Abstract
Prolonged and excessive inflammation is implicated in resistance to the biological actions of IGF-I and contributes to the pathophysiology of neurodegenerative, metabolic, and muscle-wasting disorders. IL-10 is a critical anti-inflammatory cytokine that restrains inflammatory responses in macrophages and T cells by inhibiting cytokine and chemokine synthesis and reducing expression of their receptors. Here we demonstrate that IL-10 plays a protective role in nonhematopoietic cells by suppressing the ability of exogenous IL-1β to inhibit IGF-I-induced myogenin and myosin heavy chain expression in myoblasts. This action of IL-10 is not caused by impairment of IL-1β-induced synthesis of IL-6 or the ability of IL-1β to activate two members of the MAPK family, ERK1/2 and p38. Instead, this newly defined protective role of IL-10 occurs by specific reversal of IL-1β activation of the JNK kinase pathway. IL-10 blocks IL-1β-induced phosphorylation of JNK, but not ERK1/2 or p38, indicating that only the JNK component of the IL-1β-induced MAPK signaling pathway is targeted by IL-10. This conclusion is supported by the finding that a specific JNK inhibitor acts similarly to IL-10 to restore IGF-I-induced myogenin expression, which is suppressed by IL-1β. Collectively, these data demonstrate that IL-10 acts in a novel, nonclassical, protective manner in nonhematopoietic cells to inhibit the IL-1β receptor-induced JNK kinase pathway, resulting in prevention of IGF-I resistance.
Keywords: inflammation, cytokines, MAPK, c-Jun NH2-terminal kinase, skeletal muscle, myogenin, nonhematopoietic cells
IL-1β plays a critical role in host defense and tissue remodeling following injury (16). However, prolonged activation of inflammatory mediators has now been recognized to impair activity of a variety of hormones, including IGF-I, thereby inducing hormone resistance (25, 26). A review of several recent studies of muscoskeletal disorders linked local and systemic inflammation to myopathic changes that are accompanied by pain and a decline in function (6). Elevated expression of proinflammatory cytokines, such as IL-1β and TNFα, is also implicated in muscle wasting and fatigue that is characteristic of normal aging (21), AIDS, and cancer cachexia (3, 25). These actions of proinflammatory cytokines are in part mediated by inhibiting the expression and activity of hormones such as growth hormone and IGF-I, the two most important contributors to postnatal growth (25, 33). Similar events occur in muscle progenitor cells at very low, physiologically relevant concentrations of IL-1β and TNFα (0.1–1 ng/ml). Proinflammatory cytokine-induced IGF-I resistance is characterized by the inability of IGF-I to induce global protein synthesis and expression of muscle-specific differentiation factors such as myogenin (9, 52). Promising recent results indicate that the anti-anabolic actions of proinflammatory cytokines may be mediated by common signaling intermediates, such as c-Jun NH2-terminal kinase (JNK), that lie downstream of TNFα type 1 receptor (TNF-R1) and IL-1 receptor-1 (IL-1R1). JNK is a stress-activated protein kinase that serves as a convergence point in a variety of inflammatory disorders and induces resistance to several hormones, including IGF-I (34, 53).
IL-10 is the best-characterized anti-inflammatory cytokine to date. It is elevated in most major diseases and is presumed to limit the clinical manifestations of inflammation by acting on hematopoietic cells (35, 55). The mechanism of IL-10 action is widely accepted to occur by inhibiting expression of proinflammatory cytokines and their receptors and by inducing synthesis of cytokine receptor antagonists (35, 55). Although the manner in which IL-10 regulates activity of hematopoietic cells is well established, its actions in the physiology of nonhematopoietic tissues are just beginning to be elucidated.
Several recent reports have established that IL-10 is synthesized by murine myoblasts (2) and is elevated in skeletal muscle following exercise (37, 44). The increase in IL-10 expression as a result of exercise is accompanied by a rise in proinflammatory cytokines, including IL-1β, TNFα, and IL-6 as well as the IL-1β receptor antagonist and soluble TNFα receptor (36, 37, 43, 44). Consequently, IL-10 produced by exercising muscle is thought to limit an exuberant inflammatory response. This hypothesis is consistent with the observation that intramuscular injection of an adenoviral vector encoding IL-10 inhibits the synthesis of proinflammatory cytokines TNFα, IL-1β, and IL-6 in the diaphragms of mice infected with Pseudomonas aeruginosa (14). Furthermore, administration of recombinant human IL-10 reduces hypoxia-induced skeletal muscle injury and myocyte necrosis in newborn rats (41). Additional support for the protective activity of IL-10 comes from animal studies in which IL-10-expressing plasmids targeted to skeletal muscle ameliorate the clinical severity of inflammatory conditions such as collagen-induced arthritis (45), diabetes (60), and bacterial infections (14). These findings establish that nonhematopoietic tissues are capable of synthesizing and responding to IL-10 and that IL-10 acts in a protective manner. Proinflammatory cytokines have been amply demonstrated to regulate the biological activity of hormones through receptor cross talk (25, 26), and JNK appears to be a critical mediator as established for growth hormone (30), insulin (22), IGF-I (53), and cortisol (42, 58). However, the possibility that the anti-inflammatory cytokine IL-10 regulates muscle development by overcoming IL-1β-induced IGF-I resistance rather than simply reducing the synthesis of proinflammatory cytokines has not yet been explored.
