Abstract
Background
Studies in schizophrenics have reported dopaminergic abnormalities in striatum, substantia nigra, thalamus, anterior cingulate, hippocampus and cortex which have been related to positive symptoms and cognitive impairments.
Methods
[18F]fallypride PET studies were performed in off medication or never medicated schizophrenic subjects [N = 11, 6 M, 5 F; mean age of 30.5 ± 8.0 (S.D.); 4 drug naive] and age matched healthy subjects [N = 11, 5M, 6F, mean age of 31.6 ± 9.2 (S.D.)] to examine dopamine D2 receptor (DA D2r) levels in the caudate, putamen, ventral striatum, medial thalamus, posterior thalamus, substantia nigra, amygdala, temporal cortex, anterior cingulate, and hippocampus.
Results
In schizophrenic subjects increased DA D2r levels were seen in the substantia nigra bilaterally; decreased levels were seen in the left medial thalamus. Correlations of symptoms with region of interest data demonstrated a significant correlation of disorganized thinking/nonparanoid delusions with the right temporal cortex region of interest (r = 0.94, P = 0.0001) which remained significant after correction for multiple comparisons (P<0.03). Correlations of symptoms with parametric images of DA D2r levels revealed no significant clusters of correlations with negative symptoms, but significant clusters of positive correlations of total positive symptoms, delusions and bizarre behavior with the lateral and anterior temporal cortex, and hallucinations with the left ventral striatum.
Conclusions
The results of this study demonstrate abnormal DA D2r mediated neurotransmission in the substantia nigra consistent with nigral dysfunction in schizophrenia and suggest that both temporal cortical and ventral striatal DA D2r mediate positive symptoms.
Keywords: Dopamine D2 receptors, Schizophrenia, fallypride, substantia nigra, thalamus, delusions, hallucinations
Abnormal dopaminergic neurotransmission has been implicated in the positive symptoms and cognitive deficits seen in schizophrenia (1-5). Recent studies suggest abnormal function of GABAergic/glutamatergic cortical microcircuits in schizophrenia resulting in dysfunction of cortical pyramidal glutamatergic neurons (6) which provide a major excitatory afferent projection to the substantia nigra (7). Dysfunction of this projection results in nigral dysfunction and increased striatal DA release (8, 9, and 10) which has been positively correlated with positive symptoms (11). Prefrontal cortical glutamatergic afferents to the ventral tegmental area (VTA) synapse directly on mesocortical DA neurons; it has been hypothesized that dysfunction of this projection leads to decreased cortical DA release(12) which is believed to be a factor in the cognitive impairments seen in schizophrenia(4). As dopamine D2 receptors (DA D2r) directly modulate cortical GABAergic interneurons which modulate cortical pyramidal neurons (13, 14), ventral midbrain DA neurons (15, 16), and DA release in striatal and extrastriatal regions (17), DA D2r are of considerable interest in schizophrenia.
Consistent with the hypothesis of decreased cortical DA release in schizophrenia, post mortem studies have reported decreased dopaminergic innervation in medial temporal cortex, dorsolateral prefrontal cortex and hippocampus (18-20), and DOPAC levels in the anterior cingulate (21). Some imaging studies of extrastriatal DA D2r in schizophrenic subjects have reported decreased DA D2r levels in the anterior cingulate and temporal cortex, but most have not (22-27); the most frequent finding is decreased medial thalamic DA D2r levels (24,25,26). While there have been variable results, a recent study of DA D1r in schizophrenic subjects reported increased frontal cortical levels which were negatively correlated with performance on a working memory task (28-30), The increased DA D1r levels were interpreted as being consistent with decreased frontal cortical DA levels. Post mortem studies of dopaminergic function in the the substantia nigra in schizophrenic subjects have reported increased levels of tyrosine hydroxylase (31), tyrosine hydroxylase mRNA (32), homovanillic acid (31), and DA D2r (33) consistent with nigral dysfunction. Imaging studies have largely failed to examine substantia nigra DA D2r. Both post mortem and imaging studies have reported increased striatal DA synthesis, DA levels, and DA release which has been correlated with positive symptoms (34-41). In contrast to post mortem studies which have reported increased striatal DA D2r levels (42, 43), most imaging but not all studies of striatal DA D2r have reported unaltered levels in schizophrenia (44, 45, 46). However, one imaging study of striatal DA D2r performed before and after DA depletion with alphamethylparatyrosine demonstrated normal levels prior to DA depletion but increased DA D2r levels after depletion consistent with both increased striatal DA release and increased total DA D2r levels(36). The discrepancy between post mortem studies and imaging studies with benzamide radioligands may be due to the occupancy of striatal DA D2r by increased levels of extracellular DA. Overall, the available post mortem and imaging data are consistent with the hypothesis of decreased cortical DA release, nigral DA neuronal dysfunction and increased striatal DA release in schizophrenia.
Previous imaging studies of DA D2r in medication free schizophrenic subjects have evaluated either striatal or extrastriatal DA D2r levels (22-27, 44, 45). In the current study, positron emission tomography (PET) with [18F]fallypride was utilized. [18F]Fallypride is a very high affinity, specific benzamide PET radioligand for the DA D2 receptor (KD = 0.03nM) and is the only currently available radioligand which allows estimation of both striatal and extrastriatal DA D2r levels (22,24, 27, 44-48). Given the hypothesis of nigral dysfunction in schizophrenia(1,8-10,12), the lack of previous imaging studies of the substantia nigra DA D2r, post mortem findings consistent with nigral dysfunction(31-33), and the ability of PET [18F]fallypride studies to estimate nigral DA D2r levels(48,49), we specifically examined this region. Other regions previously reported to have altered DA D2r levels, the anterior cingulate, temporal cortex, and medial thalamus(22-26), were examined. As significant correlations of symptoms with regional DA D2r levels have been reported (22-27), correlations of positive and negative symptoms with regional DA D2r levels were assessed.
Methods
Subjects
This study was conducted under protocols approved by the Vanderbilt University and Centerstone Mental Health Center Institutional Review Boards. All subjects were judged capable of giving informed consent by a senior research psychiatrist, and provided informed consent for this study. Subjects meeting the DSM IV criteria (American Psychiatric Association, 1994), and Research Diagnostic Criteria (50) for the diagnosis of schizophrenia between the ages of 18 and 45 were recruited. The diagnosis of schizophrenia was established by the Structured Clinical Interview for DSM IV Axis I disorders (51) and checklist. Schizophrenic subjects [N = 11, 6 M, 5 F; mean age of 30.5 ± 8.0 (S.D.) years and age range of 20–45 years] were either never treated (N=4) or were off medication for at least three weeks (Table 1). The BPRS (6 item scales), SAPS and SANS were administered to each subject; mean total BPRS, SAPS and SANS scores were 28.8 ± 7.0 (S.D.), 9.8 ± 3.1 (S.D.), and 9.4 ± 4.0(S.D.) respectively. Age matched healthy subjects [N = 11, 5M, 6F, mean age of 31.6 ± 9.2 (S.D.) years and age range of 21 -45 years] were recruited as well. Significant medical conditions, and previous or current substance abuse were exclusion criteria for all subjects.
