Abstract
Cross-sectional surveys of human blood and breast milk show increasing concentrations of polybrominated diphenyl ethers (PBDEs) that parallel the expanded use in consumer products, but longitudinal studies are lacking. We compared levels of major BDE congeners in archived 1994–1995 blood samples collected from a cohort of frequent and infrequent Great Lakes fish consumers with levels in the blood collected from the same individuals in 2001–2003 and 2004–2005. In mixed linear regression models controlling for multiple measurements per individual and covariates, statistically significant increases were seen from 1994–1995 to 2001–2003 for ΣPBDEs and BDE-47, 99, and 153 and from 1994–1995 to 2004–2005 for ΣPBDEs and BDE-99, 100, and 153, but ΣPBDEs and BDE congeners did not change significantly between 2001–2003 and 2004–2005. Changes in body burdens of ΣPBDEs and BDE-47, 100, and 153 in men were modified by BMI, with greater increases in men with higher BMI. Increases in BDE-153 were greater for women than men, and a greater increase in BDE-100 was found in older participants. There was a shift in the congener distribution with a significant increase in the proportion of BDE-153 relative to BDE-47 from 2001–2003 to 2004–2005.
Keywords: PBDE, biomonitoring, time trends, fish consumers, Great Lakes
1. Introduction
Since the 1960s, polybrominated diphenyl ethers (PBDEs) have been added as flame retardants to a wide variety of consumer goods including electronics, building materials, foams and upholstery in furnishings, automobiles, and aircraft. There are three types of commercial PBDE products referred to as pentaBDE, octaBDE and decaBDE. Although the manufacture of pentaBDE and octaBDE was voluntarily discontinued by the U.S. manufacturer in 2004, and decaBDE will be phased out in the U.S. by 2013, treated materials will continue to be in use for several years or decades posing an ongoing exposure hazard (Robinson, 2009).
PentaBDE is the major source of BDE-47 and BDE-99, and also contains BDE-100, BDE-153 and BDE-154 (Birnbaum and Cohen Hubal, 2006). BDE-153 and BDE-154 are also breakdown products of BDE-209 (Soderstrom et al., 2004). Half-lives of these congeners vary, with estimates of 1.8, 2.9, 1.6, 3.3, and 6.5 years for BDE-47, 99, 100, 154, and 153, respectively (Geyer et al., 2004). The dominant congener in most people is BDE-47, although 10.5% of a sample of the U.S. population had higher levels of BDE-153 than BDE-47 (Sjodin et al., 2008). While the human exposure pathway for many persistent pollutants, such as dioxins, is predominantly through contaminated food consumption, the indoor environment is a substantial contributor to PBDE exposure (Wu et al., 2007; Imm et al., 2009). However, in select individuals exposures may be higher from consumption of animal products and fish (Jones-Otazo et al., 2005) and intake of red meat and poultry also contribute significantly to PBDE exposure in the US, with a potentially stronger contribution from BDE-153 than other congeners (Fraser et al., 2009).
PBDE levels in human serum have increased exponentially by a factor of about 100 from 1970 to 2002 (Hites, 2004), with the concentrations in residents in North America an order of magnitude higher than those reported from Europe or Japan (Hites, 2004; Frederiksen et al., 2009). Human PBDE body burdens have been monitored in both serum and breast milk. Researchers in Sweden were alarmed to find a rapid rise in the PBDE levels in breast milk collected between 1972 and 1997 (Meironyte et al., 1999). In response the Swedish government halted PBDE production. Continued monitoring of PBDEs in breast milk from 1998 to 2002 demonstrated that concentrations of lower brominated PBDE levels, particularly BDE-47, have decreased significantly, but concentrations of BDE-153 continue to increase (Fangstrom et al., 2008). Similarly, increases in PBDEs have been found in pooled breast milk from the Faroe Islands from 1987 to 1999 (Fangstrom et al., 2005) and Japan from 1973 to 1988 (Akutsu et al., 2003). Serum PBDE increased in Japan from the early 1980s to the mid 1990s (Koizumi et al., 2005) and in the US from 1973 to 2003 (Schecter et al., 2005) and 1985 to 2002 (Sjodin et al., 2004).
Concern over human exposure to PBDEs stems from their structural similarity to PCBs, which disrupt memory and learning, growth, endocrine function, and immune function, and is supported by evidence of associations of PBDEs with congenital cryptorchidism (Main et al., 2007), lower birth weight (Chao et al., 2007), neurodevelopmental delays (Roze et al., 2009; Herbstman et al., 2010), increased time to pregnancy (Harley et al., 2010), and thyroid hormone abnormalities (Turyk et al., 2008).