IGF-I, in combination with growth hormone, accounts for >80% of postnatal growth (33). IL-1β induces IGF-I resistance in muscle progenitors, as defined by its ability to prevent IGF-I from inducing synthesis of myogenin and myosin heavy chain (MHC) (9, 52), but the potential anti-inflammatory actions of IL-10 in these cells are unknown. We hypothesized that IL-10 would reverse the ability of exogenous IL-1β to inhibit IGF-I-induced expression of myogenin protein in skeletal muscle myoblasts, and this would occur by IL-10 specifically targeting the JNK kinase pathway. Here we confirm this hypothesis, thereby defining a new biological activity of IL-10 by showing for the first time that IL-10 acts in a protective manner in skeletal muscle progenitors to restore IGF-I-induced myogenin expression that is inhibited by IL-1β.
MATERIALS AND METHODS
Reagents
Fetal bovine serum (FBS; <0.25 EU/ml endotoxin), DMEM containing 0.584 g/l glutamine and 4.5 g/l glucose, sodium pyruvate, and antibiotics (penicillin/streptomycin) were purchased from HyClone (Logan, UT). Recombinant murine IL-10 was obtained from Pharmingen (2–8 × 106 units/mg protein; San Diego, CA); recombinant murine IL-1β was from Serologicals (Norcross, GA), and recombinant human IGF-I was from Intergen (Purchase, NY). The JNK peptide inhibitor-1, d-stereoisomer (I-JNK), was purchased from Alexis Biochemicals (San Diego, CA). Enzyme-linked immunosorbent assay (ELISA) kits were from Pierce Biotechnology (Rockford, IL). Primary antibodies were obtained as follows: mouse monoclonal antibodies to phosphorylated ERK1/2 (IgG2a, E-4) and to myogenin (IgG1, F5D) were from Santa Cruz Biotechnology (Santa Cruz, CA), the antibody to α-tubulin (IgG1, B-5-1-2) was from Sigma Aldrich (St. Louis, MO), and the antibody to embryonic MHC (IgG1, F1.652) was from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). The rabbit polyclonal antibody to the subdomain XI of ERK1 (K-23) was purchased from Santa Cruz Biotechnology, and the antibodies specific for JNK (9252), phosphorylated JNK (P-JNK, 9251), p38 (9212), phosphorylated p38 (9211), and phosphorylated MKK7 (P-MKK7, 4171) were purchased from Cell Signaling Biotechnology (Danvers, MA). The secondary horseradish peroxidase (HRP)-linked antibodies (mouse, NA931V, and rabbit, NA934V) were purchased from GE Healthcare Biosciences (Piscataway, NJ). Other reagents and chemicals were obtained from Sigma Aldrich.
Cell culture
Murine skeletal muscle progenitor cells, C2C12 myoblasts, were obtained from American Type Culture Collection (ATCC; Manassas, VA) and cultured as previously described (10, 52). Before treatment, myoblasts were washed three times in complete DMEM devoid of FBS and incubated in this medium for a minimum of 4 h before initiation of experiments.
In ELISA time course experiments, C2C12 myoblasts were incubated for 24 h in serum-free medium, with IL-1β present for 24, 16, 8, 4, or only the final 2 h, or left untreated (0 h). In ELISA experiments that tested the ability of IL-10 to inhibit cytokine synthesis, C2C12 myoblasts were pretreated with IL-10 at 0, 10, 25, or 50 ng/ml for 1 h before addition of IL-1β, followed by a 24-h incubation and collection of samples. As a control, C2C12 myoblasts were treated for 24 h with the highest concentration (50 ng/ml) of IL-10 alone or remained untreated. In ELISA experiments testing the ability of ERK1/2 and JNK antagonists to inhibit IL-1β-induced expression of IL-6, myoblasts were pretreated with an ERK1/2 antagonist (PD98059, 10 µM) or a JNK antagonist (SP600125, 10 µM) for 1 h before addition of IL-1β and incubation for 24 h. To measure myogenin and MHC expression, C2C12 myoblasts were treated with IL-1β for 1 h before addition of IGF-I and incubation for 24 h. For experiments measuring JNK and ERK1/2 phosphorylation, C2C12 myoblasts were treated with IL-1β for 60, 30, 15, 10, and 5 min to determine the timing of JNK and ERK1/2 phosphorylation. To determine whether IL-10 inhibits IL-1β-induced JNK, ERK1/2, p38 or MKK7 phosphorylation, myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h before adding IL-1β and then incubating for another 10 or 15 min. To determine whether JNK is required for IL-1β inhibition of myogenin expression, myoblasts were pretreated with IL-10 (10 ng/ml) or the inhibitor of JNK, I-JNK (2 µM), for 1 h before treatment with IL-1β and IGF-I. Optimal concentration of I-JNK inhibitor was determined in a recent study from our laboratory (53) to be 2 µM.