Table 1. Demographic data for OFF Medication Schizophrenic Subjects.
| Subject Number | Sex | Age | Medication Free period | Previous medications | BPRS Score (6 item scales) |
|---|---|---|---|---|---|
| 1 | F | 36 | 3 weeks | quetiapine | 30 |
| 2 | F | 32 | 9 weeks | quetiapine | 32 |
| 3 | M | 22 | 5 weeks | olanzapine | 38 |
| 4 | M | 20 | Never Medicated | N.A. | 27 |
| 5 | F | 36 | 21 weeks | olanzapine | 40 |
| 6 | F | 39 | 10 weeks | olanzapine | 19 |
| 7 | M | 22 | Never Medicated | N.A. | 32 |
| 8 | F | 35 | Never Medicated | N.A. | 18 |
| 9 | M | 23 | 40 weeks | olanzapine | 30 |
| 10 | M | 45 | Never Medicated | N.A. | 29 |
| 11 | M | 26 | 3 weeks | olanzapine | 22 |
Data Acquisition and Analysis
MRI scans of the brain were performed using a G.E. 1.5T Signa LXi MRI scanner. High resolution T1-weighted gradient echo acquisitions in the sagittal plane (1.2 -1.3 mm thick slices) and coronal planes (1.4–1.5 mm thick slices), axial spin density weighted and T2-weighted (3 mm thick slices) acquisitions were obtained. PET scans were performed using a GE Advance PET scanner in the 3-D acquisition mode. [18F]Fallypride (4–5 mCi, specific activity >2,000 Ci/mmol, maximum mass dose of less than 2.5 nanomoles) was injected intravenously over a 20 second period; serial scans of increasing duration were obtained for 210 minutes, allowing stable estimates of binding potentials in all regions (47-49). A measured attenuation correction was utilized.
Serial PET scans were coregistered to each other and to thin section T1 weighted MRI images using a rigid body, mutual information algorithm (52, 53), and reoriented to the ACPC line. Regions of interest were identified on thin section T1 weighted MRI images, and transferred to coregistered PET studies. The putamen, and caudate were manually drawn by a neuroradiologist (RMK) on axial slices from 2 to 12 mm above the ACPC line. The ventral striatum was defined using the criteria of Mawlawi (54). Sobel filtering was performed on high resolution gradient echo MRI images of the brain (55), but did not provide reliable boundaries for delineation of the dorsomedial thalamus and pulvinar. We used anatomic landmarks to delineate the medial thalamus and posterior thalamus, which approximated the boundaries of the dorsomedial thalamus and pulvinar (56). The medial thalamus was delineated on slices from 2 to 12 above the ACPC line; the posterior border was the coronal plane of the posterior commissure, the medial border the midline, the anterior boundary the foramen of Monro and the lateral border extended up to 1 cm from the midline. The anterior border of the posterior thalamus was the coronal plane of the posterior commissure, the medial and posterior borders the edge of the thalamus as it projects into the quadrigeminal plate cistern, and the lateral border the posterior limb of the internal capsule. The substantia nigra/VTA is located in the ventral midbrain 9-14 mm below the ACPC line (56) and can be readily visualized in the midbrain on PET [18F]fallypride scans (57). Substantia nigra regions of interest were manually drawn to adjust for inter-individual variability by a neuroradiologist (RMK); the intersubject coefficient of variation for the substantia nigra region was 8.7 % (57). The amygdala can be visualized in the on MRI scans just anterior to the tip of the temporal horn of the lateral ventricle and deep to the uncus (57); the amygdala is located 6 to 20 mm below the ACPC line, 12 to 28 mm lateral to the midline, and from 2 to 12 mm behind the plane of the anterior commissure (56). To decrease partial voluming from the striatum, amygdala regions of interest were drawn on MRI images from 10 to 16 mm below the plane of the ACPC. Temporal cortical regions of interest were manually drawn on axial MRI images from 35 to 25 mm below the ACPC. Our previous studies have shown excellent inter-subject reliability for these regions of interest, i.e. inter-subject coefficients of variation of 6.8 to 15.9 percent (57). The anterior cingulate was delineated as extending from superior to the axial plane through the ACPC in the pregenual region superiorly and posteriorly to the coronal plane through the anterior commissure. The hippocampus was manually delineated on the coronal MRI images from the tip of the temporal horn anteriorly to the last coronal slice in which it would be reliably identified posteriorly. Regional DA D2r levels were estimated using the reference region method with a cerebellar reference region (58). The cerebellum is an appropriate reference region as less than 3 percent of cerebellar uptake is specific binding to DA D2 receptors and reference region method estimates of binding potentials are highly correlated (r>0.99) with modeled estimates using a metabolite corrected plasma input function (47, 59- 61). Parametric images of DA D2r were coregistered across subjects using an elastic deformation algorithm (62).
Statistical Analysis
Region of interest data were analyzed using a repeated measures MANOVA with region and hemisphere as within subject factors, and group (schizophrenic, healthy control) as a between subject factor with age as a covariate. Definition of the hippocampal region of interest was problematic in one subject, and this subject was left out of the analysis. When the MANOVA was performed with this subject but without the hippocampus as a region, no conclusion was changed. Because age has a significant effect on DA D2r levels, independent group two-tailed t-tests covaried for age were used to test for group differences in regional binding potentials. To evaluate positive symptoms, the total SAPS scores, and global SAPS scores for hallucinations, delusions and bizarre behavior as well the BPRS positive symptom score, and BPRS scores for suspiciousness (Item 11), hallucinations (Item 12), and disorganized thinking/nonparanoid delusions (Item 15) were measured. Negative symptoms were examined using the SANS. Correlations of symptom scores with regions of interest were performed using a Pearson product moment correlation and significance evaluated using a two-tailed t-test. Bonferroni correction was used to correct for multiple comparisons. Correlations of symptom scores with parametric DA D2r images were calculated on a voxel basis using a Pearson product moment correlation and significance evaluated using a two-tailed t-test. To assess the significance of clusters of significant correlations, corrections for multiple comparisons were made using the method of Forman as implemented in the AFNI program (63). The critical threshold for the voxelwise analysis was P<0.01 and a minimum cluster size of 24 voxels. These clusters were significant at P<0.001 corrected for multiple comparisons.
