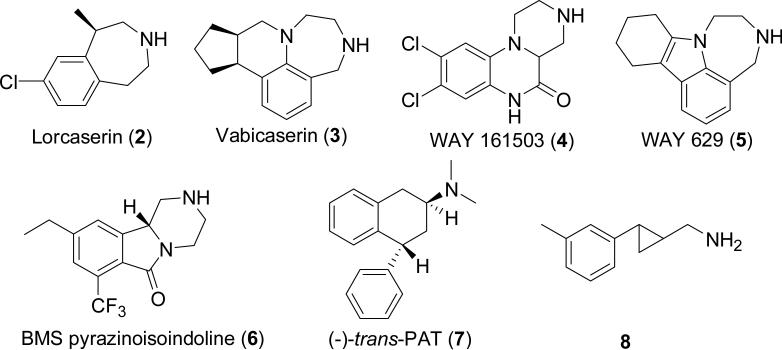
The 5-hydroxytryptamine (1, 5-HT) 2C receptor (5-HT2C), a prominent central serotonin receptor subtype, is widely distributed throughout the central nervous system (CNS) and is thought to play a role in regulating a wide variety of behavioral processes such as mood, appetite, and sexual behavior.[1-4] The 5-HT2A receptor mediates the hallucinogenic activity of drugs such as lysergic acid diethylamide (LSD) and is a major target for treating schizophrenia, insomnia and other disorders.[5-8] The 5-HT2B receptor mediates the potentially lethal valvulopathic side effects of several compounds that were used as prescription drugs.[9, 10]
5-HT2C agonists have demonstrated efficacy in preclinical models of depression, obesity, addiction, and psychosis.[11-13] Targeting the 5-HT2C receptor thus appears to offer a promising means for developing novel therapeutics for the treatment of CNS related disorders. However, as this receptor is homologous to the two other family members, 5-HT2A and 5-HT2B,[14] it is essential that 5-HTHT2C agonists being developed for clinical use show little if any activity at these subtypes.[15] To date, several 5-HTHT2C agonists have shown efficacy in preclinical animal models (Figure 1),[16-18] and are currently undergoing human trials.[16] In particular, one of the most advanced 5-HT2C ligands is Lorcaserin (2) which is being developed by Arena Pharmaceuticals and which has been demonstrated in two Phase III trials to be an orally active, antiobesity medication.[19]
Figure 1.
Several recently developed 5-HT2C agonists.
Based upon the identification of tranylcypromine as the initial hit from an HTS campaign employing a library of FDA approved drugs, we undertook a structural optimization campaign that led to a potent, but moderately selective agonist 8 with 120- and 14-fold selectivity over 5-HT2A and 5-HT2B, respectively (EC50 = 585, 65, and 4.8 nM at the 2A, 2B, and 2C subtypes, respectively). Compound 8 (10–60 mg/kg) was also demonstrated to exhibit moderate antidepressant-like effects in a commonly used behavioral assay.[18]
However, because compound 8 fails to exhibit sufficient selectivity over the 5-HT2B receptor, further optimization was required to identify a potential clinical candidate. Currently, the degree of selectivity that is actually needed to avoid side effects is unknown, however, this is a question that can ultimately be addressed only by studies in humans. Thus, we explored additional, selected modifications of these trans-2-phenylcyclopropylmethylamine analogues in order to improve upon this subtype selectivity issue. Our efforts to modify the 5-HT2C agonist 8 led to the discovery of several new drug candidates with an increased subtype selectivity including dual 5-HT2B antagonism / 5-HTHT2C agonism in the functional assays, and which were thus found suitable for in vivo testing as detailed herein.
