PP), and in contralateral diffusion negative control, presumed normal region (PN) of gray and white matter separately (GM and WM).
Results
In both GM and WM, qCBF measures distinguished the studied brain regions with the most markedly reduced values in regions corresponding to extent of likely ischemic injury. In planned comparisons, only qCBF measurements differed significantly between CI and PP tissues. ROC analysis supported the utility of qCBF for discriminating brain regions differing in the likely extent of ischemic injury (CI and PN regions – qCBF: AUC=0.96, Tmax: AUC=0.96, MTT: AUC=0.72). Importantly, qCBF afforded the best discrimination of CI and PP regions (qCBF: AUC=0.82, Tmax: AUC=0.65, MTT: AUC=0.52).
Conclusions
This initial evaluation indicates that quantitative MRI perfusion is feasible in ischemic stroke patients. qCBF derived with this strategy provide enhanced discrimination of CI and PP compared to time-based imaging metrics. This approach merits investigation in larger clinical studies.
Keywords: MR quantification, cerebral blood flow, ischemic stroke
INTRODUCTION
Stroke is the leading cause of disability and the third leading cause of death in the United States (1). A critical predictor of stroke severity and potential efficacy of reperfusion therapy is the degree to which cerebral blood flow (CBF) is compromised. The goal of reperfusion therapy is restoration of normal CBF in hypoperfused yet viable brain tissue (ischemic penumbra) (2–7). Currently, thrombolytic therapy for acute ischemic stroke is restricted to a three hour window from the onset of symptoms due to the potential complication of cerebral hemorrhage (8). Hemorrhage must be ruled out and ischemia must be demonstrated by imaging with CT and/or MRI before thrombolytics can be administered. Recent studies suggest a potential benefit within up to six hours after onset of stroke (9). The ability to determine ischemic penumbra may allow clinicians to optimize therapy while decreasing potential complications of therapy past the three hour window. The combination of diffusion weighted imaging (DWI) and perfusion weighted imaging (PWI) provides an imaging marker of the presence and extent of the ischemic penumbra (10–14). Current MRI perfusion images provide only relative values of blood flow or transit time. Despite these shortcomings, the DWI/PWI mismatch is used to select patients for treatment in large clinical trials of thrombolytic agents (15–16). Time-based indicators of tissue status such as time to peak (Tmax) and mean transit time (MTT) tend to overestimate the infarcted region, thereby representing a ‘worst case scenario’ (17) as they are sensitive indicators for ischemia. Clinically, these measurements may systematically underestimate the presence and extent of brain tissue potentially salvageable by reperfusion therapy; these measurements cannot be used to distinguish infarct from ischemia. Quantification of cerebral perfusion with MRI has been identified as a priority by the American Heart and Stroke Association (18).
A novel approach to quantify CBF using dynamic susceptibility MRI (DSC-MRI) has been reported (19–20). This approach, the ‘Bookend’ technique, is based on a mathematical model that includes parenchymal T1 changes in the fast water exchange limit and first-pass contrast kinetics yielding qCBF images. The Bookend technique, which does not rely on population averaged CBF or empirical correction factors, affords more direct measurement of CBF for an individual patient (19–20). Quantification of CBF with the Bookend technique has been shown to be reproducible (21), accurate (22), and reliable (21) in patient studies. The potential of a direct quantitative measurement of CBF in the ischemic stroke setting would provide a powerful aid in the management of patients.
This investigation examined the accuracy of qCBF in distinguishing normally perfused from critically ischemic tissue in ischemic stroke patients. qCBF images were compared with traditional time-based metrics of cerebral perfusion: Tmax and MTT. We hypothesized that qCBF is more accurate at distinguishing normally perfused from critically ischemic tissue than either Tmax or MTT (23). The goal of this work was to technically evaluate a means of quantifying CBF in the stroke setting.
MATERIALS AND METHODS
This HIPAA (Health Insurance Portability and Accountability Act) compliant study was approved by our institutional review board.
Patient Selection
Nine ischemic stroke patients (4 Male, 5 Female, 63±16 years old) were included in the study. Patients were scanned within seven days of symptom onset using the quantitative MRI perfusion protocol (between September 2005 and February 2007) (Table 1). Patients who received or were eligible for reperfusion therapy, which would alter local perfusion within the region affected by the stroke, were excluded from the study. In addition, patient with auto recanalization were identified by diffusion abnormalities co localized with regions of normal or hyper perfusion relative to the contralateral anatomy. These subjects were excluded from the study as they represent an unstable perfusion abnormality. Patients were also excluded based on the presence of posterior fossa or lacunar infarcts or other associated neurological disorders (e.g. subarachnoid or intracerebral hemorrhage).
Table 1.