Results
A repeated measures MANOVA was performed with region and hemisphere as within subject factors, group (schizophrenic, healthy control) as a between subject factors, and with age as a covariate. No significant main effect of hemisphere, no group × hemisphere interaction, or group × hemisphere × region interaction was seen. There was a significant effect of region, F(7,13) = 36,78, p<0.00001, reflecting the large differences in regional binding potentials (Table 2). There was no main effect of group. However, there was a significant region × group interaction, F(7,13) = 6.00, p<0.005. There was also a region × age interaction, F(7,13) = 4.60, p<0.010 reflecting decreases in regional binding potentials with age which were greater for cortical than subcortical regions. To explore the regions responsible for the significant region × group interaction, independent group two tailed t-tests covaried for age were performed to examine which region(s) might account for this interaction. These tests demonstrated significantly increased DA D2r levels in the substantia nigra/VTA bilaterally and decreased levels in the left medial thalamus (Table 2). No other region of interest demonstrated a significant difference in DA D2r levels between schizophrenic and healthy subjects.
Table 2. Binding potentials for regions of interest sampled in 11 unmedicated schizophrenic subjects and 11 age matched healthy subjects. Significance level was estimated using an independent group, two tailed t-test covered for age.
| Region | Schizophrenic | Normal | Significance level | |||
|---|---|---|---|---|---|---|
| R | L | R | L | R | L | |
| Medial Thalamus | 4.21±0.58 | 4.17±0.55 | 4.30±0.62 | 4.55±0.58 | 0.51 | 0.04 |
| Posterior Thalamus | 2.28±0.41 | 2.40±0.30 | 2.36±0.32 | 2.54±0.36 | 0.41 | 0.11 |
| Anterior Cingulate | 0.79±0.15 | 0.75±0.15 | 0.78±0.17 | 0.77±0.16 | 0.52 | 0.55 |
| Substantia Nigra | 2.87±0.33 | 2.75±0.34 | 2.44±0.22 | 2.41±0.19 | 0.002 | 0.009 |
| Hippocampus | 1.57±0.41 | 1.68±0.38 | 1.48±0.26 | 1.59±0.34 | 0.59 | 0.68 |
| Temporal Cortex | 1.52±0.33 | 1.63±0.33 | 1.59±0.18 | 1.72±0.24 | 0.30 | 0.20 |
| Caudate | 30.70±3.30 | 31.81±4.12 | 32.25±2.08 | 32.33±2.13 | 0.19 | 0.72 |
| Putamen | 36.52±4.36 | 35.00±4.46 | 37.02±2.56 | 36.94±2.77 | 0.68 | 0.18 |
| Ventral Striatum | 18.80±3.66 | 19.49±3.49 | 18.10±2.81 | 18.36±3.41 | 0.60 | 0.38 |
| Amygdala | 3.28±0.43 | 3.20±0.61 | 3.23±0.27 | 3.26±0.24 | 0.85 | 0.64 |
Correlation of region of interest data with SAPS scores, SANS scores, BPRS positive symptom score, and BPRS scores for suspiciousness (Item 11), hallucinations (Item 12), and disorganized thinking/nonparanoid delusions (Item 15) demonstrated one correlation which survived Bonferroni correction and a second trend level correlation. DA D2r levels in the right temporal cortex region of interest were positively correlated with the BPRS score for disorganized thinking/nonparanoid delusions, r = 0.94, P = 0.0001 uncorrected for multiple comparisons, P<0.03 after correction for multiple comparisons. The left temporal cortical region of interest demonstrated a trend level correlation with the BPRS score for disorganized thinking/nonparanoid delusions after corrections for multiple comparisons, r = 0.92, P = 0.0003, uncorrected for multiple comparisons, P<0.08, corrected for multiple comparisons.
Correlations of symptoms with regional DA D2r levels performed using voxelwise analysis revealed no significant clusters of correlations of regional DA D2r levels with either the total SANS score or individual SANS scores Significant clusters of highly positive correlations of regional DA D2r levels were seen with the total SAPS score, and global SAPS scores for delusions, hallucinations and bizarre behavior. Two clusters of highly positive correlations (146 voxels on the right, mean r =0.85; 131 voxels on the left, mean r=0.86) were seen with total SAPS scores; these clusters involved the posterior portions of the inferior, middle and superior temporal gyri and extended superiorly into the supramarginal gyrus of the parietal lobe in both cerebral hemispheres (Figure 1). Significant clusters of correlations of SAPS global delusions scores with DA D2 r levels was seen in the lateral aspects of the right and left anterior temporal cortex extending into the temporal tips laterally (50 voxels on the right, mean r=0.84; 80 voxels on the left, mean r=0.86) (Figure 2). Similarly, correlations of the BPRS score for disorganized thinking/nonparanoid delusions (Item 15) with DA D2 receptor levels demonstrated a similar cluster of positive correlations in the left anterior temporal cortex (80 voxels, mean r = 0.85). The SAPS global bizarre behavior scores demonstrated bilateral clusters of positive correlations (184 voxels on the right, mean r=0.85; 179 voxels on the left, mean r=0.84). The cluster on the left involved the posterior portions of the inferior and superior temporal gyri as well the mid to posterior portions of the middle temporal gyrus with extension into the inferior parietal lobule. The cluster on the right also involved the inferior, middle, and superior temporal gyri and inferior parietal lobule; it extended further anteriorly in the sulcus between the superior and middle temporal gyrus, but had less extension into the posterior superior temporal gyrus and inferior parietal lobule than the cluster on the left (Figure 3). In contrast, the SAPS global scores for hallucinations demonstrated positive correlations with the left ventral striatum (31 voxels, mean r = 0.84) but not with cortical regions (Figure 4). BPRS scores for hallucinations demonstrated a similar left ventral striatal cluster of positive correlations (41 voxels, mean r = 0.87).
Figure 1.

Sagittal (A,B), axial (C), and coronal (D) images through significant clusters of correlations of total SAPS scores with regional DA D2r levels. Two significant clusters are seen involving the posterior portions of the inferior, middle and superior temporal gyri with extension superiorly into the supramarginal gyrus of the parietal lobe in both cerebral hemispheres. The cluster on the right (146 voxels, mean r =0.85) was similar in size to the cluster on the left (131 voxels, mean r=0.86)
Figure 2.