The Great Lakes Fish Consumption Study is a longitudinal cohort of frequent and infrequent sport fish consumers that was established to assess exposure to persistent contaminants found in Great Lakes sport fish in. In 1994–1995, PCB and p,p’-diphenyldichloroethene (DDE) levels were highest among men who were frequent consumers of Great Lake sport fish and lowest among women in the infrequent consumers group (Hanrahan et al., 1999). Reevaluations in the early 2000s found decreases in PCBs, DDE, and Great Lakes sport fish consumption (Knobeloch et al., 2009). In contrast to PCBs and DDE, PBDEs were not strongly related to sport fish consumption, but multivariate models identified age, years consuming sport fish, shellfish intake rates, and computer use as independent positive predictors and recent weight loss as an independent negative predictor of PBDEs (Anderson et al., 2008). A follow up study in which x-ray fluorometers were used to assess bromine levels in household furnishings and automotive interiors identified foam bed pillows and automobile upholstery to be correlated with serum PBDE levels among a sub-cohort of 44 individuals (Imm et al., 2009). The purpose of this report is to summarize the temporal trends in body burdens in 168 frequent and infrequent sport fish consumers with 2 or 3 repeated PBDE measurements between 1994 and 2005.
2. Subjects and Methods
The protocol for this investigation was approved by the University of Wisconsin-Madison and University of Illinois at Chicago Human Subjects institutional review boards. All subjects provided informed consent authorizations before participation.
2.1 Study population and sampling in 1994–1995, 2001–2003, and 2004–2005
A cohort of 4,206 frequent and infrequent sport fish consumers was established in 1993–1994 that included charter boat captains on the Great Lakes (captain group), infrequent Great Lakes fish consumers from similar geographic areas as the captains (referent group) and Wisconsin anglers (angler group). Blood samples were collected in 1994–1995 from 445 frequent and 99 infrequent fish consumers for PCB and DDE analysis. Information about fish consumption habits and demographics was obtained. In 2007–2008, 118 archived serum samples were analyzed for PBDEs. In 2001–2003, participants who donated blood in 1994–1995 were contacted. A survey on fish consumption was administered and blood samples were collected analyzed for PCBs, DDE and PBDEs from 213 participants. In 2004–2005, 515 participants provided a blood sample for exposure measurements and completed an additional survey on fish consumption. Participants with 2 or 3 repeated PBDE measurements (n=168) were included in the analysis. This includes 118 participants with paired samples from 1994–1995 and 2001–2003 and 112 participants with paired samples from 2001–2003 and 2004–2005 (Table 1). A total of 62 participants had measurements at all 3 time points.
Table 1.
Characteristics of Study Participants at Sample Collections
| Group | Characteristic | 1994–1995 | 2001–2003 | 2004–2005 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | N | Median | Range | N | Median | Range | N | ||
| All Participants | Age (years) | 48 | 26–74 | 118 | 56 | 34–82 | 168 | 61 | 39–79 | 112 |
| BMI (kg m−2) | 28.1 | 18.4–46.3 | 118 | 28.5 | 17.3–47.2 | 168 | 28.9 | 19.5–52.5 | 112 | |
| Serum lipids (mg dL−1) | 626 | 495–789 | 13 | 667 | 370–2362 | 147 | 685 | 435–1172 | 112 | |
| Sport fish meals | 24 | 0–156 | 105 | 24 | 0–156 | 150 | 16.5 | 0–153 | 112 | |
| Great Lake fish meals | 12 | 0–156 | 105 | 12 | 0–156 | 147 | 11 | 0–131 | 112 | |
| Participants Measured in1994–1995 & 2001–2003a | Age (years) | 48 | 26–74 | 118 | 55 | 34–82 | 118 | |||
| BMI (kg m−2) c | 28.1 | 18.4–46.3 | 118 | 28.5 | 19.4–47.2 | 118 | ||||
| Serum lipids (mg dL−1) | 626 | 495–789 | 13 | 666 | 370–1544 | 99 | ||||
| Sport fish meals | 24 | 0–156 | 105 | 24 | 0–156 | 104 | ||||
| Great Lake fish meals | 12 | 0–156 | 105 | 12 | 0–156 | 102 | ||||
| Participants Measured in2001–2003 & 2004–2005b | Age (years) | 58 | 36–75 | 112 | 61 | 39–79 | 112 | |||
| BMI (kg m−2) d | 28.5 | 17.3–46.6 | 112 | 28.9 | 19.5–52.5 | 112 | ||||
| Serum lipids (mg dL−1) | 676 | 424–2362 | 102 | 685 | 435–1172 | 112 | ||||
| Sport fish meals | 21 | 0–156 | 100 | 16.5 | 0–153 | 112 | ||||
| Great Lake fish meals | 12 | 0–150 | 101 | 11 | 0–131 | 112 | ||||
| Participants Measured in1994–1995, 2001–2003 & 2004–2005a | Age (years) | 49 | 31–68 | 62 | 56 | 39–75 | 62 | 59 | 41–79 | 62 |
| BMI (kg m−2) d | 28.1 | 18.4–46.3 | 62 | 28.5 | 20.2–46.2 | 62 | 28.9 | 19.5–44.8 | 62 | |
| Serum lipids (mg dL−1) | 626 | 495–789 | 9 | 677 | 465–1007 | 54 | 669 | 435–1172 | 62 | |
| Sport fish meals | 24 | 0–104 | 57 | 24 | 0–156 | 54 | 22 | 0–137 | 62 | |