Patient Demographics
| Patient | Sex | Age | Age of Stroke | Vascular Distribution |
|---|---|---|---|---|
| 2 | F | 88 | < 7 days | LMCA |
| 9 | M | 62 | 7.5 hrs | LMCA |
| 11 | F | 31 | 5.5 hrs | LMCA |
| 12 | M | 63 | 7 days | RMCA |
| 16 | M | 61 | 3–6 hrs | RMCA |
| 32 | F | 62 | 5 hrs | RMCA |
| 50 | F | 80 | < 7 days | LMCA |
| 55 | M | 62 | 1 hr 55 min | LMCA |
| 63 | F | 55 | 2 hrs | LMCA |
Image Acquisition
The imaging protocol included T1, T2, FLAIR, TOF MRA, DWI, and Bookend CBF scans. Diffusion weighted images were used to define regions of critically ischemic tissue (DWI – Acquisition Plane=Axial, TR/TE=3000 ms/75 ms, 256×256 matrix, 250 mm field of view, 5 mm thickness, 1.5 mm skip, 24 slices, b=0, 500,1000 mm −2, for a total 72 images). The Bookend imaging protocol requires three consecutive pulse sequences to be run: (1) a pre-contrast Look-Locker EPI scan (LL-EPI), a gradient recalled EPI (GRE-EPI) DSC perfusion scan, and (3) post-contrast LL-EPI scan. The T1-changes seen in the LL-EPI scans, which result from the residual systemic T1-shortening contrast agent, provide values for perfusion quantification. The parameters of pre- and post- contrast LL-EPI sequences were identical: nonselective inversion pulse, followed by 2D readout, 20° flip angle, TR=21 ms, TE=9.9 ms, TI=15 ms, 128×128 matrix, 220 mm field of view, 21 lines in k-spaces per acquisition, a single 5 mm thick slice, 120 time points, intra-scan delay=2000 ms, total scan time=45 s. The LL-EPI sequence was designed without regard to scanner specific parameters and falls within specifications of all of the scanners used in the data accrual (1.5 T, Avanto, Sonata, Symphony, Siemens Medical Solutions, Erlangen, Germany). This sequence was designed with 4400 ms between inversion pulses to allow full regrowth of magnetization. The pre-contrast LL-EPI scan was followed by a DSC perfusion scan (single shot, GRE-EPI, TR/TE=1500/40 ms, 128×128 matrix, 220 mm field of view, 5 mm thickness, 13 slices, 50 measurements). A single dose injection of gadolinium-based contrast agent (0.1 mmol/kg, Magnevist, Berlex, Princeton, NJ) was injected during PWI at a rate of 5 ml/sec by a power injector. The post-contrast LL-EPI was run immediately following the DSC perfusion scan. The slice for LL-EPI is co-registered to one of the 13 slices of the DSC perfusion images containing a major WM region at the scan prescription time.
Image Post-Processing
All post-processing and image analysis was done using commercially available software (MATLAB R2006a; Mathworks, Inc., Natick, MA). Relative cerebral blood volume (CBV), CBF, and MTT images were calculated using standard singular value decomposition (SVD) analysis (24). Tmax is calculated as the time point of maximum residue function which is only measured at intervals of repetition time yielding quantized Tmax values. A correction was approximated by adding a time delay factor determined from the acquisition time of the corresponding slice within the normal stack of 2D DSC perfusion scan slices which mitigates quantization of repetition time. Parametric qCBF images were calculated using the Bookend technique.
The Bookend technique is based on the premise that relative perfusion images (relative CBV, relative CBF) can be quantified if a proper calibration can be determined (20,25–27). The Bookend technique determines a calibration constant from post-gadolinium T1 changes (19,25,28), which are dependent on water exchange between intra- and extra-vascular spaces (29). This method includes a term which explicitly accounts for water-exchange effects with a Water Correction Factor (WCF) (20). The expression for the quantification of CBF (i.e. qCBF) is shown below:
| [Eq 1] |
| [Eq 2] |
where T1 values are measured before and after the DSC scan (1/T1,Pre, 1/T1,Post ) in the blood pool and WM, KH is hematocrit correction factor, 0.71 with hematocrit values 0.25/0.45 in the large/small vessel size of arteries, ρ is average density in brain tissue (1.04g/100ml), < CBVDSC >WM is average relative CBV value in a region of normal appearing WM selected from the DSC perfusion images, and CBFDSC are whole brain relative CBF images from the DSC analysis. Initial results in moyomoya patients have shown that qCBF values derived using the Bookend technique are highly correlated (r=0.74, p<10−3) with [15O]-H2 PET qCBF images acquired in the same patients on the same day (22).