In this study, we evaluated the ability of a low concentration of IL-10 (10 ng/ml) to inhibit IL-1β-induced IGF-I resistance in skeletal muscle progenitors. Whereas high concentrations of recombinant IL-10 up to 300 ng/ml are commonly used for in vitro and ex vivo experiments (5, 20, 28, 31, 38, 59), the in vitro endogenous expression of IL-10 rarely exceeds 1 ng/ml, even when cells are stimulated with high concentrations of LPS (100–1,000 ng/ml) (24, 47). Similarly, endogenous IL-10 concentration is even lower in plasma of humans afflicted with various diseases, such as malaria (15) and human African trypanosomiasis (11), or in healthy males injected with 4 ng/kg LPS (13). We utilized a low, more physiologically relevant concentration of IL-10 (10 ng/ml) to evaluate how it regulates IL-1β-induced signaling activity in myoblasts. At this low concentration, IL-10 inhibits IL-1β signaling, but not IL-1β-induced cytokine synthesis, which requires higher concentrations of IL-10 (25–50 ng/ml).
ELISA and Western blotting
IL-6 and TNFα were measured with specific ELISA assays (Pierce Biotechnology) by adding 50 µl of each sample in duplicate to ELISA plates precoated with an IL-6 or a TNFα capture antibody. Recombinant murine IL-6 standards ranged from 0 to 2,000 pg/ml, and the lower assay limit of detection was 7 pg/ml. Recombinant murine TNFα standards ranged from 0 to 2,450 pg/ml. Absorbance was measured on an OPTImax ELISA plate reader from Molecular Devices (Sunnyvale, CA). IL-6 and TNFα concentrations were expressed as picograms per milliliter. All Western blotting experiments were conducted as previously described (10, 52). Proteins for Western blotting experiments to measure myogenin (mol mass = 35 kDa), JNK (mol mass = 46 and 54 kDa), ERK1/2 (mol mass = 44 and 42 kDa), p38 (mol mass = 38 kDa) and MKK7 (mol mass = 48 kDa) were separated using SDS-containing 10% polyacrylamide gels, and proteins for measuring MHC (mol mass = 250 kDa) were separated with 6% polyacrylamide gels. Proteins were then transferred to Trans-Blot polyvinylidene difluoride (PVDF) membranes, which were incubated overnight at 4°C with primary antibodies to myogenin, MHC, α-tubulin, phosphorylated JNK, JNK, phosphorylated ERK1/2, ERK1/2, phosphorylated p38, p38, or MKK7 diluted 1/1,000 or 1/5,000 (for α-tubulin) in 2% BSA. Densitometric analysis of scanned autoradiograms was performed using publically available IMAGE-J software from the National Institutes of Health (Bethesda, MD). To adjust for interassay variation between experiments, data were standardized by dividing individual sample values by the mean of the entire experiment. Densitometric summaries were expressed as ratios of myogenin to α-tubulin, MHC to α-tubulin, Thr183/Tyr185 phosphorylated JNK to α-tubulin, Tyr204 phosphorylated ERK1/2 to α-tubulin, Ser271/Thr275 phosphorylated MKK7 to α-tubulin, Thr183/Tyr185 phosphorylated JNK to total JNK, Tyr204 phosphorylated ERK1/2 to total ERK1/2, and Thr180/Tyr182 phosphorylated p38 to total p38.
Statistical analysis
For all experiments, data were analyzed using the Statistical Analysis System (SAS; version 8) (46). Differences between treatments were detected by an F-protected Duncan’s multiple range test. Results are expressed as means ± SE of at least three independent experiments.