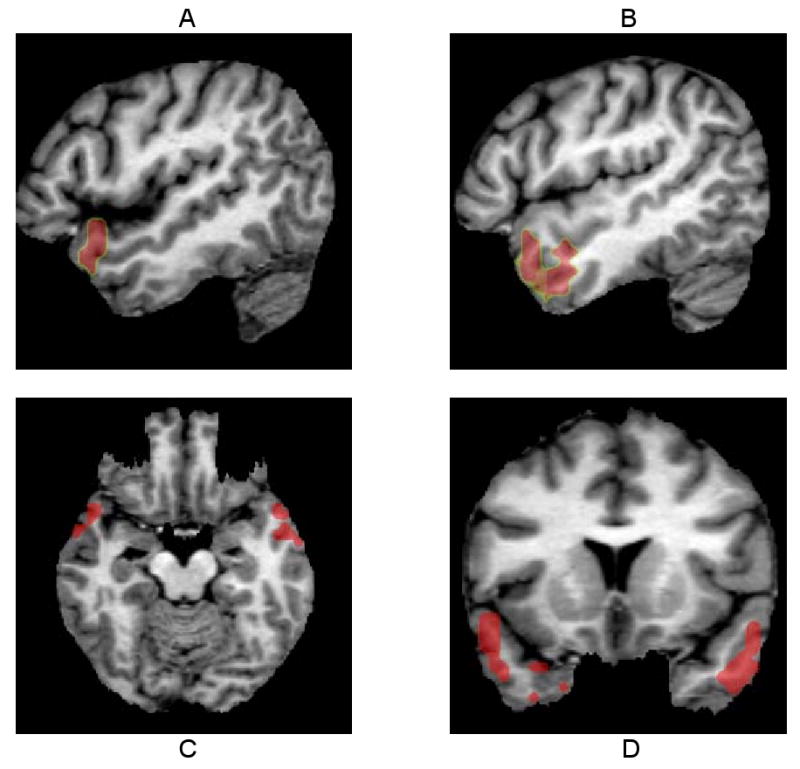
Two significant clusters of correlations of the SAPS global score for delusions with DA D2r levels are seen in the right and left anterolateral temporal cortex extending into the temporal tips. The cluster on the left (80 voxels on the left, mean r=0.86) is larger than the cluster on the right (50 voxels on the right, mean r=0.84).
Figure 3.
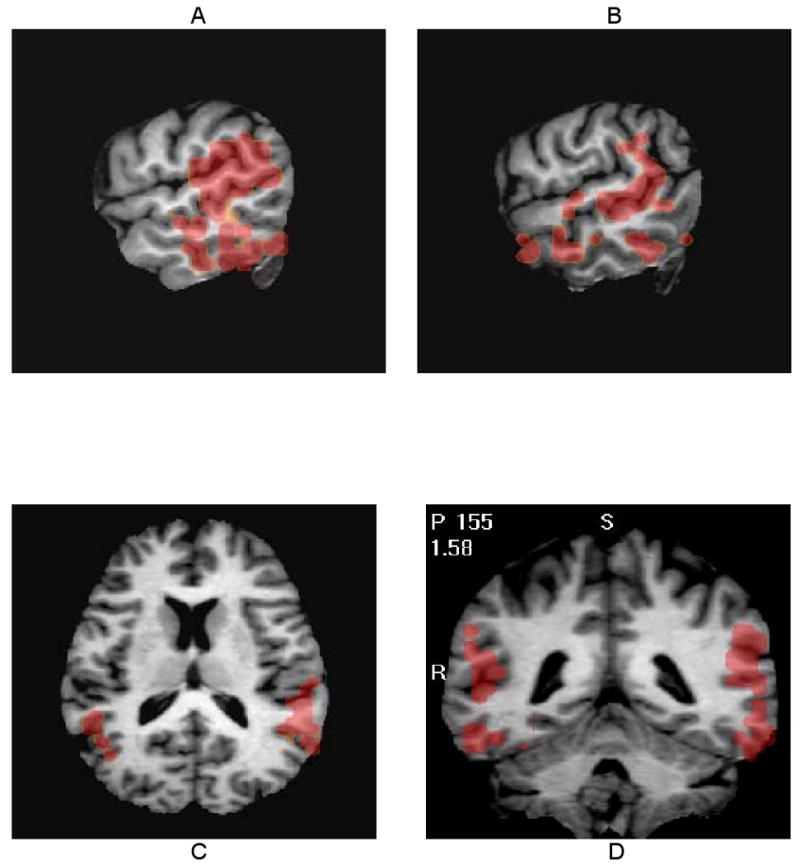
Sagittal left (A) and right (B), axial (C), and coronal (D) images through significant clusters of correlations of SAPS global scores for bizarre behavior with regional DA D2r levels. Two significant clusters of highly positive correlations (184 voxels on the right, mean r=0.85; 179 voxels on the left, mean r=0.84) involve the mid to posterior lateral aspects of the temporal lobes with extension into the inferior parietal lobule
Figure 4.
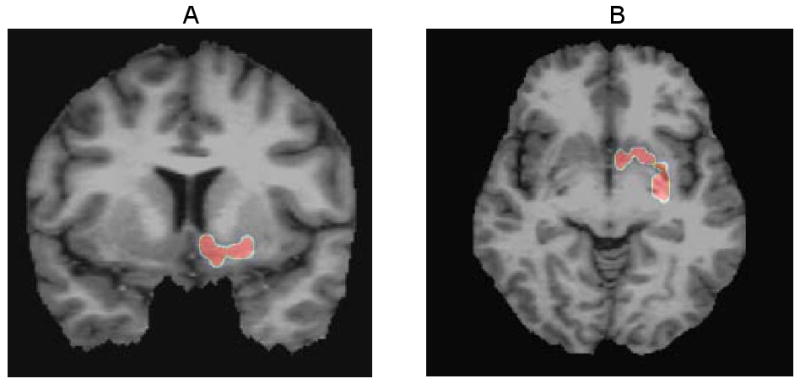
A significant cluster of correlations (31 voxels, mean r = 0.84) of the SAPS global score for hallucinations with DA D2r levels is seen in the left ventral striatum. No other significant clusters of correlations were seen.
Discussion
The results of this study indicate that there are increased DA D2r levels in the substantia nigra/VTA and decreased DA D2r levels in the left medial thalamus. The increased levels of nigral/VTA DA D2r seen in the current study are consistent with the one post mortem study of nigral DA D2r in schizophrenics which also reported increased levels (33). DA D2r in the substantia nigra are largely inhibitory autoreceptors on nigral DA neurons (15, 16). As discussed above, post mortem studies have also reported increased nigral levels of tyrosine hydroxylase, tyrosine hydroxylase mRNA, and homovanillic acid (31,32) in the substantia nigra of schizophrenic subjects. The findings in both the current study and previous post mortem studies demonstrate both increased inhibitory nigral DA D2 autoreceptor levels and increased DA synthesis and release suggesting dysregulation of midbrain dopaminergic neurons in schizophrenic subjects. Similar findings, i.e. increased total DA D2r levels and increased DA synthesis and release (34-43), have been reported in the striatum of schizophrenic subjects and suggest that similar dysregulation of dopaminergic neurotransmission occurs in both nigra and striatum. The factor(s) responsible for increased nigral and striatal DA D2r levels when increased extracellular DA levels are preset are unclear.