Region of Interest Analysis
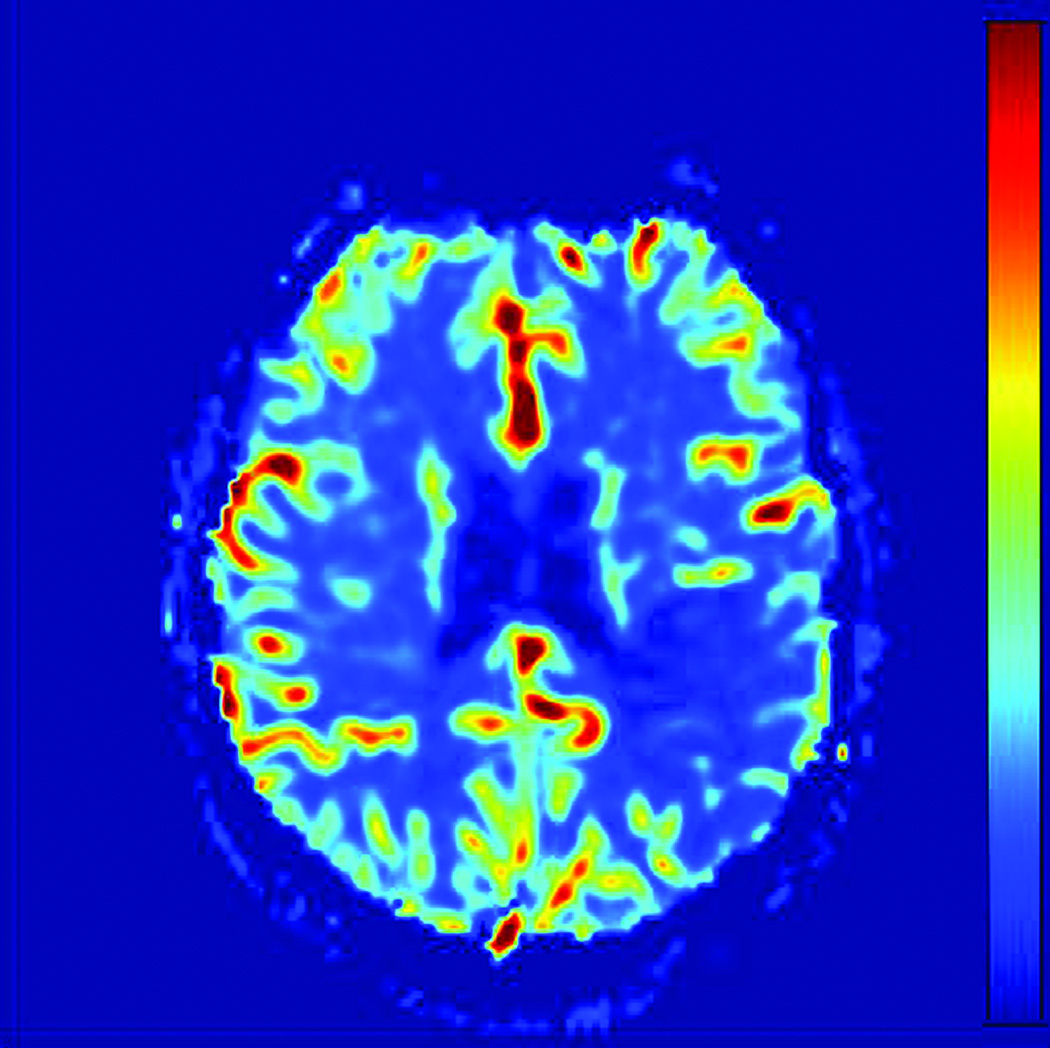
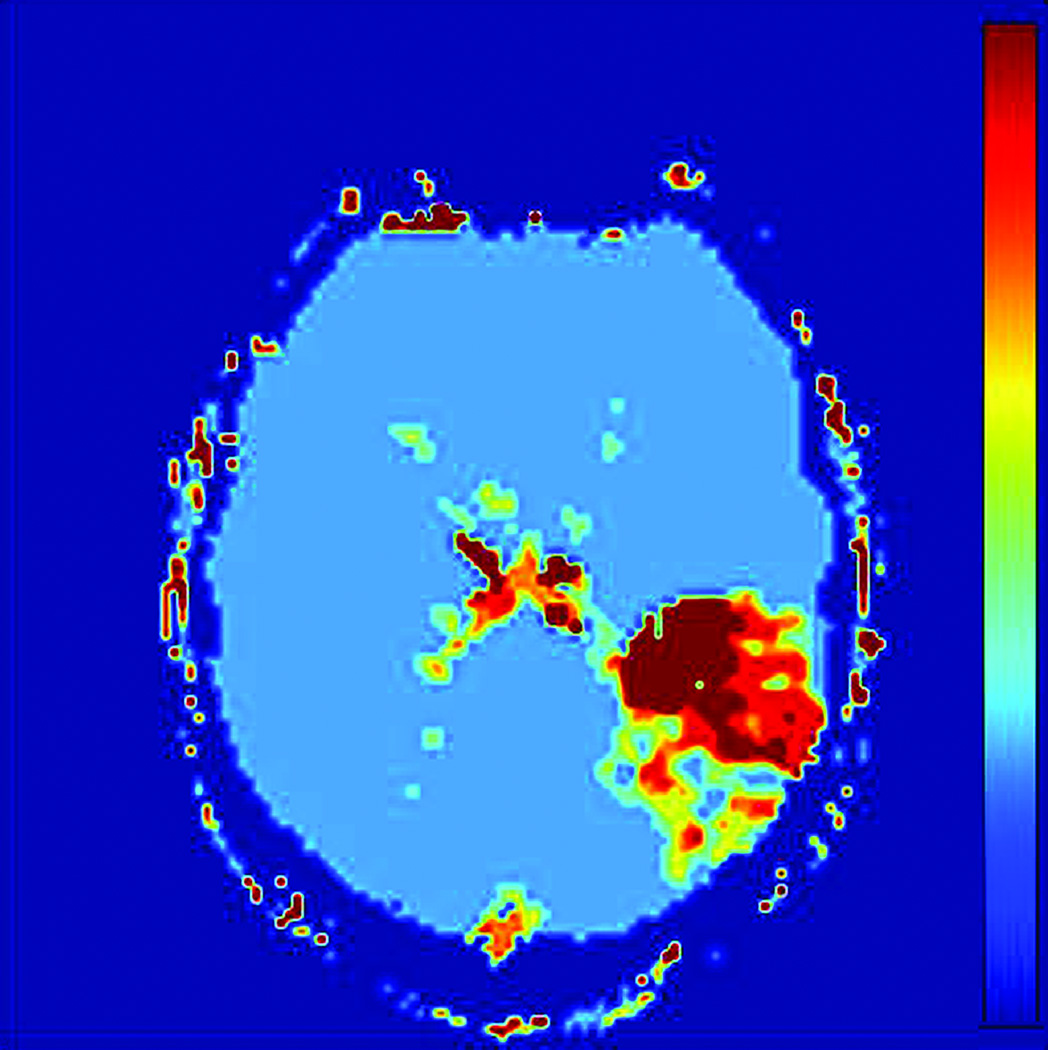
Prior to region of interest (ROI) analysis, DWI images were coregistered to the CBF images using Statistical Parametric Mapping (SPM Version 5; Wellcome Department of Cognitive Neurology, London, England). Images with high diffusion weighting (b=1000 mm −2) were reviewed by a resident physician in consensus with a board certified neuroradiologist to determine the extent of the diffusion abnormality (HB, MW). T2 weighted images (b=0 mm−2) were inspected to ensure regions of T2 shine through were avoided. Reviewers were blinded to the perfusion images when placing ROIs. ROIs were visually drawn by a resident physician (in consensus with a board certified neuroradiologist) in three separate regions corresponding to the likely extent of brain injury: (1) a diffusion positive or critically ischemic region (CI), (2) an ipsilateral normal region, approximately 2–3 cm surrounding critically ischemic region, the presumed penumbra (PP), and (3) a contralateral diffusion negative, presumed normal region (PN). The ipsilateral normal region surrounding the critically ischemic region is hypoperfused within the same vascular territory of the diffusion positive region; consequently, the region represents the presumed penumbra, tissue at risk for infarction. Serial MRIs need to be used to identify the actual penumbra as collaterals and occlusion site can vary the penumbra. However, we assumed that qCBF and Tmax would be equally affected by the ROI. ROIs were drawn in gray matter (GM) and white matter (WM) regions separately. Figure 1 shows ROI placement on a representative diffusion image and corresponding qCBF, Tmax, and MTT images.
Figure 1.
ROI Analysis Methodology: A) Diffusion (DWI) image with and without ROIs of Presumed Normal (PN), Presumed Penumbra (PP), and Critically Ischemic (CI) regions. B) DWI ROIs applied to qCBF image. C) DWI ROIs applied to Tmax image. D) DWI ROIs applied to MTT image.
Statistical Analysis