RESULTS
IL-10 restores myoblast differentiation by fully suppressing the ability of IL-1β to inhibit IGF-I-induced myogenin expression
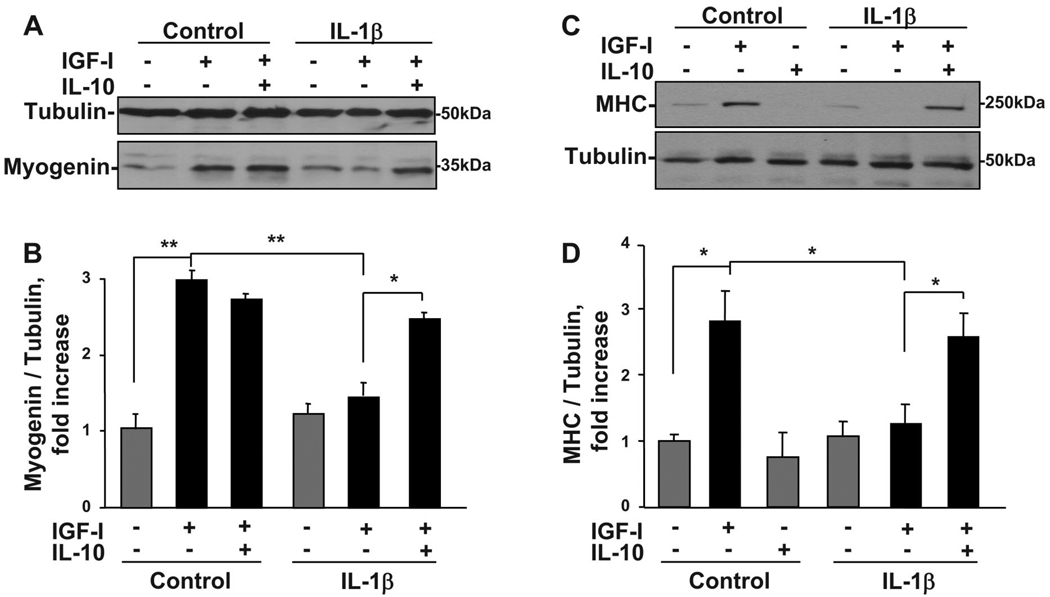
Muscle tissue development is dependent on a well-orchestrated, sequential expression of muscle-specific transcription factors, including myogenin and MyoD, that are promoted by IGF-I. Very low (0.1–1 ng/ml) concentrations of proinflammatory cytokines completely inhibit the ability of IGF-I to induce expression of myogenin and MyoD as well as MHC, a marker of more terminal differentiation (10, 52). To explore the possibility that IL-10 interacts with IGF-I and IL-1β to regulate muscle cell progenitor development, C2C12 myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h before treatment with IL-1β (1 ng/ml) and IGF-I (50 ng/ml). A representative Western blot for myogenin is shown in Fig. 1A and for MHC in Fig. 1C. A densitometric summary of three independent experiments expressed as ratios of myogenin to α-tubulin is presented in Fig. 1B and as ratios of MHC to α-tubulin in Fig. 1D. Consistent with our previous findings (9, 52), the ability of IGF-I to increase myogenin expression was completely blocked by IL-1β (P < 0.01; Fig. 1, A and B). More importantly, IL-10 (10 ng/ml) restored myoblast differentiation by suppressing the ability of IL-1β to inhibit IGF-I-induced myogenin expression (P < 0.05; Fig. 1, A and B). Similar results were obtained for another early transcription factor, MyoD (data not shown). Finally, IL-10 suppressed the ability of IL-1β to inhibit IGF-I-induced expression of a more terminal marker of differentiation, MHC (P < 0.05; Fig. 1, C and D). IL-10 was ineffective when added just after or at 2 h following addition of IL-1β (data not shown). These data are among the first to show a direct protective role of IL-10 in the early events that occur during the differentiation of skeletal muscle progenitor cells.
Fig. 1.
IL-10 reverses the ability of IL-1β to inhibit the ability of IGF-I to increase expression of both myogenin and myosin heavy chain (MHC). C2C12 myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h before treatment with IL-1β (1 ng/ml) for an additional 1 h. Myoblasts were then incubated in the presence or absence of IGF-I (50 ng/ml) for 24 h. Myogenin and MHC expression was determined by Western blots using monoclonal antibodies specific for myogenin and MHC. An anti-α-tubulin antibody was used to detect α-tubulin expression, demonstrating that equal amounts of protein from each sample were loaded. Densitometric summaries were calculated as ratios of myogenin to α-tubulin and MHC to α-tubulin. Representative Western blots (A and C) and densitometric summaries (B and D) of 3 independent experiments show that IL-1β completely blocked the ability of IGF-I to induce the expression of myogenin (A and B) and MHC (C and D). More importantly, pretreatment with IL-10 completely reversed this inhibition by IL-1β and restored the expression of myogenin and MHC. IL-10 was inactive alone and did not alter IGF-I-induced myogenin and MHC expression in the absence of IL-1β. *P < 0.05; **P < 0.01.