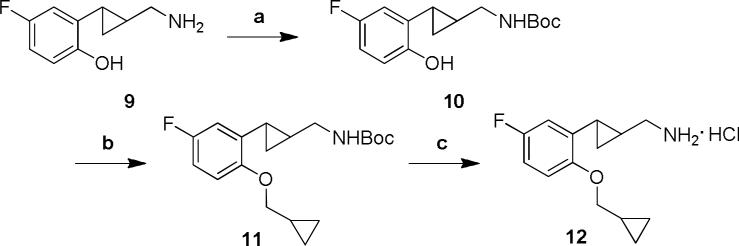
By stepwise structural modifications of the trans-(2-arylcyclopropyl)methylamine aromatic moiety, we found that potent, 5-HT2C-selective compounds could be produced using 5-hydroxy or 5-fluoro substituted systems with a medium sized 2-alkyloxy group. The 2-cyclopropylmethyloxy-5-fluoro substituted derivative 12 was synthesized according to the steps shown in Scheme 1. Thus, the starting compound 9 was prepared by employing a standard sequence of reactions as previously reported.[18] The amino group of the phenolic derivative 9 was protected using Boc-anhydride. The N-Boc protected derivative 10 was then alkylated with cyclopropylmethyl bromide followed by subsequent deprotection to provide the racemic compound 12.
Scheme 1.
Reagents and conditions: (a) Boc2O, triethylamine, CH2Cl2, 0 °C to rt, 5 h. (b) (bromomethyl)cyclopropane, K2CO3, DMF, 60 °C, 20 h. (c) 2M HCl, rt, 48 h.
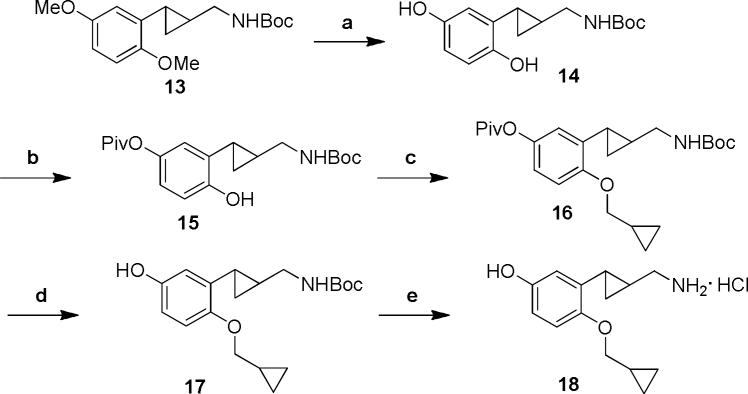
To synthesize the 2-cyclopropylmethyloxy-5-hydroxy substituted derivative 18, the 2-cyclopropylmethyloxy intermediate 16 was prepared in a straightforward manner through a sequence of selective protection and alkylation steps.[20] Next, the pivaloyl and Boc protecting groups were removed sequentially to afford the final product 18 (Scheme 2).
Scheme 2.
Reagents and conditions: (a) (i) BBr3, CH2Cl2, −78 °C to rt, 6 h; (ii) Boc2O, triethylamine, CH2Cl2, 0 °C to rt, 1h. (b) Piv-Cl, CH2Cl2, triethylamine, 0 °C to rt, 6 h. (c) (bromomethyl)cyclopropane, K2CO3, DMF, 60 °C, 20 h. (d) NaOtBu, MeOH, rt, 1 h. (e) 2 M HCl, rt, 48 h.