Primary measures for analysis included mean values of qCBF, Tmax, and MTT determined for the CI, PP, and PN regions in both GM and WM separately. Statistical analyses were accomplished using repeated measures analysis of variance with planned comparisons (SPSS Version 17, Chicago, IL). Receiver Operator Characteristic (ROC) curves were used to evaluate qCBF, Tmax, and MTT for discriminating tissue types (MATLAB R2006a) (30–31). For qCBF, sensitivity and specificity were calculated on a voxel-by-voxel basis for values between 0 and 120 ml/100g-min, in intervals of 2 ml/100g-min. Similarly for Tmax and MTT, sensitivity and specificity were calculated for values between 0 and 20 s, in intervals of 0.333 s. ROC curves were generated by plotting the True Positive Fraction versus the False Positive Fraction and were fitted to a binormal curve (31). The ischemic threshold was defined as the equality point between sensitivity and specificity on the ROC curve (32).
RESULTS
Table 2 presents means and standard deviations for qCBF, Tmax, and MTT for the three tissue types. PN mean values are 53.44±12.41 ml/100g-min, 2.13±0.97 s, and 4.49±0.87 s in GM and 21.50±5.18 ml/100g-min, 2.51±1.39 s, and 4.91±0.91 s in WM for qCBF, Tmax, and MTT, respectively.
Table 2.
Mean Values for Presumed Normal (PN), Critically Ischemic (CI), and Presumed penumbra (PP).
| qCBF GM (ml/100g-min) |
qCBF WM (ml/100g-min) |
Tmax GM (s) |
Tmax WM (s) |
MTT GM (s) |
MTT WM (s) |
|
|---|---|---|---|---|---|---|
| PN | 53.44±12.41 | 21.50±5.18 | 2.13±0.97 | 2.51±1.39 | 4.49±0.87 | 4.91±0.91 |
| PP | 26.24±11.981 | 14.12±0.982 | 5.46±1.751 | 7.38±0.761 | 6.04±1.292 | 5.15±2.03 |
| CI | 12.09±6.4313 | 7.27±2.9513 | 6.54±1.811 | 7.82±0.801 | 6.41.±1.812 | 5.57±3.47 |
|
F Test Region |
F(2,14) = 57.9 | F(2,8)=14.9 | F(2,14)=12.7 | F(2,8)=160.0 | F(2,14)=5.35 | NS |
|
F Test Trend |
F(1,7)=77.1 | F(1,4)=17.7 | NS | NS | NS | NS |
We have denoted CI and PP values that are significantly different than the PN values with 1 for p≤0.01 and 2 for p≤0.05 and CI values that are significantly different than PP values with 3 for p≤0.01 using paired t-tests.