| Great Lake fish meals | 12 | 0–104 | 57 | 12 | 0–104 | 56 | 12 | 0–131 | 62 | |
50% males
59% males
p<0.05 for signed rank tests on paired samples from 1994–1995 and 2001–2003
p<0.05 for signed rank tests on paired samples from 2001–2003 and 2004–2005
2.2 Laboratory Analysis of persistent pollutants
Sera were analyzed at the Wisconsin State Laboratory of Hygiene (Imm et al., 2009). A sample of 4 to 5 ml of serum was extracted with hexane/ethyl ether, with clean-up and fractionation using florisil, silica-gel and concentrated sulfuric acid. PBDEs were analyzed by gas chromatography-mass spectrometry (GC/MS) and PCBs and DDE by gas chromatography-electron capture detector (GC-ECD). Quality control was monitored by the use of method blanks, spikes of bovine serum, duplicates of bovine serum spikes or sample duplicates, surrogate spikes, and confirmation of the analytes by second column or GC/MS as appropriate. Mean PBDE recoveries for tri- to deca-BDE congeners ranged from 67 to 90%. Mean PCB recoveries for di- to nona-PCB congeners ranged from 71 to 102%, while p,p’-DDE recovery averaged 94%.
We analyzed 24 congeners (BDE 17, 28, 47, 49, 66, 71, 77, 85, 99, 100, 119, 126, 138, 153, 154, 156, 183, 184, 191, 196, 197, 206, 207, and 209), and quantified seven (BDE conge-ners 28, 47, 85, 99, 100, 153, and 154). BDE-154 was excluded because it was found to co-elute with polybrominated biphenyl (PBB)-153, which is a concern because 35% of our cohort resided in Michigan, where accidental contamination of meat, milk and poultry in the 1970s lead to measurable general population PBB human body burdens not seen elsewhere in the US (Wolff et al., 1982).
2.3 Stability of Stored Sera
Serum samples from 1994–1995 study participants were archived in long term cold storage at −20ºC prior to analysis for PBDEs in 2007–2008. To examine storage-related changes, we analyzed eight archived serum samples for PCBs and DDE in 2007–2008 and compared the results with those determined in 1994–1995. To facilitate comparison with PBDE analyses, we calculated annualized percent change in paired samples, assuming 13 years of storage (1994–2007). Median annualized percent change during storage was: DDE=0.2%; PCB 132/153/105=−1.0%; PCB 163/138=−0.6%; PCB 180=−1.1%. Using paired t-tests on natural log-transformed analytes, original and archived serum measurements did not differ significantly, with the exception of PCB 180 (p=0.05).
2.4 Measurement of Serum Lipids
Total cholesterol and triglycerides were measured by Quest Diagnostics (Auburn Hills, MI and Wood Dale, IL) for samples collected in 2004–2005 and by Meriter Laboratories (Madison, WI) for samples collected in 2001–2003. Lipids were measured in one third of the 1994–1995 samples by the Centers for Disease Control. Total serum lipids were calculated by the formula: .
2.5 Statistical Methods
ΣPBDEs is the sum of BDE-28, 47, 49, 99, 100,138, and 153. When individual congeners were below the limit of detection (LOD), we imputed the values below the LOD as the LOD/2. Because ΣPBDEs followed a log normal distribution, we used a natural log transformation for mixed-effect models. A natural log transformation was also use for fish meals, congeners and the ratio of BDE-153:47; however a normal distribution was not achieved for BDE-99, 100 and 153.
Associations among ΣPBDEs and individual congeners were examined using Spearman’s correlation coefficients. Non-parametric signed rank tests were used to assess differences in exposures and demographics in paired samples.
Annual percent change was used to estimate changes in PBDEs over time (Knobeloch et al., 2009).
Data across the three time periods was combined using mixed-effects models with random intercepts (PROC MIXED, SAS version 9.1 for Windows; SAS Institute Inc., Cary, NC) to evaluate effects of time and covariates on measures of ΣPBDEs, BDE congeners, the ratio of BDE-153:47, and the percent contribution of BDE-47 and 153 to total PBDEs. These models account for the lack of independence among measurements collected on the same individual. Estimates of least square geometric means and 95% confidence intervals for exposures at the three sampling times were derived from these models, with post hoc analysis using Tukey-Kramer adjustment to assess differences in exposures between sampling times. All models included age in 1994–1995, body mass index (BMI), and gender. Because previous studies have found that PBDEs vary non-linearly with age (Sjodin et al., 2008; Toms et al., 2009), we explored models which included categorical variables for age (26–44 years, 45–54 years, ≥ 55 years) as well as quadratic models with the variables age and age2. We also included fish consumption variables in the models when significant (study group, annual sport fish meals, and annual Great Lakes sport fish meals).