IL-10 inhibits IL-1β-induced cytokine expression in a dose-dependent manner
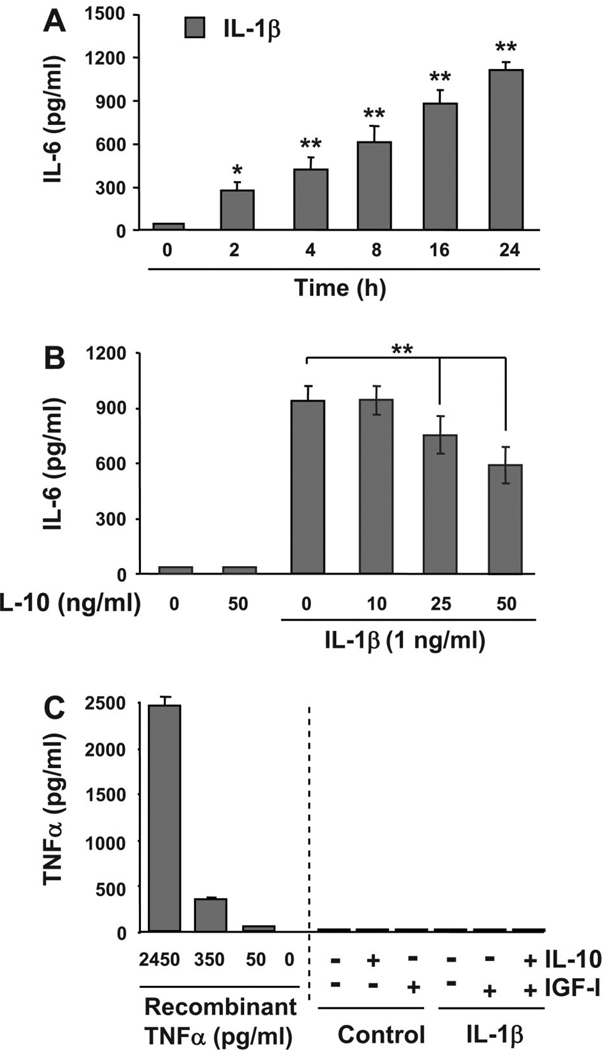
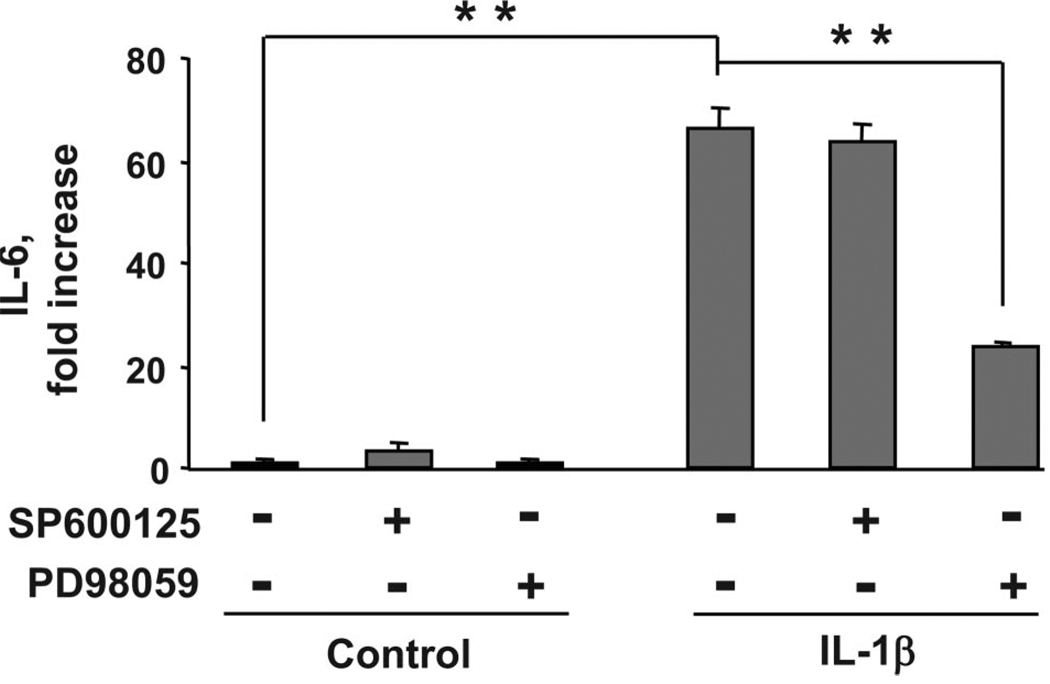
To further explore the potential role of IL-10 on nonhematopoietic tissues, we examined its classical role of inhibiting cytokine synthesis in an established model for skeletal muscle development, murine C2C12 myoblasts. For these experiments, C2C12 myoblasts were first treated with IL-1β (1 ng/ml) for 0, 2, 4, 8, 16, and 24 h. Protein concentrations of IL-6 in the supernatant were determined by ELISA. Consistent with published results (19), IL-1β induced a time-dependent increase in IL-6 expression (Fig. 2A). A significant elevation in IL-6 concentration was detected following a 2-h treatment with IL-1β (P < 0.05; Fig. 2A), and the largest increase in IL-6 expression was observed 24 h following treatment (P < 0.01; Fig. 2A). IL-6 was not detected in untreated control cells (lower limit of sensitivity of 7 pg/ml). To determine whether IL-10 acted in a classical manner in muscle progenitors by inhibiting cytokine synthesis, we pretreated C2C12 myoblasts with 0, 10, 25, and 50 ng/ml IL-10 for 1 h before treatment with IL-1β, followed by a 24-h incubation. Consistent with the previous results, IL-1β induced a significant increase in IL-6 expression following 24 h of treatment. This increase in IL-6 expression was significantly impaired by IL-10 at 25 ng/ml and at 50 ng/ml (P < 0.01; Fig. 2B) but not by the lower, more physiologically relevant concentration of IL-10, 10 ng/ml, which was used for the experiments presented in Fig. 1. Because we have previously established that TNFα inhibits the biological activity of IGF-I in myoblasts (10, 52), we next tested the possibility that IL-1β induces expression of TNFα. A summary of these results established that IL-1β (1 ng/ml) did not induce detectable levels of TNFα (lower limit of sensitivity of 9 pg/ml) (Fig. 2C). Similarly, IGF-I or IL-10 or the combination of IGF-I, IL-10, and IL-1β did not induce detectible TNFα. Additionally, IGF-I (50 ng/ml) did not increase cell sensitivity to IL-10 as determined by equivalent IL-1β-induced (1 ng/ml) expression of IL-6 in the presence and absence of IL-10 (10 ng/ml) and IGF-I (50 ng/ml; data not shown). Finally, as we have previously established for TNFα (54), neither IL-1β (1 ng/ml) nor IGF-I (50 ng/ml) affected expression of IL-10R1 (data not shown). These data confirm and extend previous findings by showing that IL-10 can act in a classical manner to inhibit the ability of IL-1β to induce synthesis of IL-6 in nonhematopoietic cells. However, this inhibition of cytokine synthesis occurs only at concentrations ≥25 ng/ml. Therefore, all subsequent experiments were conducted with IL-10 at 10 ng/ml.