For F-tests all significant results are show with p<0.01 and NS stands for not significant.
Repeated measures analysis of qCBF values in GM indicated a significant main effect for region (F(2,14)=57.9, p<0.001) with a significant linear trend in qCBF measures (F(1,7)=77.1; p<0.001). Blood flow was most markedly reduced in the CI region and highest in PN tissue. PP tissue was characterized by intermediate qCBF values (Table 2). A consistent pattern was identified for qCBF measures in WM. qCBF differed significantly across the three regions as indicated by a significant main effect for brain region (F(2,8)=14.9; p=0.002) as well as a significant linear trend (F(1,4)=17.7; p=0.01) with most marked blood flow reduction measured in the CI region, intermediate values in the PP region, and generally higher values in PN WM. While main effects of region were also observed for the other measures: Tmax (GM: F(2,14)=12.7, p=0.001 and WM: F(2,8)=160.0; p<0.001) and MTT (GM: F(2,14)=5.35, p=0.02), MTT did not distinguish blood flow in WM. Moreover, the main effects observed for Tmax and MTT were accounted for by significant differences between CI and PN tissue. Neither Tmax nor MTT values differed significantly between CI and PP regions (Table 2). Planned comparisons, accomplished with paired t-tests, indicated that qCBF values distinguished CI from PN regions in GM (t(8)=9.90; p<0.001) and WM (t(4)=4.2; p=0.01). More importantly, for both GM and WM, qCBF values measured in the PP regions differed significantly from those of both CI regions (GM: t(7)=3.41; p=0.01; WM: t(4)=5.4; p=0.006) and PN regions (GM: t(7)=10.8; p<0.001; WM: t(4)=2.7; p=0.05).
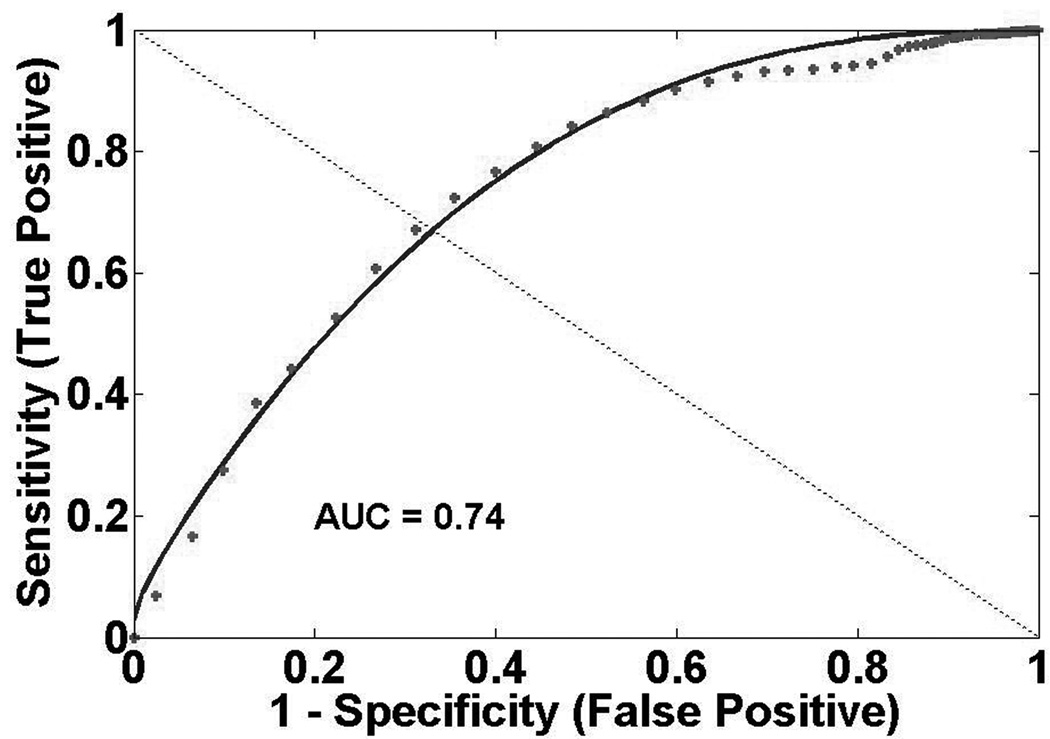
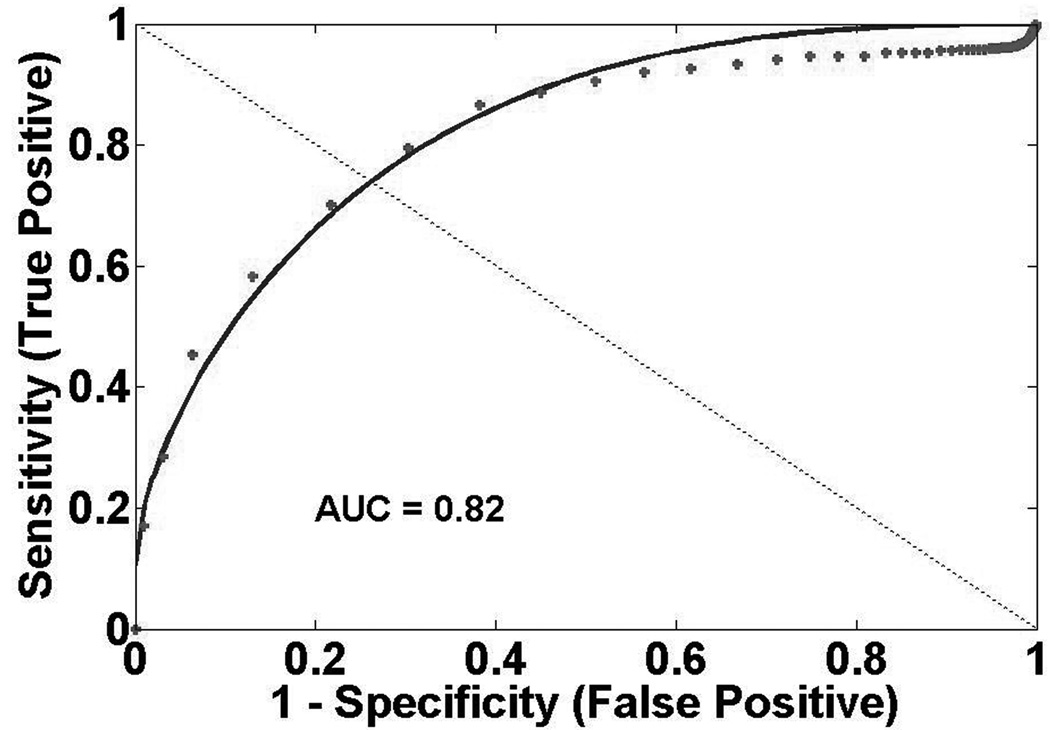
Figure 2 presents GM ROC curves. ROC analysis indicates qCBF best discriminates CI and PN regions (qCBF: AUC=0.95, Tmax: AUC=0.94, MTT: AUC=0.87). qCBF also best discriminates between CI and PP regions (qCBF: AUC=0.74, Tmax: AUC=0.64, MTT: AUC=0.61). In GM, threshold points between CI and PN regions are 26 ml/100g-min, 3.0 s, and 2.8 s for qCBF, Tmax, and MTT respectively. Threshold points between CI and PP regions are 17 ml/100g-min, 5.3 s, and 6.0 s for qCBF, Tmax, and MTT respectively. Figure 3 shows WM ROC curves. qCBF is equivalent to Tmax and better than MTT (qCBF: AUC=0.96, Tmax: AUC=0.96, MTT:AUC=0.72) at identifying critically ischemic tissue. qCBF measurements better discriminate CI and PP tissue than other measurements (qCBF: AUC=0.82, Tmax: AUC=0.65, MTT: AUC=0.52). WM threshold points between PN and CI regions are 14 ml/100g-min, 2.5 s, and 4.7 s for qCBF, Tmax, and MTT respectively. Threshold points between CI and PP regions are 11 ml/100g-min, 7.4 s, and 5.5 s for qCBF, Tmax, and MTT respectively.
Figure 2.
Gray Matter ROC Curves A) Presumed Normal (PN) versus Critically Ischemic (CI) qCBF B) PN versus CI Tmax C) Presumed Penumbra (PP) versus CI qCBF D) PP versus CI Tmax.
Figure 3.
White Matter ROC Curves A) Presumed Normal (PN) versus Critically Ischemic (CI) qCBF B) PN versus CI Tmax C) Presumed Penumbra (PP) versus CI qCBF D) PP versus CI Tmax.
DISCUSSION
These findings indicate that quantitative measurement of CBF using the Bookend method is feasible in ischemic stroke patients. When compared with time-based imaging parameters, direct measurement of CBF better discriminated between brain regions. These findings suggest that quantitative measurement of cerebral perfusion with MRI may have considerable utility in assessment of severity and extent of an ischemic stroke.
Arterial spin tagging (ASL) is an MRI technique that also has the potential to determine quantitative CBF (33). A plethora of tagging and readout schemes have been developed (18,34–36) and have shown the potential for measuring qCBF (33,37–38). The signal changes observed in ASL are small, typically 1–2%, so that acquiring useful images requires multiple signal averages and scan times of several minutes. Furthermore, with ASL perfusion imaging, calculation of qCBF depends on a variety of physiologic parameters (T1 of blood, T1 of tissue, water diffusion rate, etc) (39). In particular, the accuracy of CBF depends on the time it takes for the blood to flow from the labeling region to the readout slice. Some ASL techniques can minimize this dependence when transit times are within “normal ranges”. In patients with severely altered hemodynamics, this transit time can confound the accurate determination of CBF (28). Therefore, although ASL has been shown to measure qCBF, its utility in the ischemic stroke setting, where label-to-tissue transit times cannot be predicted, has not been established.