To determine if age at baseline, BMI, gender and fish meals affected changes in exposures across time, we estimated interaction terms (the product of time with covariate) in mixed effects models. When the beta estimate for an interaction was significant, exposure changes across time were estimated in mixed effect models stratified by the median level of the effect modifier. Estimates for effect modification by BMI were also modeled after stratification by gender.
3. Results
3.1 Participants
A total of 74 women and 94 men who had at least 2 PBDE measurements were included in the analysis. At recruitment, the participants had been consuming Great Lakes sport fish consumption for 21 years on average and 90% were frequent fish consumers from the captain or angler groups. Demographic characteristics of study participants at the times of the blood collections are shown in Table 1.
3.2 PBDE Exposures
The most commonly detected congeners were BDE-47, 99, 100, and 153 (Table 2). ΣPBDEs were most strongly associated with BDE-47 at all collections, while associations of BDE-99, 100 and 153 with ΣPBDEs became stronger at later sampling times (Table S1). Associations of ΣPBDEs with individual congeners were generally weakest for BDE-153. The association of ΣPBDEs measured in 2004–2005 and 2001–2003 (r=0.64, p<0.0001) was stronger than associations of 1994–1995 levels with 2004–2005 levels (r=0.40, p=0.001) and 2001–2003 levels (r=0.27, p=0.0004) (Table S2). Similar patterns of associations were seen for BDE-47, 99, and 100, but associations of BDE-153 measured at the three time points did not differ to the same extent (r=0.42 to r=0.57, Table S2).
Table 2.
Distribution of BDE and Congeners ΣPBDEs (ng g−1 whole serum) in Participants
| Contaminant | LOD | Year | N | %<LOD | Arithmetic Mean | Percentile: | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | 100th | ||||||
| BDE 28 | 0.025 | 1994–1995 | 118 | 94.1 | 0.019 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.240 |
| 2001–2003 | 168 | 90.5 | 0.017 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.160 | ||
| 2004–2005 | 112 | 88.4 | 0.018 | 0.013 | 0.013 | 0.013 | 0.013 | 0.0037 | 0.110 | ||
| BDE 47 a,b | 0.025 | 1994–1995 | 118 | 1.7 | 0.257 | 0.031 | 0.047 | 0.067 | 0.110 | 0.330 | 6.000 |
| 2001–2003 | 168 | 4.8 | 0.293 | 0.037 | 0.062 | 0.108 | 0.190 | 0.610 | 6.800 | ||
| 2004–2005 | 112 | 0 | 0.200 | 0.048 | 0.063 | 0.090 | 0.165 | 0.310 | 3.900 | ||
| BDE 49 | 0.025 | 1994–1995 | 118 | 96.6 | 0.033 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 2.200 |
| 2001–2003 | 168 | 98.8 | 0.017 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.730 | ||
| 2004–2005 | 112 | 99.1 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.033 | ||
| BDE 85 | 0.025 | 1994–1995 | 118 | 95.8 | 0.016 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.150 |
| 2001–2003 | 168 | 95.2 | 0.017 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.250 | ||
| 2004–2005 | 112 | 97.3 | 0.014 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.120 | ||
| BDE 99 a | 0.025 | 1994–1995 | 118 | 66.1 | 0.072 | 0.013 | 0.013 | 0.013 | 0.028 | 0.067 | 2.200 |
| 2001–2003 | 168 | 35.7 | 0.088 | 0.013 | 0.013 | 0.032 | 0.053 | 0.150 | 1.900 | ||
| 2004–2005 | 112 | 28.6 | 0.057 | 0.013 | 0.013 | 0.031 | 0.047 | 0.087 | 1.100 | ||
| BDE 100 a | 0.025 | 1994–1995 | 118 | 85.1 | 0.041 | 0.013 | 0.013 | 0.013 | 0.013 | 0.039 | 1.000 |
| 2001–2003 | 168 | 76.8 | 0.053 | 0.013 | 0.013 | 0.013 | 0.013 | 0.120 | 1.800 | ||
| 2004–2005 | 112 | 60.7 | 0.047 | 0.013 | 0.013 | 0.013 | 0.044 | 0.070 | 1.300 | ||
| BDE 153 a | 0.05 | 1994–1995 | 118 | 90.7 | 0.049 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.620 |
| 2001–2003 | 168 | 73.2 | 0.071 | 0.025 | 0.025 | 0.025 | 0.052 | 0.120 | 2.200 | ||
| 2004–2005 | 112 | 68.8 | 0.079 | 0.025 | 0.025 | 0.025 | 0.082 | 0.130 | 2.200 | ||
| ΣPBDE a | 1994–1995 | 118 | 0.486 | 0.119 | 0.135 | 0.158 | 0.243 | 0.582 | 12.330 | ||
| 2001–2003 | 168 | 0.557 | 0.128 | 0.165 | 0.218 | 0.365 | 1.087 | 13.098 | |||
| 2004–2005 | 112 | 0.428 | 0.139 | 0.166 | 0.210 | 0.382 | 0.630 | 8.763 | |||
p<0.05 for signed rank tests on paired samples from 118 individuals collected in 1994–1995 and 2001–2003
p<0.05 for signed rank tests on paired samples from 112 individuals collected in 2001–2003 and 2004–2005
3.3 Changes in PBDE Exposure
In this cohort the number of serum samples with detectable levels of BDE congeners increased over time (Table 2). In univariate analyses, ΣPBDEs, BDE-47, 99, 100, and 153 increased significantly from 1994–1995 to 2001–2003 in paired samples from 118 individuals, while BDE-47 decreased significantly from 2001–2003 to 2004–2005 in paired samples from 112 individuals (Table 2). Annualized percent change and the proportion of participants with increases in ΣPBDEs and BDE congeners between the sampling times are shown in Table S3.