Fig. 2.
IL-1β-induced cytokine synthesis in muscle progenitors is inhibited only by high concentrations of IL-10 (≥25 ng/ml). A: C2C12 myoblasts were treated with IL-1β (1 ng/ml) for 24, 16, 8, 4, and 2 h or with medium alone for 24 h (zero time point). The amount of IL-6 protein was measured with an ELISA specific for murine IL-6. IL-1β significantly increased IL-6 expression in a time-dependent manner with a statistically significant increase as early as 2 h (n = 3). B: C2C12 myoblasts were pretreated with 0, 10, 25, or 50 ng/ml IL-10 before incubation with IL-1β (1 ng/ml) for an additional 24 h or with medium alone or IL-10 alone for 24 h. Results obtained with an IL-6-specific ELISA demonstrated that IL-10 significantly inhibited IL-6 expression only at concentrations ≥25 ng/ml but not at the lower concentration (10 ng/ml) (n = 4). IL-6 expression was not detected in supernatants from untreated cells or cells treated with IL-10 alone (lower limit of sensitivity of 7 pg/ml). C: IL-10 (10 ng/ml), IGF-I (50 ng/ml), IL-1β (1 ng/ml), or the combination of IL-10 and IGF-I did not induce detectible levels of TNFα expression (lower limit of sensitivity of 9 pg/ml). Recombinant murine TNFα was used as a positive control (2,450, 350, and 50 ng/ml) (n = 3). *P < 0.05; **P < 0.01.
IL-1β induces the phosphorylation of MAPK proteins in muscle progenitors
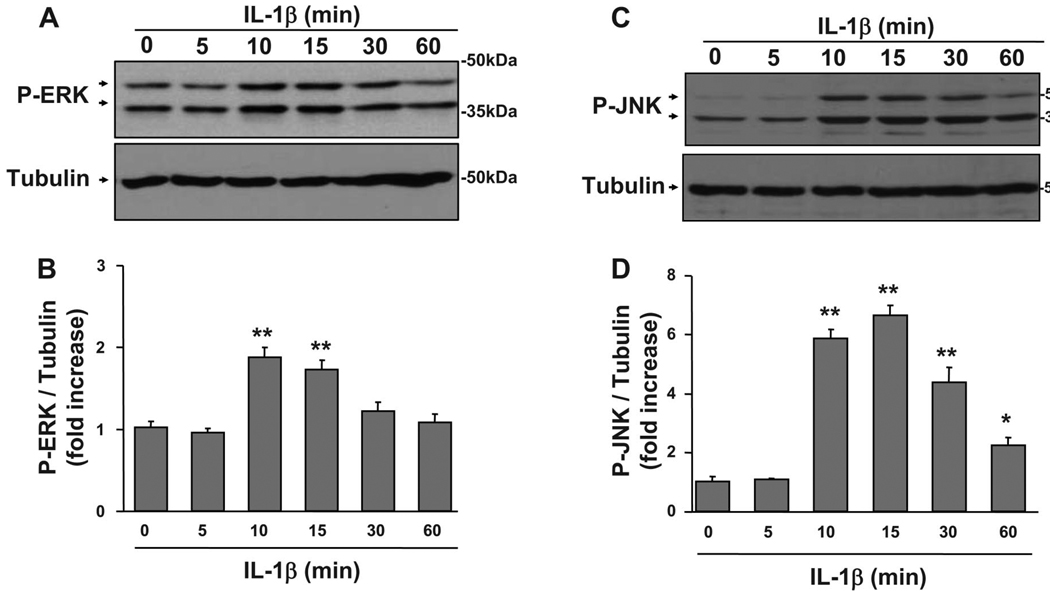
All three of the major MAPKs, p38, ERK1/2, and JNK, are activated by IL-1β in hematopoietic cells (39), but the ability of IL-1β to activate these kinases in skeletal muscle progenitor cells is not clear. For these experiments, C2C12 myoblasts were treated with IL-1β (1 ng/ml) for 0, 5, 10, 15, 30, and 60 min. We evaluated both JNK and ERK1/2 activity in more detail than for p38 because prolonged activation of these kinases has been shown to disrupt skeletal muscle development (53, 57), whereas p38 has been shown to promote differentiation (12). A densitometric summary of seven independent experiments measuring ERK1/2 phosphorylation is presented in Fig. 3B as a ratio of slower migrating isomer (44 kDa) of P-ERK to α-tubulin, and a summary of four independent experiments measuring JNK phosphorylation is shown in Fig. 3D as a ratio of slower migrating (54 kDa) isomer of P-JNK to α-tubulin. IL-1β increased Tyr204 phosphorylation of ERK1/2 at 10 min, and it remained elevated at 15 min and diminished within 30 min (P < 0.01; Fig. 3, A and B). Comparable results were observed for IL-1β-induced phosphorylation of the faster migrating band of ERK1/2 (42 kDa) (data not shown). Similarly, IL-1β induced a sixfold increase in JNK Thr183/Tyr185 phosphorylation within 10 min (P < 0.01; Fig. 3, C and D). JNK phosphorylation remained elevated at 15 and 30 min (P < 0.01; Fig. 3, C and D) but decreased