Average qCBF values (GM = 53.44±12.41 ml/100g-min and WM = 21.50±5.18 ml/100g-min) are in agreement with historical reference values based on [15O]-H2 PET (GM = 49.9±9.6 ml/100g-min and WM = 22.3±4.9 ml/100g-min) (40). While PET perfusion images were not acquired in this investigation, Bookend perfusion scans have been compared to PET in patients with angiographically confirmed hemodynamic impairment (22). In this PET study comparing [15O]-H2 PET with Bookend perfusion scans in moyamoya patients, a strong correlation was observed (r=0.74, p<10−3) (22). This result is important since these patients exhibit significant alterations in hemodynamics and flow. The performance of the Bookend technique in Moyamoya patients supports the assertion that qCBF in stroke patients, i.e. in the setting of altered flow, delay, and dispersion, is accurate. Other combined MRI perfusion/PET evidence indicates considerable variation between patients in CBV and consequently CBF (41). The Bookend technique corrects this source of measurement error to provide a more stable (21) and accurate (22) measurement of perfusion in an individual patient.
In GM, the qCBF ischemic threshold of 26 ml/100g is slightly higher than other reported values (32). In WM, the qCBF threshold between PN and CI regions of 14 ml/100g-min is in agreement with other findings indicating a WM ischemic threshold of 12.3 ml/100g-min (32), but is lower than those reporting threshold of 20.8 ml/100g-min (42). However, the latter threshold correlates with normal WM values from studies in PET and MRI (32,42–43). The ischemic threshold in GM is higher than in WM, consistent with other findings (32,42).
The work is not without limitations. The Bookend technique requires three separate scans. A newer MRI pulse sequence includes the LL-EPI calibration scans with the DSC perfusion scan (44). The combination of a single self-calibrating pulse sequence and fully automated post-processing (21) may provide means of producing qCBF images in near real-time. Another limitation is that this was a retrospective study and we were unable to have follow-ups. With serial MRIs, we could determine the true penumbra instead of presumed penumbra. In addition, due to the small sample size, the study did not have any patients with transhemispheric diachisis (45), which results in a lower blood flow in the contralateral hemisphere after a stroke. Quantitative perfusion could possibly be used to evaluate transhemisphreic diachisis as relative measures of flow can be misleading in these patients. Furthermore, to truly understand the role that qCBF images can play in the triage of ischemic stroke patients, a larger sample of patients within the critical three hour window for reperfusion therapy would be beneficial.
In conclusion, this investigation indicates that quantitative MRI perfusion is feasible in ischemic stroke patients, and the direct quantification afforded by these measurements enhances discrimination of critically ischemic tissue compared to time-based imaging metrics. An ischemic stroke is a dynamic process in which perfusion and diffusion change throughout the evolution of the infarct. Only a single window into this process may be available when critical management decisions must be made. Quantitative measurement of perfusion represents a promising incremental step forward in the development of diffusion-perfusion imaging to assist efforts aimed at preservation of brain and function in an ischemic stroke. This approach merits more investigation in larger, clinical studies.
Acknowledgments
Grant Support:
NIH/NINDS: 1R01 NS0493395
American Heart Association 0515256Z, 0655758Z
REFERENCES
- 1.Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke. 1999;30(12):2523–2528. doi: 10.1161/01.str.30.12.2523. [DOI] [PubMed] [Google Scholar]
- 2.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37(5):1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 3.Hakim AM. Ischemic penumbra: the therapeutic window. Neurology. 1998;51(3 Suppl 3):S44–S46. doi: 10.1212/wnl.51.3_suppl_3.s44. [DOI] [PubMed] [Google Scholar]
- 4.Hill MD, Barber PA, Demchuk AM, et al. Acute intravenous--intra-arterial revascularization therapy for severe ischemic stroke. Stroke. 2002;33(1):279–282. doi: 10.1161/hs0102.101900. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann AM, Firlik AD, Fukui MB, Wechsler LR, Jungries CA, Yonas H. Ischemic core and penumbra in human stroke. Stroke. 1999;30(1):93–99. doi: 10.1161/01.str.30.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14(3):332–341. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- 7.Schellinger PD, Kaste M, Hacke W. An update on thrombolytic therapy for acute stroke. Curr Opin Neurol. 2004;17(1):69–77. doi: 10.1097/00019052-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 8.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 10.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 11.Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14(2):249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 12.Rohl L, Ostergaard L, Simonsen CZ, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001;32(5):1140–1146. doi: 10.1161/01.str.32.5.1140. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer PW, Ozsunar Y, He J, et al. Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol. 2003;24(3):436–443. [PMC free article] [PubMed] [Google Scholar]
