Abstract
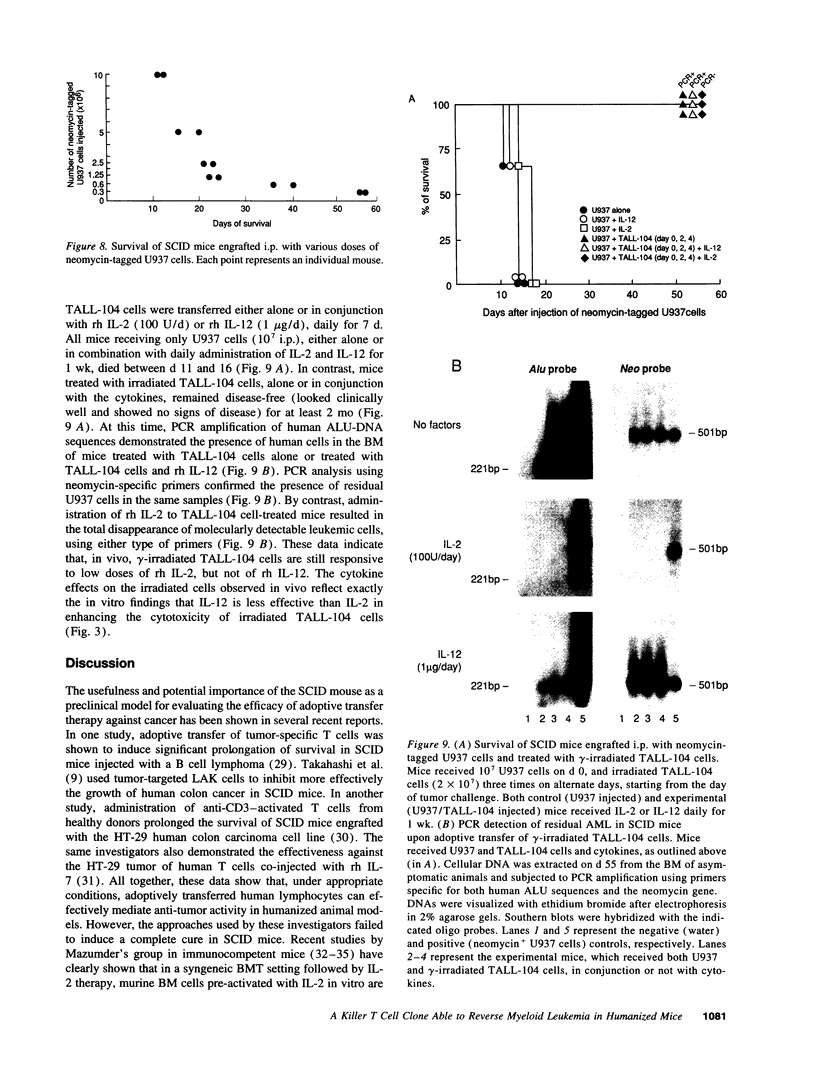
Advanced human malignancies cannot be permanently cured by adoptive transfer of autologous lymphokine-activated killer (LAK) cells. Moreover, administration of high doses of IL-2 to maintain LAK activity in vivo is associated with severe toxicity. In this study, we tested the anti-tumor efficacy of a uniquely potent MHC non-restricted human killer T cell clone (TALL-104) in humanized severe combined immunodeficient (SCID) mice bearing acute myelogenous leukemia (AML). We show that, in appropriate experimental conditions, TALL-104 cells could effectively reverse the aggressive growth of the myelomonocytic leukemia cell line U937 in SCID mouse tissues, leading to complete abrogation of AML. A single transfer of TALL-104 cells at the time of tumor challenge in combination with recombinant human (rh) IL-12 (1 microgram/d) prolonged significantly the life of the mice. However, eradication of leukemia, as monitored both clinically and by PCR, was achieved by repeated injection of the effectors at close intervals. Complete cure was obtained also upon transfer of lethally irradiated (non-proliferating) TALL-104 cells together with low doses of rh IL-2 (100 U/d). Most notably, of the mice that received multiple transfers of TALL-104 cells without cytokines in an advanced disease stage, 50% were clinically cured, and 50% survived significantly longer. The potential of TALL-104 cells as an effective and safe leukemia purging agent is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher A. L., Mulé J. J., Kasid A., Restifo N. P., Salo J. C., Reichert C. M., Jaffe G., Fendly B., Kriegler M., Rosenberg S. A. Murine tumor cells transduced with the gene for tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991 May 1;146(9):3227–3234. [PMC free article] [PubMed] [Google Scholar]
- Cesano A., Hoxie J. A., Lange B., Nowell P. C., Bishop J., Santoli D. The severe combined immunodeficient (SCID) mouse as a model for human myeloid leukemias. Oncogene. 1992 May;7(5):827–836. [PubMed] [Google Scholar]
- Cesano A., O'Connor R., Lange B., Finan J., Rovera G., Santoli D. Homing and progression patterns of childhood acute lymphoblastic leukemias in severe combined immunodeficiency mice. Blood. 1991 Jun 1;77(11):2463–2474. [PubMed] [Google Scholar]
- Cesano A., O'Connor R., Nowell P. C., Lange B., Clark S. C., Santoli D. Establishment of a karyotypically normal cytotoxic leukemic T-cell line from a T-ALL sample engrafted in SCID mice. Blood. 1993 May 15;81(10):2714–2722. [PubMed] [Google Scholar]
- Cesano A., Santoli D. Two unique human leukemic T-cell lines endowed with a stable cytotoxic function and a different spectrum of target reactivity analysis and modulation of their lytic mechanisms. In Vitro Cell Dev Biol. 1992 Sep-Oct;28A(9-10):648–656. doi: 10.1007/BF02631041. [DOI] [PubMed] [Google Scholar]
- Cesano A., Visonneau S., Clark S. C., Santoli D. Cellular and molecular mechanisms of activation of MHC nonrestricted cytotoxic cells by IL-12. J Immunol. 1993 Sep 15;151(6):2943–2957. [PubMed] [Google Scholar]
- Charak B. S., Agah R., Brynes R. K., Chogyoji M., Groshen S., Chen S. C., Mazumder A. Interleukin-2 (IL-2) and IL-2-activated bone marrow in transplantation: evaluation from a clinical perspective. Bone Marrow Transplant. 1992 Jun;9(6):479–486. [PubMed] [Google Scholar]