The VTA, dorsal tier of the zona compacta of the substantia nigra and retrorubal fields provide dopaminergic innervation to limbic and cortical regions and so are of considerable interest in schizophrenia (64, 65). The resolution of the PET scanner used in this study is insufficient to distinguish changes in these areas from the ventral tier of the zona compacta which provides dopaminergic innervation to the striatum. While the PET scanner used in this study does not have sufficient resolution to provide complete quantitative recovery of DA D2r levels in the substantia nigra, published calculated estimates of quantitative recovery for the substantia nigra indicate that the the 5-6 mm resolution of the scanner does allow substantial recovery of quantitation (66). Consistent with these calculations are studies which indicate the ability of the scanner used to estimate SN DA D2r occupancies by a number of antipsychotic drugs as well as the changes in apparent SN DA D2r levels following DA release and DA depletion (49, 57, 59, 67). There has been one recent [123I]epidepride SPECT study which has reported decreased levels of midbrain uptake in schizophrenic subjects (68). The low resolution of SPECT relative to the size of the substantia nigra does not allow separation of nigral DA D2r from those in other structures. In addition, the lack of a scatter correction, the use of a ratio method using cerebellum as a reference region prior to the attainment of a transient equilibrium, and the variability in this ratio due to lipophilic metabolites of [123I]epidepride in the cerebellum makes interpretation of these results difficult (69-71).
The results of this study confirm the previously reported finding of decreased left medial thalamic DA D2 receptor levels in schizophrenic subjects (24-26). An autoradiographic study of human thalamic DA D2r has reported a heterogenous and nuclear specific distribution of DA D2r with highest levels in the midline and intralaminar nuclei of the thalamus; levels in the dorsomedial nucleus were at least two fold lower than in the midline and intralaminar nuclei (72). While the dorsomedial nucleus accounts for most of the medial thalamic region of interest, the midline and intralaminar nuclei are included in this region of interest. As a number of cognitive functions and behaviors which are impaired in schizophrenia are mediated by prefrontal cortical/basal ganglia/ medial thalamic circuits (73), a loss of DA D2r in the dorsomedial nucleus of the thalamus may contribute to these impairments. The thalamic intralaminar nuclei project to frontal cortex, striatum and limbic regions providing feedback from the thalamus to these regions (74, 75); this feedback is affected by DA D2r in these nuclei providing an additional site for modulation of prefrontal cortical/basal ganglia/ medial thalamic circuit function. The apparent reduction in medial thalamic DA D2r levels may reflect loss of medial thalamic neurons expressing DA D2r consistent with imaging and post mortem studies reporting decreased medial thalamic volume and neuronal numbers (55, 76-79), a loss of autoreceptors on medial thalamic dopaminergic projections, or an increase in thalamic DA release (80). However, increases in thalamic DA release are unlikely to cause the decrease in apparent left medial thalamic DA D2 receptor levels as d-amphetamine administration produces only a 3% decline in medial thalamic [18F]fallypride binding potentials in humans (57).
The current results suggest that different positive symptoms are mediated by DA D2r in different regions. Scores for delusions and bizarre behavior are positively correlated with anterior temporal/temporal tip, and lateral temporal/inferior parietal cortical DA D2r, respectively, while hallucinations are positively correlated with left ventral striatal but not cortical DA D2r. Consistent with these correlations are cerebral blood flow studies in schizophrenic subjects which found positive correlations of left ventral striatal and left temporal tip blood flow in schizophrenic subjects with a reality distortion factor principally related to hallucinations and delusions.(81). A comprehensive review of neurophathological lesions producing schizophrenic symptoms reported an association of striatal lesions with auditory hallucinations, whereas left temporal lobe lesions were associated with delusions (82). The differences in regional correlations for hallucinations and delusions raise the possibility that hallucinations and delusions may be differentially affected by antipsychotic drugs which produce preferential occupancy of temporal cortical versus striatal DA D2r (60, 67, 83, 84). The lack of significant clusters of correlations with negative symptoms suggests that these symptoms may not be mediated by DA D2r neurotransmission.
The positive correlations of positive symptoms with cortical DA D2r levels are similar to a recent [123I]epidepride SPECT study which reported a positive correlation of positive symptoms with frontal cortical DA D2r levels in males but not females(27). In subjects with bipolar disorder psychosis has been correlated with increased striatal DA D2r (85) consistent with the results of the current study. Although previous studies, (23-26) have reported negative correlations of medial, lateral and/or total thalamic DA D2 receptor levels with positive symptoms as measured by the BPRS or with the PANSS general psychopathological scores, no significant correlations of symptom scores with medial thalamic regions of interest were seen.
There a number of potential limitations in this study. These include the small number of subjects studied and the fact that seven of the eleven subjects studied had received previous neuroleptic treatment. While the number of schizophrenic subjects examined in the current study is similar to other PET studies of extrastriatal DA D2r which have studied 7 to 15 subjects, a larger cohort may provide more reliable estimates of DA D2r levels in schizophrenia (22-26). The largest study of extrastriatal DA D2r in unmedicated schizophrenics, a SPECT study of 25 subjects, did not evaluate the regions found to be abnormal in the current study (27). As increased, decreased, and unchanged levels of DA D2r are seen in the current study, it is unlikely that the increased levels seen reflect receptor upregulation due to previous therapy or the decreased levels residual antipsychotic drug effects. Although subjects were not carefully matched for smoking status, it is unlikely that the current results were affected by smoking status as extrastriatal DA D2r levels are not affected by smoking status (86). While females were not carefully matched for menstrual status, one study of the effect of menstrual status on DA D2r levels in humans (86) found no statistically significant effect while a second, older study (87) reported a small effect but no statistical significance was reported; the effect, if any, is small. Finally, extracellular DA levels may be altered in schizophrenia and affect the apparent levels of DA D2r as [18F]fallypride has been shown to be sensitive to extracellular DA levels (49,57).
In conclusion, the results of this study demonstrate increased substantia nigra and decreased left medial thalamic DA D2r levels in off medication schizophrenic subjects. Positive correlations of positive symptoms with temporal cortical and left ventral striatal striatal DA D2r levels were found. The increased substantia nigra DA D2r levels are consistent with the hypothesized nigral dysfunction in schizophrenia. The positive correlations of hallucinations with ventral striatal DA D2r levels, and delusions and bizarre behavior with temporal cortical receptor levels provides additional evidence for the role of DA D2r mediated neurotransmission in these key psychotic symptoms, and suggests that these symptoms may be mediated by DA D2r in different brain regions.