- 14.Wu O, Koroshetz WJ, Ostergaard L, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32(4):933–942. doi: 10.1161/01.str.32.4.933. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowley HA, Roberts TP. Clinical perspectives in perfusion: neuroradiologic applications. Top Magn Reson Imaging. 2004;15(1):28–40. doi: 10.1097/00002142-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke. 2003;34(4):1084–1104. doi: 10.1161/01.STR.0000064840.99271.9E. [DOI] [PubMed] [Google Scholar]
- 19.Sakaie KE, Shin W, Carroll TJ. Comparison of Analysis Methods for Measureing T1 by TrueFISP Readout of Inversion Recovery. Proc Intl Soc Magn Reson Med. 2004;12:2118. [Google Scholar]
- 20.Shin W, Cashen TA, Horowitz SW, Sawlani R, Carroll TJ. Quantitative CBV measurement from static T1 changes in tissue and correction for intravascular water exchange. Magn Reson Med. 2006;56(1):138–145. doi: 10.1002/mrm.20937. [DOI] [PubMed] [Google Scholar]
- 21.Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med. 2007;58(6):1232–1241. doi: 10.1002/mrm.21420. [DOI] [PubMed] [Google Scholar]
- 22.Parikh V, Lee J, Shin W, et al. qCBF: A comparison of the accuracy between the Bookend technique, empirical reference values and [O15]-H2O PET in moyamoya patients. Proc Int Soc Magn Reson Med. 2009;Volume 2015 [Google Scholar]
- 23.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47(4):462–469. [PubMed] [Google Scholar]
- 24.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn Reson Med. 1996;36(5):726–736. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- 25.Kuppusamy K, Lin W, Cizek GR, Haacke EM. In vivo regional cerebral blood volume: quantitative assessment with 3D T1-weighted pre- and postcontrast MR imaging. Radiology. 1996;201(1):106–112. doi: 10.1148/radiology.201.1.8816529. [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Celik A, Derdeyn C, et al. Quantitative measurements of cerebral blood flow in patients with unilateral carotid artery occlusion: a PET and MR study. J Magn Reson Imaging. 2001;14(6):659–667. doi: 10.1002/jmri.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostergaard L, Johannsen P, Host-Poulsen P, et al. Cerebral blood flow measurements by magnetic resonance imaging bolus tracking: comparison with [(15)O]H2O positron emission tomography in humans. J Cereb Blood Flow Metab. 1998;18(9):935–940. doi: 10.1097/00004647-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Moseley ME, Chew WM, White DL, et al. Hypercarbia-induced changes in cerebral blood volume in the cat: a 1H MRI and intravascular contrast agent study. Magn Reson Med. 1992;23(1):21–30. doi: 10.1002/mrm.1910230104. [DOI] [PubMed] [Google Scholar]
- 29.Donahue KM, Weisskoff RM, Chesler DA, et al. Improving MR quantification of regional blood volume with intravascular T1 contrast agents: accuracy, precision, and water exchange. Magn Reson Med. 1996;36(6):858–867. doi: 10.1002/mrm.1910360608. [DOI] [PubMed] [Google Scholar]
- 30.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229(1):3–8. doi: 10.1148/radiol.2291010898. [DOI] [PubMed] [Google Scholar]
- 31.Obuchowski NA. ROC analysis. AJR Am J Roentgenol. 2005;184(2):364–372. doi: 10.2214/ajr.184.2.01840364. [DOI] [PubMed] [Google Scholar]
- 32.Bristow MS, J.E S, R.A B, et al. Determining Ischemic Thresholds for Gray and White Matter in Stroke Penumbra. Proc Int Soc Magn Reson Med. 2004;12:408. [Google Scholar]
- 33.Roberts DA, Detre JA, Bolinger L, Insko EK, Leigh JS., Jr Quantitative magnetic resonance imaging of human brain perfusion at 1.5 T using steady-state inversion of arterial water. Proc Natl Acad Sci U S A. 1994;91(1):33–37. doi: 10.1073/pnas.91.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16(6):1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Carroll TJ, Rowley HA, Haughton VM. Automatic calculation of the arterial input function for cerebral perfusion imaging with MR imaging. Radiology. 2003;227(2):593–600. doi: 10.1148/radiol.2272020092. [DOI] [PubMed] [Google Scholar]
- 36.Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31(3):680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- 37.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 38.Lin W, Celik A, Paczynski RP. Regional cerebral blood volume: a comparison of the dynamic imaging and the steady state methods. J Magn Reson Imaging. 1999;9(1):44–52. doi: 10.1002/(sici)1522-2586(199901)9:1<44::aid-jmri6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt P, Griswold MA, Jakob PM, et al. Inversion recovery TrueFISP: quantification of T(1), T(2), and spin density. Magn Reson Med. 2004;51(4):661–667. doi: 10.1002/mrm.20058. [DOI] [PubMed] [Google Scholar]
- 40.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 41.Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39(3):870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 42.Arakawa S, Wright PM, Koga M, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke. 2006;37(5):1211–1216. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee P, Kang HC, Videen TO, McKinstry RC, Powers WJ, Derdeyn CP. Measurement of cerebral blood flow in chronic carotid occlusive disease: comparison of dynamic susceptibility contrast perfusion MR imaging with positron emission tomography. AJNR Am J Neuroradiol. 2003;24(5):862–871. [PMC free article] [PubMed] [Google Scholar]
- 44.Shin W, Carroll TJ. A Self Calibrating Pulse Sequence of Real Time Quantitative Cerebral Perfusion. Proc Intl Soc Magn Reson Med. 2007;Volume 155 [Google Scholar]
- 45.Andrews RJ. Transhemispheric diaschisis. A review and comment. Stroke. 1991;22(7):943–949. doi: 10.1161/01.str.22.7.943. [DOI] [PubMed] [Google Scholar]