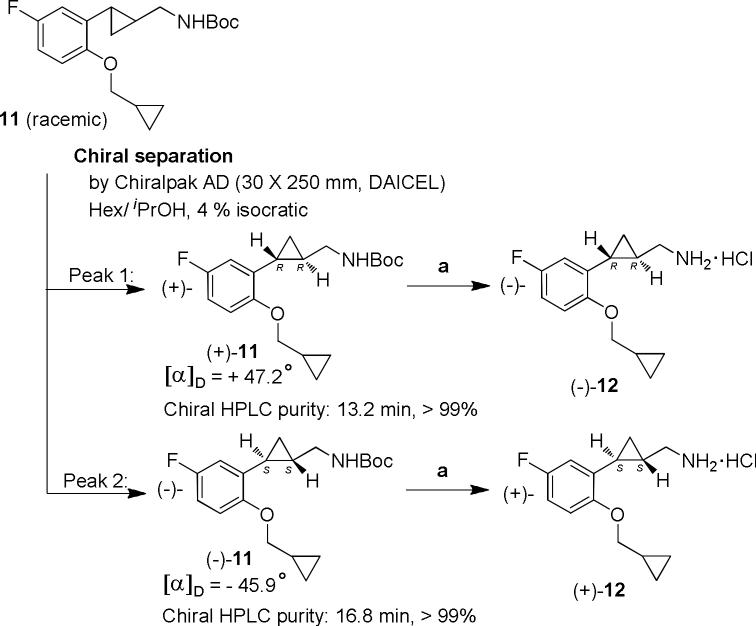
To prepare the optically pure enantiomers of 12 (Scheme 3), we first carried out a chiral separation of the N-Boc protected fluoro derivative 11 using a Chiralpack AD column. Under isocratic conditions (4% isopropanol in hexane), the individual enantiomers could be conveniently separated in pure form (> 99%). Due to the high resolution, stacked injections could be employed in order to increase throughput. The choice of intermediate 11 on which to carry out the chiral separation was based on the ease of the separation and the ready cleavage of the resulting enantiomers under acidic conditions.
Scheme 3.
Reagents and conditions: (a) 2M HCl, rt, 48 h.
The resulting enantiomers (+)-11 and (−)-11 were then converted individually to (−)- and (+)-trans-[2-(2 cyclopropylmethyloxy-5-fluorophenyl)cyclopropyl]-methylamine hydrochlorides ((−)-12 and (+)-12), respectively, using the same method as described above for the racemate. For preparation of the pure enantiomers of compound 18, intermediate 17 was used for the chiral separation.
The functional activity of these two sets of compounds was determined by measuring Gαq mediated intracellular calcium mobilization in HEK-293 cells stably expressing the human 5-HT2A, human 5-HT2B, and human 5-HT2C (INI) receptors.[21] The results are summarized in Table 1. In the first round of the functional assays, racemic 12 was found not to activate either the 5-HT2A or 5-HT2B receptors and to have an EC50 of 19 nM at the 5-HT2C receptor. The more active enantiomer (+)-12 also showed the expected selectivity profile in these functional assays. In contrast, the less active isomer (−)-12 had an EC50 of 918 nM in the 5-HT2C assay. For comparison purposes we also tested the 5-HT2C ligand 2, which is in Phase III clinical trials for obesity. In our hands this compound has a low nM potency at the 5-HT2C receptor, however, it is also fairly active at the 5-HT2B subtype, with an EC50 of 85 nM and an Emax of 93%. As such, there may be some risk for possible valvulopathic side effects induced by this drug. Compound (+)-12 has an even lower selectivity margin towards the 5HT2B-subtype, but due to its low maximal activity, 21%, we believe this will be a very minor concern, especially in view of the 2B-antagonist activity described below. Three other known 5-HT2C agonists Vabicaserin (3) and the Way compounds 4 and 5 along with 5-HT (1) are also included in Table 1 for reference purposes.
Table 1.
Functional Activity and Selectivity of Racemic 12 and 18, their Enantiomers, and Comparison Compounds at the Human 5-HT2A, 5-HT2B, and 5-HT2C Receptors in Calcium Flux Assays Using Stably Transfected HEK-293 Cells.