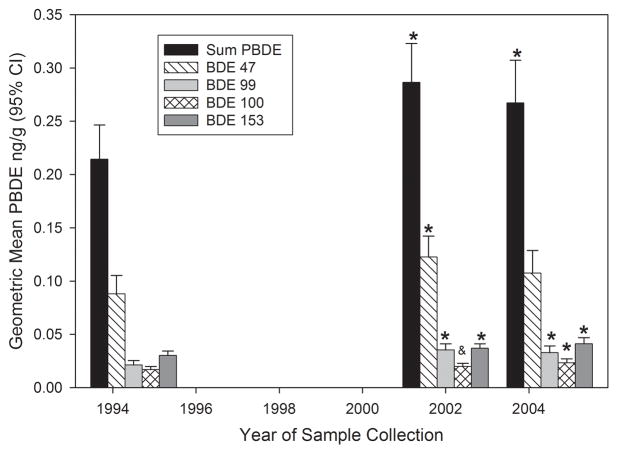
Least square geometric means for ΣPBDEs and BDE congeners, adjusted for age in 1994–1995, gender, and BMI are shown in Figure 1. In 2001–2003, ΣPBDEs and BDE-47, 99, and 153 increased significantly compared with 1994–1995 levels, while in 2004–2005 ΣPBDEs and BDE-99, 100, and 153 increased significantly compared with 1994–1995 levels. However, ΣPBDEs and BDE congeners did not differ significantly between 2001–2003 and 2004–2005.
Figure 1. Geometric Means for ΣPBDEs and BDE Congeners Across Time.
Least square means estimated in mixed-effect models of natural log transformed ΣPBDEs and BDE congeners, with post hoc analysis using Tukey-Kramer adjustment to determine significant differences in exposures among sampling times. ΣPBDEs and all congeners adjusted for age at 1994–1995, gender, and BMI; BDE 153 also adjusted for angler group membership.
* = greater than geometric mean in 1994–1995 (p<0.05).
& = greater than geometric mean in 1994–1995 (0.05<p<0.10).
Changes in ΣPBDEs and BDE congeners across time were modified by age in 1994–1995, BMI and gender, but not by annual intake of Great Lakes sport fish or annual sport fish consumption (not shown). Change in BDE-100 was modified by age, with significant increases in older participants (p=0.01 for interaction term beta, not shown). Change in BDE-153 was modified by gender, with significant increases seen in women but not men (p=0.01 for interaction term beta, not shown). In both genders, greater increase in BDE-100 concentrations were associated with higher BMIs (p=0.053 for interaction term beta, not shown). In gender-stratified analyses, changes in ΣPBDEs and BDE-47, 100, and 153 were modified by BMI in men, with significant increases in men with higher BMI (p<0.05 for male interaction term beta, not shown).
3.4 Changes in BDE 47 Relative to BDE 153
The proportion of participants with higher body burdens of BDE-153 than BDE-47 increased from 3.3% in 1994–1995 to 8.9% in 2004–2005 (Table 3). The ratio of BDE 153:47 across time is shown in Table 3. The least square geometric mean ratio was significantly higher in 2004–2005 than in 2001–2003. The percent contribution of BDE-153 to ΣPBDEs increased significantly between 2001–2003 and 2004–2005, while the percent contribution of BDE-47 to ΣPBDEs decreased significantly over this time period. Changes in the ratio of BDE153:47, percent BDE-153 and BDE-47 across time were not modified by demographic or fish consumption variables.