Acknowledgments
This study was supported by NIH grants RO1MH60890 and R21MH68757, and by grants from the Ritter Foundation, Mr. and Mrs. Edward Hintz, and Mr. and Mrs. Robert Peterson.
Footnotes
Dr. Kessler has investigator initiated research support from Janssen, consults with and is a shareholder in PharmorRx. Dr. Herbert Meltzer has been a consultant to and received grants from Abbot, ACADIA, ARYx, BioLine Rx, Bristol Myers Squibb, Cephalon, Eli Lilly, Janssen, Litmus Molecular Design, Memory, Minster, and Pfizer. Dr Meltzer has been a consultant to Astellas, Glaxo Smith Kline, Lundbeck, Merck, Otsuka, Roche, and Solvay, and has received grants from Dainippon Sumitomo, Sepreacor, and SK Pharma. Drs. Woodward, Riccardi, Dawant, and Zald, Mr. Ansari and Li, and Ms. Anderson have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in Schizophrenia: A Review and Reconceptualization. Am J Psychiatr. 1991;148:1474–86. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal Neurons and the Genetics of Schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 3.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. Neurosci. 1991;11:1907–17. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman-Rakic PS. The Relevance of the Dopamine-D1 Receptor in the Cognitive Symptoms of Schizophrenia. Neuropsychopharmacology. 1999;21 6:S171–S177. [Google Scholar]
- 5.Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Robinson D, Lieberman JA, Kane JM. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28(2):270–82. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DA, Hashimoto T, Volk DW. Cortical Inhibitory Neurons and schizophrenia. Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 7.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;8(320 (2)):145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 8.Deutch AY. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm [suppl] 1992;36:61–89. doi: 10.1007/978-3-7091-9211-5_5. [DOI] [PubMed] [Google Scholar]
- 9.Taber MT, Das S, Fibiger HC. Cortical Regulation of Subcortical Dopamine Release: Mediation via the Ventral Tegmental Area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 10.Harden DG, King D, Finlay JM, Grace AA. Depletion of dopamine in the prefrontal cortex decreases the basal electrophysiological activity of mesolimbic dopamine neurons. Brain Research. 1998;794:96–102. doi: 10.1016/s0006-8993(98)00219-4. [DOI] [PubMed] [Google Scholar]
- 11.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single Photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sesack SR, Carr DB. Selective Prefrontal Cortex inputs to dopamine cells:implications for schizophrenia. Physiology & Behavior. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 13.Khan ZU, Gutiérrez A, Martín R, Peňafiel A, Rivera A, DeLa Calle A. Differential Regional and Cellular Distribution of Dopamine D2- Like Receptors: An Immunocytochemical Study of Subtype-Specific Antibodies in Rat and Human Brain. The Journal of Comparative Neurology. 1998;402:353–371. doi: 10.1002/(sici)1096-9861(19981221)402:3<353::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Tseng KY, O'Donnell P. Dopamine-Glutamate Interactions Controlling Prefrontal Cortical Pyrmidal Cell Excitability Involve Multiple Signaling Mechanisms. 2004;24(22):5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor- like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci. 1997;17:5738–46. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. 1990;604:323–43. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- 18.Akil M, Edgar CL, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina - Specific Alterations in the Dopamine innervation of the Prefrontal Cortex in Schizophrenic Subjects. Am J Psychiatry. 1999;156:10. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 19.Akil M, Edgar CL, Pierri JN, Casali Sherry, Lewis DA. Decreased Density of Tyrosine Hydroxylase – Immunoreactive Axons in the Enthorhinal Cortex of Schizophrenic Subjects. Biol Psychiatry. 2000;47:361–370. doi: 10.1016/s0006-3223(99)00282-6. [DOI] [PubMed] [Google Scholar]
- 20.Benes FM, Todtenkopf MS. Effect of age and neuroleptics on tyrosine hydroxylase- IR in sector CA2 of schizophrenic brain. Neuro Report. 1999;10:3527–3530. doi: 10.1097/00001756-199911260-00012. [DOI] [PubMed] [Google Scholar]
- 21.Wyatt RJ, Karoum F, Casanova MF. Decreased DOPAC in the anterior cingulate cortex of individuals with schizoprenia. Biol Psychiatry. 1995;38:4–12. doi: 10.1016/0006-3223(94)00236-V. [DOI] [PubMed] [Google Scholar]
- 22.Suhara T, Yoshiro O, Fumihiko Y, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L. Decreased Dopamine D2 Receptor Binding in the Anterior Cingulate Cortex in Schizophrenia. Arch Gen Psychiatry. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Tuppurainen H, Kuikka J, viinamäki H, Husso-Saastamoinen M, Bergstrom K, Tiihonen J. Extrastriatal dopamine D2/3 receptor density and distributution in drug-naïve schizophrenic patients. Molecular Psychiatry. 2003;8:453–455. doi: 10.1038/sj.mp.4001334. [DOI] [PubMed] [Google Scholar]
- 24.Talvik M, Nordström AL, Olsson H, Halldin C, Farde L. Decreased thalamic D2/D3 receptor binding in drug- naïve patients with schizophrenia: a PET study with [11C] FLB 457. Intl J Neuropsychopharm. 2003;6:361–170. doi: 10.1017/S1461145703003699. [DOI] [PubMed] [Google Scholar]
- 25.Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T, Takano A, Nakayama K, Halldin C, Farde L. Low Dopamine D2 Receptor Binding in Subregions of the Thalamus in Schizophrenia. AM J Psychiatry. 2004;161:1016–1022. doi: 10.1176/appi.ajp.161.6.1016. [DOI] [PubMed] [Google Scholar]
- 26.Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J, Kemether E, Oakes TR, Mukherjee J. D2/D3 dopamine receptor binding with [F-18] fallypride in thalamus and cortex of patients with Schizophrenia. Schizophrenia Research. 2006;85:232–244. doi: 10.1016/j.schres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Glenthoj BY, Mackeprang T, Svarer C, Rasmussen H, Pinborg LH, Friberg L, Baaré W, Hemmingsen R, Videbaek C. Frontal Dopamine D2/3 Rerceptor Binding inn Drug –Naïve First-Episode Schizophrenic Patients Correlates with Postive Psychotic Symptoms and Gender. Biol Psychiatry. 2006;60:621–629. doi: 10.1016/j.biopsych.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hawang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;1(22 (9)):3708–19. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo Y, Suhara T, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y. Decreased prefrontal dopamine D1 receptors in Schizophrenia revealed by PET. Nature. 1997;13(385 (6617)):634–6. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry. 2002;159(5):761–7. doi: 10.1176/appi.ajp.159.5.761. [DOI] [PubMed] [Google Scholar]