| 5-HT2A | 5-HT2B | 5-HT2C | Selectivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound[a] | EC50 ± SEM (nM) | %MAX[b] ± SEM (nM) | N[c] | EC50 ± SEM (nM) | %MAX[b] ± SEM (nM) | N[c] | EC50 ± SEM (nM) | %MAX[b] ± SEM (nM) | N[c] | 2A/2C | 2B/2C |
| 1 (5-HT) | 6.9 ± 0.42 | 100% | 15 | 0.7 ± 0.02 | 100% | 16 | 0.1 ± 0.01 | 100% | 16 | 63 | 6.7 |
| (+/-)-12 | NA | 7% ± 1.8% | 3 | NA | 6% ± 0.6% | 3 | 19 ± 3.5 | 68% ± 5.2% | 3 | - | - |
| (+/-)-18 | 761 ± 160 | 11% ± 1.9% | 3 | NA | 2% ± 0.9% | 3 | 9.9 ± 1.7 | 68% ± 4.5% | 3 | 77 | - |
| 2 (Lorcaserin) | 264 ± 31 | 24% ± 0.9% | 6 | 85 ± 7.0 | 93% ± 1.2% | 6 | 2.1 ± 0.29 | 99% ± 1.0% | 7 | 123 | 40 |
| 3 (Vabicaserin) | NA | 2% ± 0.1% | 6 | NA | 5% ± 1.0% | 6 | 6.0 ± 1.0 | 95% ± 0.8% | 7 | - | - |
| 4 (WAY-161503) | 76 ± 9.1 | 80% ± 1.0% | 3 | 15 ± 1.6 | 92% ± 1.3% | 3 | 1.1 ± 0.16 | 97% ± 1.6% | 4 | 70 | 14 |
| 5 (WAY 629) | NA | 1% ± 0.2% | 6 | NA | 6% ± 1.3% | 6 | 286 ± 40 | 80% ± 1.1% | 7 | - | - |
| | |||||||||||
| 1 (5-HT) | 6.1 ± 0.38 | 100% | 9 | 0.8 ± 0.08 | 100% | 9 | 0.1 ± 0.0 | 100% | 9 | 62 | 7.7 |
| (+)-12 | 894 ± 91 | 29 ± 1.6% | 3 | 289 ± 34 | 21% ± 4.9% | 3 | 21 ± 2.2 | 71% ± 4.4% | 3 | 42 | 14 |
| (–)-12 | NA | 0% ± 0.0% | 3 | NA | 4% ± 2.7% | 3 | 918 ± 83 | 35% ± 2.4% | 3 | - | - |
| (+)-18 | 372 ± 131 | 18% ± 3.0% | 3 | NA | 6% ± 2.8% | 3 | 9.3 ± 0.05 | 70% ± 5.4% | 3 | 40 | - |
| (–)-18 | NA | 3% ± 0.6% | 3 | NA | 0% ± 0.5% | 3 | 361 ± 21 | 51% ± 2.4% | 3 | - | - |
| 2 (Lorcaserin) | 136 ± 24 | 31% ± 0.5% | 3 | 50 ± 10 | 86% ± 2.3% | 3 | 1.7 ± 0.09 | 94% ± 0.8% | 3 | 79 | 29 |
| 3 (Vabicaserin) | NA | 7% ± 0.1% | 3 | 47 ± 6.4 | 15% ± 3.3% | 3 | 8.0 ± 1.6 | 88% ± 1.8% | 3 | - | 5.8 |
| 4 (WAY-161503) | 61 ± 8.3 | 79% ± 4.5% | 3 | 16 ± 3.2 | 86% ± 4.7% | 3 | 1.5 ± 0.18 | 94% ± 3.6% | 3 | 40 | 11 |
| 5 (WAY 629) | NA | 7% ± 2.0% | 3 | > 5 μM | 17% ± 2.7% | 3 | 451 ± 52 | 82% ± 3.5% | 3 | - | - |
Tested in two independent screening campaigns using different cell lines / passages; direct comparisons of the potencies and efficacies are only valid within the bounds of each particular table section.
Percent of maximal activation by 5-HT; activation at 10 μM for compounds without EC50 value.
n: Number of concentration curves from ≥ 2 (typically ≥ 3) independent experiments. NA: Emax ≤ 12%. In contrast to binding affinities, the potencies in functional assays can vary strongly depending on cell type, receptor expression level, and passage number.