Table 3.
Relative Levels of BDE 47 and BDE 153 in Participants Across Time
| 1994–1995 | 2001–2003 | 2004–2005 | |
|---|---|---|---|
| Percent with BDE 153 > BDE 47 | 3.3% | 3.6% | 8.9% |
| Geo Mean Ratio BDE 153/BDE47 (95% CI) a | 0.35 (0.30, 0.41) | 0.31 (0.27, 0.35) | 0.39 (0.33, 0.45) b |
| Mean Percent BDE 153/Sum PBDEs (95% CI) a | 16.4 (14.6, 18.2) | 15.4 (13.9, 16.9) | 18.3 (16.5, 20.1) b |
| Mean Percent BDE 47/Sum PBDEs (95% CI) a | 43.0 (40.1, 45.3) | 45.5 (43.6, 47.5) | 41.8 (39.4, 44.1) b |
Least square means estimated in random intercept mixed-effect models adjusted for age, BMI, and gender, with post hoc analysis using Tukey-Kramer adjustment to determine significant differences in exposures among sampling times.
significantly greater than geometric mean in 2001–2003 (p<0.05).
3.5. Associations of PBDE Exposures with Demographics and Fish Consumption
In univariate analyses, age was positively associated with ΣPBDEs and BDE, and most correlation coefficients reached significance (Table S4). BMI was inconsistently associated with ΣPBDEs and BDE congeners, and several associations reached significance in 2001–2003 (Table S4). Serum lipids, fish consumption (Table S4) and gender (not shown) were not consistently associated with ΣPBDEs and BDE congeners. In 2001–2003 and 2004–2005 BDE-153 levels varied by enrollment group, being highest in the captain group (p=0.05, p=0.07, respectively, not shown).
In multivariate models examining exposure levels, and relative conger levels across time (sections 3.3 and 3.4), age was modeled as a categorical variable. On average across all time periods, ΣPBDEs, BDE-47, and BDE-100 were significantly higher in ages 45–54 and ≥ 55 years than 26–44 years, BDE-99 was significantly higher in ages ≥ 55 years than 26–44 years, BDE-153 was significantly higher in ages 45–54 than ≥ 55 years and 26–44 years, BDE153:47 and percent BDE-47 were higher in participants ≥ 55 years than 26–44 years and 45–54 years, and the percent BDE-153 was higher in participants 45–54 years than ≥ 55 years (not shown). BMI was positively related to levels of ΣPBDEs, BDE-47, 99 and 100, and percent BDE-47 and inversely related to percent BDE-153 and BDE-153:47 (not shown). Gender and fish consumption were not associated with exposures, with the exception the Wisconsin angler group that had lower BDE-153 (p=0.06).
4. Discussion
We found that geometric mean levels of PBDEs increased significantly from 1994–1995 to both follow up sampling times, but not from 2001–2003 to 2004–2005. Overall, PBDEs increased in 67% of our participants between 1994–1995 and 2001–2003 and 69% of participants between 1994–1995 and 2004–2005. The median annual percent change was 3.8% from 1994–1994 to 2001–2003, 1.7% from 1994–1995 to 2004–2005, and −1.1% from 2001–2003 to 2004–2005. We recently reported that geometric mean concentrations of serum PBDEs decreased less than 2% between 2004–2005 and 2008 (p>0.05) in a subgroup of 44 participants from this cohort (Imm et al., 2009).
Several investigators have examined temporal trends in PBDEs in serial cross sectional studies of breast milk or serum in other populations. Few, if any, have examined intra-individual trends. Increasing levels of PBDEs have been noted in individual Japanese blood samples from the early 1980s to the mid 1990s (Koizumi et al., 2005) and in pooled US blood samples from 1973 to 2003 (Schecter et al., 2005) and 1985 to 2002 (Sjodin et al., 2004). However, pooled blood samples in adults from Australia did not differ significantly in 2002–2003, 2004–2005 and 2006–2007 (Toms et al., 2009). PBDEs in pooled breast milk from the Faroe Islands increased from 1987 to 1999 (Fangstrom et al., 2005). Several studies in Sweden suggest an increase in PBDEs in pooled breast milk from the 1980s, with a peak in 1998 (Lind et al., 2003) in one study, and 1995 in another (Fangstrom et al., 2008), although higher brominated PBDEs have continued to increase (Fangstrom et al., 2008). Similarly PBDEs in Japanese pooled breast milk samples collected from 1973 to 2000 peaked in 1998 (Akutsu et al., 2003). PBDE concentrations in breast milk from Spain were similar in 2002 and 2007 (Schuhmacher et al., 2009). The trends from these world-wide studies, many of which tracked PBDEs over several decades are consistent with the results of the current decade-long study and show increasing PBDE body burdens, leveling off in the late 1990s and 2000s. This leveling off preceded the 2004 discontinuation of pentaBDE use in the US and the European Union. It is possible that manufacturers decreased use well before 2004 in anticipation of the phase out. Following the 1977 ban on the manufacture of PCBs, levels declined slowly, but remain detectable in the environment, biota, and humans.