- 31.Toru M, Watanabe S, Shibuya H, Nishikawa T, Noda K, Mitsushio H, Ichikawa H, Kurumaji A, Takashima M, Mataga N, Ogawa A. Neurotransmitters, receptors and neuropeptides in postmortem brains of chronic schizophrenic patients. Acta Psychiatr Scand. 1988;78:121–137. doi: 10.1111/j.1600-0447.1988.tb06312.x. [DOI] [PubMed] [Google Scholar]
- 32.Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of th ionotropic glutamate receptor subunits and NMDA receptor- associated intracellular proteins in the substantia nigra in schizophrenia. Brain Research Mol Brain Res. 2004;121:60–69. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Owen R, Owen F, Poulter M, Crow T. Dopamine D2 receptors in substantia nigra in schizophrenia. Brain Research. 1984;299:152–154. doi: 10.1016/0006-8993(84)90798-4. [DOI] [PubMed] [Google Scholar]
- 34.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis Robert. Increased Dopamine Transmission in Schizophrenia. Biological Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 35.Breier A, SU TP, Saunders R, Carson RE, Kolachana BS, Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine- induced synaptic dopamine concentrations. Proc Nat Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. PNAS. 2000;97(NO 14) doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bird ED, Spokes EGS, Iversen LL. Increased Dopamine Concentration In Limbic Areas of Brain From Limbic Areas Of Brain From Patients Dying With Schizophrenia. Brain. 1979;102:347–360. doi: 10.1093/brain/102.2.347. [DOI] [PubMed] [Google Scholar]
- 38.Mackay AVP, Iversen LL, Rossoe M, Spokes E, Bird E, Arregui A, Creese I, Snyder H. Increased Brain Dopamine and Dopamine Receptors in Schizophrenia. Archives of General Psychiatry. 1982;39:991–996. doi: 10.1001/archpsyc.1982.04290090001001. [DOI] [PubMed] [Google Scholar]
- 39.Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve E, Etienne P, Evans AC, Lal S, Shevell M, Savard G, Wong DF, Ghounard G, Gjedde A. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hietala J, Syvälahti E, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Kirvelä O, Rutosalainen U, Salokangas RKR. Presynaptic dopamine function in striatum of neuroleptic-naïve schizophrenic patients. The Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 41.Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ. 6-18F- DOPA PET study in patients with schizophrenia. Psychiatry Research Neuroimagin. 2000;100:1–11. doi: 10.1016/s0925-4927(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 42.Seeman P, Ulpian C, Bergeron C, Riederer P, Jellinger K, Gabriel E, Reynolds GP, Tourtellotte WW. Bimodal Distribution of dopamine receptor densities in brains of schizophrenics. Science. 1984;225:728–731. doi: 10.1126/science.6147018. [DOI] [PubMed] [Google Scholar]
- 43.Seeman P, Bzowej NH, Guan HC, Bergeron C, Reynolds GP, Bird ED, Riederer P, Jellinger K, Tourtellotte WW. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer's, Parkinson's, and Huntington's diseases. Neuropsychopharmacology. 1987;1:5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- 44.Farde L, Wiesel FA, Elander SS, Halldin C, Nordstrom AL. D2 Dopamine Receptors in Neuroleptic-Naïve Schizophrenic Patients. Arch Gen Psychiatry. 1990;47:213–219. doi: 10.1001/archpsyc.1990.01810150013003. [DOI] [PubMed] [Google Scholar]
- 45.Hietala J, Syvälahtti E, Vuorio K, Någren K, Lehikoinen P, Ruotsalainen U, Räkkőäinen V, Lehtinen V, Wegelius U. Striatal D2 dopamine receptor characteristics in neuroleptic-naïve schizophrenic patients studied with positron emission tomography. Arch Gen Psychiatry. 1994;51(2):116–23. doi: 10.1001/archpsyc.1994.03950020040004. [DOI] [PubMed] [Google Scholar]
- 46.Wong DF, Wagner HN, Jr, Tune LE, Dannals Rf, Pearlson GD, Links Jm, Tamminga Ca, Broussolle EP, Ravert HT, Wilson AA, Toung JK, Malat J, Williams JA, O'Tuama LA, Snyder SH, Kuhar MJ, Gjedde A. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naïve schizophrenics. Science. 1986 Dec 19;234(4783):155–63. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 47.Kessler RM, Mason NS, Jones C, Ansari MS, Manning RF, Price RR. [18F] N-allyl-5-fluoropropylepidepride (fallypride):radation dosimetry, quantification of striatal and extrastriatal dopamine receptors in man. NeuroImage. 2000;11:S32. [Google Scholar]
- 48.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain images of 18F- fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 49.Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, Dawant B, Bauernfeind A, Schmidt D, Kessler R. Estimation of Baseline Dopamine D2 Receptor Occupancy in Striatum and Extrastriatal Regions in Humans With Positron Emission Tomography With [18F] Fallypride. Biol Psychiatry. 2008;63:241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiat. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 51.First MB, Spitzer RI, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM- IV Disorders-Patient Edition (with psychotic Screen) (SCID-I/P) (Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 52.Maes F, Collognon A, Vandermuele D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imag. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 53.Wells WM, Viola P, Atsumi H, Nakajima S, Kikinis R. Multimodal volume registration by maximization of mutual information. Med Img Anal. 1996;1:35–51. doi: 10.1016/s1361-8415(01)80004-9. [DOI] [PubMed] [Google Scholar]
- 54.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Acurracy and precision of D (2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Byne W, Buchsbaum MS, Hazlett EA, Kemether E, Shinwari A, Mitropoulou V, Siever LJ. Magnetic Resonance Imaging of the Thalamic Mediodorsal Nucxleus and Pulvinar in Schizophrenia and Schizotypal Personality Disorder. Arch Gen Psychiatr. 2001;58:133–140. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- 56.Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. Yearbook Medical Publisher Inc.; Chicago: 1977. [Google Scholar]
- 57.Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, Park S, Schoenberg E, Schmidt D, Baldwin R, Woodward NS, Kessler R. Amphetamine induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31:1016–1026. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- 58.Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods of analysis of clinical [11C] raclopride studies. J cereb Blood Flow Methab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer H. Occupancy of striatal and extrastriatal DA D2/3 receptors by olanzapine and haloperidol. Neuropsychopharmacology. 2005;30:2283–2289. doi: 10.1038/sj.npp.1300836. [DOI] [PubMed] [Google Scholar]