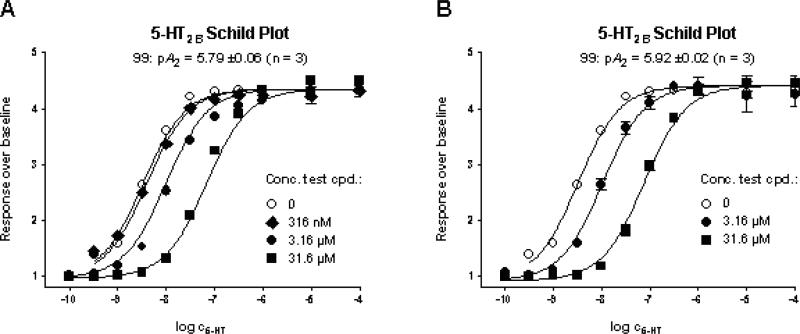
In order to further characterize the pharmacology of the lead compounds, both (+)-12 and (+)-18, which show minimal 5-HT2B activation, were tested for their ability to function as 5-HT2B antagonists. Compounds (+)-12 and (+)-18 were found to shift the concentration curve of 5-HT in calcium flux experiments rightwards without depressing the maximal 5-HT response, indicating fast binding kinetics (Figure 2). Schild analyses yielded pA2 (± SEM) values of 5.50 ± 0.06, 5.79 ± 0.07, and 5.92 ± 0.02, respectively (n = 3). These results show that the tested compounds act as moderate potency, full antagonists at the 5-HT2B receptor.
Figure 2.
5-HT2B antagonism (Schild Plot) of compounds (+)-18 (A) and (+)-12 (B). (pA2 (± SEM): 5.79 ± 0.07 and 5.92 ± 0.02 (n = 3)).
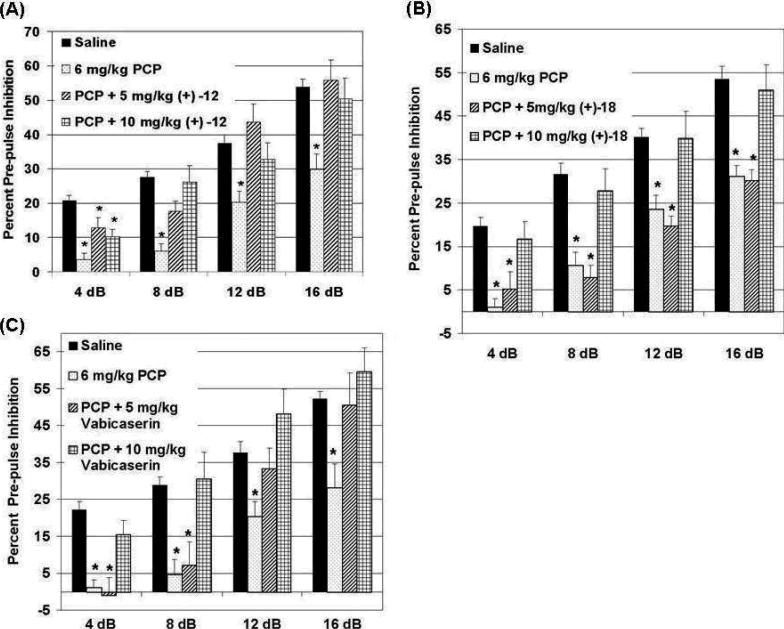
On the basis of their promising in vitro pharmacology, the active enantiomers (+)-12 and (+)-18 were selected for further in vivo studies. Specifically, we examined the ability of these compounds to normalize disrupted prepulse inhibition (PPI) in phencyclidine (PCP) treated animals.[22] The ability to normalize the effects of PCP (an NMDA receptor antagonist)-induced disruption of PPI in mice is a well accepted model of measuring atypical antipsychotic activity [23, 24] and has been used previously to characterize the antipsychotic activity of 5-HT2C agonists.[25] We have successfully used an identical PPI testing paradigm previously to demonstrate clozapine, a classic atypical antipsychotic, robustly normalizes PCP-disrupted PPI.[26] We tested the ability of compounds (+)-12 and (+)-18 as well as 3 as the reference compound, to normalize PCP effects on PPI (Figure 3). As is apparent from the accompanying figures, both (+)-12 and (+)-18 are able to normalize PCP-disrupted PPI at doses of 10 mg/kg (and are comparable in activity to 3), while at the 5 mg/kg dose level, (+)-12 and 3 are still effective.
Figure 3.