Previous studies have found that PBDEs vary non-linearly with age (Sjodin et al., 2008; Toms et al., 2009), We found positive associations of PBDEs, and BDE-47, 99 and 100 across one or more age categories, but an inverted U-shaped relationship of age with BDE-153. In the 2003–2004 NHANES cohort study of persons from 12 to 85 years of age, PBDE congeners were inversely associated with age, although a small increase was noted in older adults (Sjodin et al., 2008). The findings in the present study for PBDEs and BDE-47, 99 and 100, which included only middle age and older adults, are consistent with the NHANES findings on age and PBDE body burdens, and with our findings in the 508 participants sampled in 2004–2005 (Anderson et al., 2008). Changes in PBDEs across time were not modified by age at baseline, with the exception of BDE-100, which increased more in older participants.
BMI was not consistently associated with PBDE body burdens in univariate analyses, nor did we find an association in the 508 participants sampled in 2004–2005 (Anderson et al., 2008). However, in the mixed-effects regression models incorporating all three sampling times, BMI became significantly associated with PBDEs, and BDE-47, 99 and 100. In addition, BMI modified temporal changes in BDE-47, 100, and 153 in males, but not females, with significant increases in body burdens found only in men with higher BMI. Effect modification by BMI may be related to differential metabolism or exposure.
Our current findings are not consistent with the weak positive association of PBDEs with years of sport fish consumption in the 508 participants from this cohort sampled in 2004–2005 (Anderson et al., 2008). Only BDE-153 was related to fish consumption, with lower body burdens in members of the Wisconsin anglers group. Predictors of changes in BDE-153 also differed from those for BDE-47, 99 and 100 with respect to gender, with significant temporal increases in BDE-153 for females but not males. Variation in either exposure or metabolism could explain the effect of gender on BDE-153 changes, but it is unlikely to be related to sport fish consumption, which is greater in men than among women in the study (Hanrahan et al., 1999).
In addition to the differences in associations of age, BMI, and fish consumption with BDE-153 compared with the other BDE congeners discussed above, we noted an increase in the percent of persons with higher BDE-153 than BDE-47 and a shift in the congener proportions over time, with an increasing proportion of BDE-153 relative to BDE-47 between 2001–2003 and 2004–2005. Time trends may be related to differences in metabolism of BDE congeners, as BDE-153 has been found to be more persistent than BDE-47 in mice (Qiu et al., 2009) and humans (Geyer et al., 2004). Alternatively or additionally, relative levels of BDE-153 could be increasing because of persistence in the food supply or higher levels in the indoor environment related to the phase out of pentaBDE, but not decaBDE during the study period. Differences in the relative proportions of BDE congeners have been noted in other studies. In agreement with our findings, an increasing ratio of BDE-153 to BDE-47 was seen in breast milk from the Faroe Islands (Fangstrom et al., 2005) and Stockholm (Fangstrom et al., 2008). On the other hand, decreasing ratios of BDE-153, BDE-99 and BDE-100 to BDE-47 were noted in Japanese blood samples from the early 1980s to the mid 1990s (Koizumi et al., 2005).
This study is one of the first to investigate intra-individual changes in PBDEs over a decade. However, the serum measurements were not equally spaced during this time period, increasing the difficulty of evaluating temporal trends. Although some of the samples had been stored longer than 10 years, we validated the use of archived samples for similar lipophilic contaminants, and other investigators have successfully used archived serum samples for PBDE measurements (Koizumi et al., 2005; Schecter et al., 2005). BDE-47 was the most commonly detected congener, with >95% of samples above the limit of detection. However, a greater number of samples were below the limit of detection for other BDE congeners, which increases the uncertainty in these measurements and the estimated time trends. Furthermore, we were not able to evaluate changes in body burdens of the highly brominated BDE congeners. Lipid measurements were insufficient to allow us to analyze lipid weight PBDEs, but we do not expect that variations in lipid levels substantially altered our findings for several reasons. First, the correlation between ΣPBDEs wet weight and ΣPBDEs lipid weight in our participants is very strong (r=0.91, Spearman’s correlation coefficient). Second, lipid levels did not differ significantly in the 13 paired samples from 1994–1995 and 2001–2003 and in the 102 paired samples from 2001–3 and 2004–5 (Table 1).