- 60.Grunder G, Landvogt C, Vernaleken, Buchholz HG, Ondracek J, Siessmeier The striatal and extrastriatal D2/3 receptor binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31:1027–1035. doi: 10.1038/sj.npp.1300931. [DOI] [PubMed] [Google Scholar]
- 61.Siessmeier T, Zhou Y, Buchholz HG, Landvogt C, Vernaleken I, Piel M, et al. Parametric mapping of binding in human brain of D2 receptor ligands of different affinities. J Nucl Med. 2005;46:964–972. [PubMed] [Google Scholar]
- 62.Rohde Gk, Aldroubi A, Dawant BM. The adaptive bases algorithm for intensity- based nonridge image registration. IEEE Trans Med Imaging. 2003;22(11):1470–9. doi: 10.1109/TMI.2003.819299. [DOI] [PubMed] [Google Scholar]
- 63.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster- size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 64.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 65.Williams SM, Goldman-Rakic PS. Widespread origin of the primates mesofrontal dopamine system. Cereb Cortex. 1998;8:321–45. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 66.Kessler RM, Ellis JR, Jr, Eden M. Analysis of Emission Tomographic Scan Data: Limitations Imposed by Resolution and Background. Journal of Computer Assisted Tomography. 1984 June;8(3):514–522. doi: 10.1097/00004728-198406000-00028. [DOI] [PubMed] [Google Scholar]
- 67.Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer Hy. Occupancy of striatal and extrastriatal dopamine D2 receptors by clozapine and quetiapine. Neuropsychopharmacology. 2006;31(9):1991–2001. doi: 10.1038/sj.npp.1301108. [DOI] [PubMed] [Google Scholar]
- 68.Tuppurainen H, Kuikka J, Laakso, Viinamaki H, Husso M, Tiihonen J. Midbrain dopamine D2/3 receptor binding in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:382–387. doi: 10.1007/s00406-006-0649-3. [DOI] [PubMed] [Google Scholar]
- 69.Fujita M, Seibyl JP, Verhoeff NP, Ichise M, Baldwin RM, Zoghbi SS, Burger C, Staley JK, Jajeevan N, Charney DS, Innis RB. Kinetic and equilibrium analyses of [123I]epidepride binding to striatal and extrastriatal dopamine D2 receptors. Synapse. 1999;34:290–304. doi: 10.1002/(SICI)1098-2396(19991215)34:4<290::AID-SYN5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 70.Fujita M, Varrone A, Kim KM, Watabe H, Soghbi SS, Seneca N, Tipre D, Seibyl JP, Innis RB, Iida H. Effect of scatter correction on the compartmental measurement of striatal and extrastriatal dopamine D2 receptors using [123I]epidepride SPET. Eur J Nucl Med Mol Imaging. 2004;31:644–54. doi: 10.1007/s00259-003-1431-7. [DOI] [PubMed] [Google Scholar]
- 71.Ichise M, Fujita M, Seibyl JP, Verhoeff NPLG, Baldwin RM, Zoghbi SS, Rajeevan N, Charney DS, Innis RB. Graphical Analysis and Simplified Quantification of Striatal and Extrastriatal Dopamine D2 Receptor Binding with [123I]Epidepride SPECT. J Nucl Med. 1999;40:1902–1912. [PubMed] [Google Scholar]
- 72.Rieck RW, Ansari MS, Whetesell WO, Detch Ay, Kessler RM. Distribution of Dopamine D2- Like Receptors in the Human Thalamus: Autoradiographic and PET Studies. Neuropsychopharmacology. 2004;29:362–372. doi: 10.1038/sj.npp.1300336. [DOI] [PubMed] [Google Scholar]
- 73.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and limbic functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- 74.Bubser M, Deutch Ay. Thalamic paraventricular nucleus neurons collateralize to innervate the perfrontal cortex and nucleus accumbens. Brain Res. 1998;23(787 (2)):304–10. doi: 10.1016/s0006-8993(97)01373-5. [DOI] [PubMed] [Google Scholar]
- 75.Lis, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;10(506 (2)):263–87. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- 76.Pakkenberg B. Pronounced Reduction of Total Neuron Number in Mediodorsal Thalamic Nucleus and Nucleus Accumbens in Schizophrenics. Arch Gen Psychiatry. 1990;47 doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- 77.Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elkhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem Assessment of Thalamic Nuclear Volumes in Subjects With Schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- 78.Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic Volumes in Patients With First-Episode Schizophrenia. Am J Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 79.Kemether EM, Buchsbaum MS, Byne W, Haznedar M, Brickman AM, Pltholi J, Bloom R. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry. 2003;60:983–91. doi: 10.1001/archpsyc.60.9.983. [DOI] [PubMed] [Google Scholar]
- 80.Oke AF, Adams RN. Elevated Thalamic Dopamine: Possible Link to Sensory Dysfunctions in Schizophrenia. Schizophr Bull. 1987;13:589–604. doi: 10.1093/schbul/13.4.589. [DOI] [PubMed] [Google Scholar]
- 81.Liddle PF, Friston KJ, Frith SR, Hirsch T, Jones T, Frackowiak SR. Patterns of Cerebral Blood Flow in Schizophrenia. British Journal of Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 82.Davison K, Bagley CR. Schizophrenia- like psychoses Associated with Organic Disorders of the Central Nervous System: A review of Literature. British Journal of Psychiatry. 1963:113–182. [Google Scholar]
- 83.Pilowsky LS, Mulligan RS, Acton PD, Ell Pj, Costa DC, Kerwin RW. Limbic selectivity of clozapine. Lancet. 1997;16(350 (9076)):490–1. doi: 10.1016/S0140-6736(05)63079-6. [DOI] [PubMed] [Google Scholar]
- 84.Stephenson CM, Bigliani V, Jones HM, Mulligan RS, Acton PD, Visvikis D, Ell Pj, Kerwin RW, Pilowsky LS. Striatal and extrastriatal D(2)D(3) dopamine receptor occuoancy by quetiapine in vivo.[9123)]-epidepride single photon emission tomography (SPET) study. British Journal of Psychiatry. 2000;177:408–15. doi: 10.1192/bjp.177.5.408. [DOI] [PubMed] [Google Scholar]
- 85.Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, Dannals RF, Wilson AA, Ravert HT, Wagner HN, et al. In Vivo D2 Dopamaine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52(6):471–7. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- 86.Fehr C, Yakushev I, Homann N, Buchholz HG, Landvogt C, Deckers H. Association of low Striatal Dopamine D2 receptor Availability with Nictine Dependance Similar to That Seen With Other Drugs of Abuse. Am J Psychiatry. 2008;165-4:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 87.Nordström AL, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual Cycle. Psychiatry Res. 1998;15(83 (1)):1–6. doi: 10.1016/s0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 88.Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, links JM, Zacur HA, Harris J, Naidu Sakkubai. In Vivo Measurement of Dopamine Receptors in Human Brain By Positron Emission Tomography Age and Sex Differences. Annals New York Academy of Sciences. 2006;515:203–214. doi: 10.1111/j.1749-6632.1988.tb32986.x. [DOI] [PubMed] [Google Scholar]