Compounds (+)-12 and (+)-18 normalize PCP-disrupted PPI in mice. Mice were administered (ip) saline or 6 mg/kg PCP and immediately tested for PPI of the acoustic startle response. (A) and (B). 30 min pre-treatments of mice with (+)-12 or (+)-18 effectively normalize PCP-disruption of PPI. (C). Compound 3, a positive reference control compound, also normalizes PCP-disrupted PPI. *, p < 0.05 versus saline treated animals. Data are the mean ± SEM. N = 9, 10 or 7 animals in each testing group for (+)-12, (+)-18 or 3, respectively.
Because of its robust activity at normalizing PCP-disrupted PPI, we carried out cytochrome P-450 screening, metabolic stability studies, and hERG assays on (+)-12 in order to qualify it as a possible candidate for further development. In the recombinant CYP inhibition test, (+)-12 showed relatively low inhibition against CYP2C9 (19.6%), CYP2D6 (27.0%), and CYP3A4 (21.7% and 25.1% using midazolam and testosterone as the substrates, respectively, at 10 μM (Table 2). Furthermore, compound (+)-12 had an acceptable microsomal stability in a 1h assay (90.5% left in human microsomes and 66% in rat, respectively) compared with the control drug, verapamil (39.6% using human microsomes). Additionally, no hERG inhibition was detected for (+)-12, (–)-12, or 18, nor for the comparison compounds 2, 3, and 5. However, 4 caused very modest inhibition (3.6 mM, 97%), and the standard cisapride gave full inhibition (35 nM, 100%).
In conclusion, our optimization efforts led to the discovery of the highly selective 5-HTHT2C agonists (±)-12 and (±)-18, and to the identification of (+)-12 and (+)-18 as the more active enantiomers. Compounds (+)-12 and (+)-18 were as effective as the known reference compound 3 in the PCP-PPI animal model of antipsychotic drug activity. Compound 3 was recently in Phase II clinical trials for the treatment of acute exacerbations of schizophrenia. Compound (+)-12 has an acceptable DMPK profile while showing no adverse side effects in animals. Compound (+)-12 represents a structurally simple 5-HT2C partial agonist of excellent potency and high selectivity. The structural relationship of 12 with the marketed monamine oxidase inhibitor tranylcypromine is also noteworthy, and when combined with the information now in hand, bodes well for the further development of this class of molecules. The study of (+)-12 in other animal disease models including cocaine addiction is underway.
Supplementary Material
Acknowledgements
This work was supported by NIH grants R01 DA022317 (A.P.K.) and R01 MH61887, N01 MH80032, and U19 MH82441 (B.L.R.). S.J.C. was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2007-357-C00071) and the NIDA fellowship (2006–2007). J.A.A. was supported by fellowship NICHD T32HD040127 and the UNC Neurodevelopmental Disorders Research Center. We thank Drs. Arsen Gaysin and Rong He for technical assistance. We thank Barbara Caldarone for reviewing the article and providing comments.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author.
Contributor Information
Alan P. Kozikowski, Drug Discovery Program, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy University of Illinois at Chicago, Illinois 60612-7230 (USA)
Sung Jin Cho, Drug Discovery Program, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy University of Illinois at Chicago, Illinois 60612-7230 (USA).