at 60 min (P < 0.05; Fig. 3, C and D). A similar increase in IL-1β-induced JNK phosphorylation was observed for the faster (46 kDa) migrating band of JNK (data not shown). Finally, IL-1β also induced a time-dependent increase in phosphorylation of another major MAPK, p38 (data not shown). These data are consistent with our previous results showing that TNFα induces JNK phosphorylation in C2C12 myoblasts (53) in a time-dependent manner and suggest that IL-1β inhibition in muscle progenitors may be mediated by activation of MAPK family members.
Fig. 3.
IL-1β induces JNK and ERK1/2 phosphorylation. To determine the time course in which IL-1β activates two of the classical MAPK pathways in muscle progenitors, we treated C2C12 myoblasts with IL-1β (1 ng/ml) for 0, 5, 10, 15, 30, or 60 min. Phosphorylation of ERK1/2 and JNK was determined by probing membranes with monoclonal antibodies against phospho-ERK1/2 and polyclonal antibodies against phospho-JNK. Membranes were then stripped and reprobed with an anti-α-tubulin-specific antibody to ensure that equal amounts of protein for each sample were loaded into the SDS-PAGE gels and subsequently transferred to polyvinylidene difluoride (PVDF) membranes. Densitometric summaries were calculated as ratios of phosphorylated ERK1/2 and JNK to α-tubulin. A: densitometric summary of 7 independent experiments demonstrated that IL-1β induced an increase in ERK1/2 phosphorylation at 10 min and that this increase was sustained through 15 min. B: similarly, a densitometric summary of 4 independent experiments indicated that phosphorylation of JNK also required a 10-min induction period in muscle progenitors, but this signal diminished less rapidly than that for P-ERK1/2. *P < 0.05; **P < 0.01.
IL-10 specifically blocks the ability of IL-1β to activate the key downstream mediator, JNK, but not ERK1/2 or p38
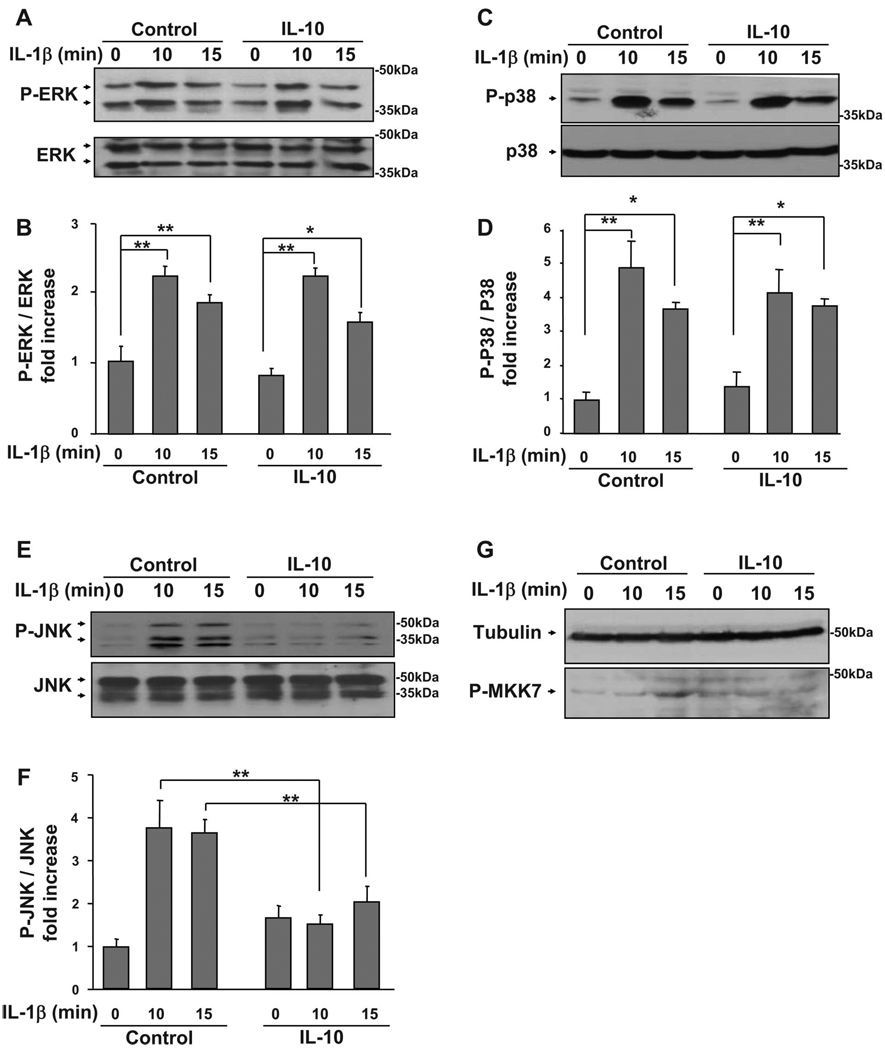
MAPK family members are expressed in virtually all eukaryotic cells and are key components in muscle development (12, 27, 54, 57). Here we address the important possibility that IL-10 can restore myoblast differentiation by suppressing the ability of IL-1 receptors to respond to IL-1β and phosphorylate key MAPK mediators downstream of IL-1R1: ERK1/2, p38, and JNK. To test this hypothesis, we examined the ability of IL-10 to inhibit IL-1β-induced ERK1/2, p38, and JNK phosphorylation. C2C12 myoblasts were pretreated with IL-10 for 1 h before treatment with IL-1β for an additional 10 and 15 min. Following termination of these experiments, cells were homogenized and proteins separated on SDS 10% polyacrylamide gels. Representative Western blots are shown in Fig. 4, A, C, and E, and densitometric summaries of at least three independent experiments in Fig. 4, B, D, and F. Densitometric summaries