5. Conclusion
In this investigation of PBDE body burdens in repeated serum samples from a cohort of Great Lakes residents, we noted increasing geometric mean levels of PBDE and BDE congeners between 1994–1995 and 2001–2003 and 1994–1995 and 2004–2005, but not from 2001–2003 to 2004–2005. These results were consistent with temporal trends in PBDEs from serial cross sectional studies of breast milk or serum in other populations in North America, Europe and Asia. Changes in body burdens of ΣPBDEs and BDE-47, 100, and 153 in men were modified by BMI, with greater increases in men with higher BMI. Increases in BDE-153 were greater for women than men and the increase in BDE-100 was greater in older participants. There was a shift in the congener proportions over time, with an increasing proportion of BDE-153 relative to BDE-47.
Supplementary Material
Acknowledgments
Funding and Acknowledgements: This research was funded by the National Institute of Environmental Health Sciences, Grant Number 1R21ES017121-01A1, the Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, Grant Number H75/ATH598322 and by the U.S. Environmental Protection Agency, Grant Number RD-83025401-1. Although the research described in this article has been funded by the U.S. Environmental Protection Agency, it has not been subjected to the Agency’s required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. We thank Dr. Heather Stapleton for her helpful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akutsu K, Kitagawa M, Nakazawa H, et al. Time-trend (1973-2000) of polybrominated diphenyl ethers in Japanese mother’s milk. Chemosphere. 2003;53:645–654. doi: 10.1016/S0045-6535(03)00764-1. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Imm P, Knobeloch L, et al. Polybrominated diphenyl ethers (PBDE) in serum: findings from a US cohort of consumers of sport-caught fish. Chemosphere. 2008;73:187–194. doi: 10.1016/j.chemosphere.2008.05.052. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114:1770–1775. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lee WJ, et al. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int. 2007;33:239–245. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Athanassiadis I, Odsjo T, et al. Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980–2004. Mol Nutr Food Res. 2008;52:187–193. doi: 10.1002/mnfr.200700182. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Strid A, Grandjean P, et al. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health. 2005;4:12. doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, McClean MD. Diet Contributes Significantly to the Body Burden of PBDEs in the General U.S. Population. Environ Health Perspect. 2009;117:1520–1525. doi: 10.1289/ehp.0900817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, et al. Human internal and external exposure to PBDEs - A review of levels and sources. Int J Hyg Environ Health. 2009;212:109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm KW, Darnerud PO, et al. Terminal elimination and half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- Hanrahan LP, Falk C, Anderson HA, et al. Serum PCB and DDE levels of frequent Great Lakes sport fish consumers-a first look. The Great Lakes Consortium. Environ Res. 1999;80:S26–S37. doi: 10.1006/enrs.1998.3914. [DOI] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, et al. PBDE Concentrations in Women’s Serum and Fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, et al. Prenatal Exposure to PBDEs and Neurodevelopment. Environ Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Imm P, Knobeloch L, Buelow C, et al. Household exposures to polybrominated diphenyl ethers (PBDEs) in a Wisconsin Cohort. Environ Health Perspect. 2009;117:1890–1895. doi: 10.1289/ehp.0900839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Otazo HA, Clarke JP, Diamond ML, et al. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39:5121–5130. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Turyk M, Imm P, et al. Temporal changes in PCB and DDE levels among a cohort of frequent and infrequent consumers of Great Lakes sportfish. Environ Res. 2009;109:66–72. doi: 10.1016/j.envres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Yoshinaga T, Harada K, et al. Assessment of human exposure to polychlorinated biphenyls and polybrominated diphenyl ethers in Japan using archived samples from the early 1980s and mid-1990s. Environ Res. 2005;99:31–39. doi: 10.1016/j.envres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lind Y, Darnerud PO, Atuma S, et al. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ Res. 2003;93:186–194. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, Virtanen HE, et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Environ Health A. 1999;58:329–341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect. 2009;117:93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH. E-waste: an assessment of global production and environmental impacts. Sci Total Environ. 2009;408:183–191. doi: 10.1016/j.scitotenv.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, et al. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, et al. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schuhmacher M, Kiviranta H, Ruokojarvi P, et al. Concentrations of PCDD/Fs, PCBs and PBDEs in breast milk of women from Catalonia, Spain: A follow-up study. Environ Int. 2009;35:607–613. doi: 10.1016/j.envint.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol. 2008;42:1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Soderstrom G, Sellstrom U, de Wit CA, et al. Photolytic debromination of decabromodiphenyl ether (BDE 209) Environ Sci Technol. 2004;38:127–132. doi: 10.1021/es034682c. [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, et al. Serum Polybrominated Diphenyl Ether (PBDE) Levels Are Higher in Children (2–5 Years of Age) than in Infants and Adults. Environ Health Perspect. 2009;117:1461–1465. doi: 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Persky VW, Imm P, et al. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Anderson HA, Selikoff IJ. Human tissue burdens of halogenated aromatic chemicals in Michigan. JAMA. 1982;247:2112–2116. [PubMed] [Google Scholar]
- Wu N, Herrmann T, Paepke O, et al. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol. 2007;41:1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.