- Charak B. S., Brynes R. K., Chogyoji M., Kortes V., Tefft M., Mazumder A. Graft versus leukemia effect after transplantation with interleukin-2-activated bone marrow. Correlation with eradication of residual disease. Transplantation. 1993 Jul;56(1):31–37. doi: 10.1097/00007890-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Charak B. S., Brynes R. K., Chogyoji M., Mazumder A. Lymphokine-activated killer cells in autologous bone marrow transplantation. Evidence against inhibition of engraftment in vivo. Transplantation. 1992 Dec;54(6):1008–1013. doi: 10.1097/00007890-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Charak B. S., Choudhary G. D., Tefft M., Mazumder A. Interleukin-2 in bone marrow transplantation: preclinical studies. Bone Marrow Transplant. 1992 Aug;10(2):103–111. [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansbacher B., Bannerji R., Daniels B., Zier K., Cronin K., Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990 Dec 15;50(24):7820–7825. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K., Desai B. B., Wolitzky A. G., Quinn P. M., Dwyer C. M., Podlaski F. J., Familletti P. C., Sinigaglia F., Chizonnite R., Gubler U. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991 Aug 1;147(3):874–882. [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Hock H., Dorsch M., Diamantstein T., Blankenstein T. Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J Exp Med. 1991 Dec 1;174(6):1291–1298. doi: 10.1084/jem.174.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Balch C. M. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986 Jun;46(6):3011–3017. [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. E., Lotze M. T., Skibber J. M., Tucker E., Bonow R. O., Ognibene F. P., Carrasquillo J. A., Shelhamer J. H., Parrillo J. E., Rosenberg S. A. Cardiorespiratory effects of immunotherapy with interleukin-2. J Clin Oncol. 1989 Jan;7(1):7–20. doi: 10.1200/JCO.1989.7.1.7. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Custer M. C., Bolton E. S., Wiebke E. A., Kawakami Y., Rosenberg S. A. Mechanisms of immunologic antitumor therapy: lessons from the laboratory and clinical applications. Hum Immunol. 1990 Jun;28(2):198–207. doi: 10.1016/0198-8859(90)90020-p. [DOI] [PubMed] [Google Scholar]
- Malkovska V., Cigel F. K., Armstrong N., Storer B. E., Hong R. Antilymphoma activity of human gamma delta T-cells in mice with severe combined immune deficiency. Cancer Res. 1992 Oct 15;52(20):5610–5616. [PubMed] [Google Scholar]
- Murphy W. J., Back T. C., Conlon K. C., Komschlies K. L., Ortaldo J. R., Sayers T. J., Wiltrout R. H., Longo D. L. Antitumor effects of interleukin-7 and adoptive immunotherapy on human colon carcinoma xenografts. J Clin Invest. 1993 Oct;92(4):1918–1924. doi: 10.1172/JCI116785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Conlon K. C., Sayers T. J., Wiltrout R. H., Back T. C., Ortaldo J. R., Longo D. L. Engraftment and activity of anti-CD3-activated human peripheral blood lymphocytes transferred into mice with severe combined immune deficiency. J Immunol. 1993 Apr 15;150(8 Pt 1):3634–3642. [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò P., Mortarini R., De Monte L. B., Mazzocchi A., Mariani M., Malavasi F., Parmiani G., Natali P. G., Anichini A. Cell retargeting by bispecific monoclonal antibodies. Evidence of bypass of intratumor susceptibility to cell lysis in human melanoma. J Clin Invest. 1992 Sep;90(3):1093–1099. doi: 10.1172/JCI115925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor R., Cesano A., Lange B., Finan J., Nowell P. C., Clark S. C., Raimondi S. C., Rovera G., Santoli D. Growth factor requirements of childhood acute T-lymphoblastic leukemia: correlation between presence of chromosomal abnormalities and ability to grow permanently in vitro. Blood. 1991 Apr 1;77(7):1534–1545. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nakada T., Puisieux I. Inhibition of human colon cancer growth by antibody-directed human LAK cells in SCID mice. Science. 1993 Mar 5;259(5100):1460–1463. doi: 10.1126/science.8451642. [DOI] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- Xiao J., Brahmi Z. Target cell-directed inactivation and IL-2-dependent reactivation of LAK cells. Cell Immunol. 1989 Sep;122(2):295–306. doi: 10.1016/0008-8749(89)90078-6. [DOI] [PubMed] [Google Scholar]