Niels H. Jensen, Departments of Pharmacology and Psychiatry, Comprehensive Cancer Center, Center for Neurobiology, Division of Medicinal Chemistry and Natural Products and NIMH Psychoactive Drug Screening Program, University of North Carolina Medical School, Chapel Hill CB 7365, North Carolina 27599 (USA)
John A. Allen, Departments of Pharmacology and Psychiatry, Comprehensive Cancer Center, Center for Neurobiology, Division of Medicinal Chemistry and Natural Products and NIMH Psychoactive Drug Screening Program, University of North Carolina Medical School, Chapel Hill CB 7365, North Carolina 27599 (USA)
Andreas M. Svennebring, Drug Discovery Program, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy University of Illinois at Chicago, Illinois 60612-7230 (USA)
Bryan L. Roth, Departments of Pharmacology and Psychiatry, Comprehensive Cancer Center, Center for Neurobiology, Division of Medicinal Chemistry and Natural Products and NIMH Psychoactive Drug Screening Program, University of North Carolina Medical School, Chapel Hill CB 7365, North Carolina 27599 (USA)
References
- 1.Chou-Green JM, Holscher TD, Dallman MF, Akana SF. Physiol. Behav. 2003;78:641. doi: 10.1016/s0031-9384(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 2.Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. J. Neurosci. 2002;22:10039. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Nature. 1995;374:542. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 4.Millan MJ, Peglion JL, Perrin-Monneyron GSL. Eur. J. Pharmacol. 1997;325:9. doi: 10.1016/s0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- 5.Nichols DE. Pharmacol. Ther. 2004;101:131. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson BM. J. Med. Chem. 2006;49:4023. doi: 10.1021/jm058240i. [DOI] [PubMed] [Google Scholar]
- 7.Lacivita E, Leopoldo M. Curr. Top. Med. Chem. 2006;6:1927. doi: 10.2174/156802606778522168. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad S, Ngu K, Miller KJ, Wu G, Hung CP, Malmstrom S, Zhang G, O'Tanyi E, Keim WJ, Cullen MJ, Rohrbach KW, Thomas M, Ung T, Qu Q, Gan J, Narayanan R, Pelleymounter MA, Robl JA. Bioorg. Med. Chem. Lett. 20:1128. doi: 10.1016/j.bmcl.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Circulation. 2000;102:2836. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 10.Roth BL. N. Engl. .J Med. 2007;356:6. [Google Scholar]
- 11.Dunlop J, Marquis KL, Lim HK, Leung L, Kao J, Cheesman C, Rosenzweig-Lipson S. CNS Drug. Rev. 2006;12:167. doi: 10.1111/j.1527-3458.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbassi S, Cervo L. Psychopharmacology (Berl) 2008;196:15. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller KJ. Mol. Interv. 2005;5:282. doi: 10.1124/mi.5.5.8. [DOI] [PubMed] [Google Scholar]
- 14.Kroeze WK, Kristiansen K, Roth BL. Curr. Top. Med. Chem. 2002;2:507. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- 15.Berger M, Gray JA, Roth BL. Annu. Rev. Med. 2009;60:355. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacker DA, Miller KJ. Curr. Opin. Drug Discovery Dev. 2008;11:438. [PubMed] [Google Scholar]
- 17.Booth RG, Fang L, Huang Y, Wilczynski A, Sivendran S. Eur. J. Pharmacol. 2009;615:1. doi: 10.1016/j.ejphar.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho JS, Jensen HN, Kurome T, Kadari S, Manzano LM, Malberg EJ, Caldarone B, Roth LB, Kozikowski PA. J. Med. Chem. 2009;52:1885. doi: 10.1021/jm801354e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfson W. Chem. Biol. 2008;15:1139. doi: 10.1016/j.chembiol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Yao Q. Angew. Chem., Int. Ed. 2000;39:3896. doi: 10.1002/1521-3773(20001103)39:21<3896::AID-ANIE3896>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. Neuropsychopharmacology. 2008;33:2303. doi: 10.1038/sj.npp.1301646. [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow NR, Braff DL, Taaid NM, Geyer MA. Arch. Gen. Psychiatry. 1994;51:139. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 23.Geyer MA, Ellenbroek B. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2003;27:1071. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Psychopharmacology (Berlin, Ger.) 2001;156:117. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 25.Marquis LK, Sabb LA, Logue FS, Brennan AJ, Piesla JM, Comery AT, Grauer MS, Ashby RC, Nguyen QH, Dawson AL, Barrett EJ, Stack G, Meltzer YH, Harrison LB, Rosenzweig-Lipson S. J. Pharmacol. Exp. Ther. 2007;320:486. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- 26.Abbas IA, Yadav NP, Yao W-D, Arbuckle IM, Grant GNS, Caron GM, Roth LB. J. Neurosci. 2009;29:7124. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.