for ERK1/2 are presented as ratios of slower migrating isomer (44 kDa) of P-ERK1/2 to total ERK1/2 (Fig. 4B), for p38 as ratios of P-p38 to total p38 (Fig. 4D), and for JNK as ratios of the slower migrating (54 kDa) isomer of P-JNK to total JNK (Fig. 4F). As expected, IL-1β caused an increase in ERK1/2, p38, and JNK phosphorylation at 10 and 15 min (P < 0.01; Fig. 4). However, IL-1β did not induce JNK phosphorylation at later time points (2 to 24 h) and did not alter total levels of JNK (data not shown). Similarly, when added alone, neither IL-10 nor IGF-I altered ERK1/2, p38, or JNK phosphorylation and expression at the early (0 to 60 min) or later (2 to 24 h) time points (data not shown). Importantly, IL-10 completely blocked the ability of IL-1β to induce JNK but not ERK1/2 or p38 phosphorylation (P < 0.01; Fig. 4). Furthermore, as demonstrated in Fig. 4G, IL-10 also suppressed IL-1β-induced phosphorylation of MKK7, a kinase that lies directly upstream of JNK and is critical for JNK activation. Consistent with these results, IL-1β-induced expression of IL-6 was inhibited by the ERK1/2 antagonist (PD98059, 10 µM) but not by a JNK antagonist (SP600125,10 µM) (P < 0.01; Fig. 5), although both pharmacological inhibitors were effective in suppressing phosphorylation of their target MAPK at these concentrations (data not shown). In addition, IL-1β-induced expression of IL-6 was not blocked by the peptide inhibitor of JNK, I-JNK (data not shown), which we have previously shown to ameliorate proinflammatory cytokine-induced IGF-I resistance (54). These data demonstrate that the mechanism of action for the protective biological activity of IL-10 in muscle progenitors is not mediated by global downregulation of IL-1R1 activity because IL-1β was fully able to activate both ERK1/2 and p38 in the presence of IL-10. Instead, the mechanism of action of IL-10 occurs by specific targeting of the JNK kinase pathway.
Fig. 4.
IL-10 blocks IL-1β-induced phosphorylation of JNK but not ERK1/2 or p38. C2C12 myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h before treatment with IL-1β (1 ng/ml) for another 10 and 15 min. Phosphorylation of ERK1/2, p38, and JNK was determined as in Fig. 3. Membranes were stripped and reblotted with antibodies specific for total ERK1/2, p38, or JNK to ensure that expression of these proteins does not change. Phosphorylation of MKK7 was determined as described in the materials and methods. Densitometric summaries were calculated as ratios of phospho-ERK1/2 to ERK1/2, phospho-p38 to p38, phospho-JNK to JNK, and phospho-MKK7 to α-tubulin. Representative Western blots (A) and a densitometric summary (B) of 6 independent experiments demonstrated that IL-10 does not inhibit IL-1β-induced phosphorylation of ERK1/2. Similarly, IL-10 did not inhibit IL-1β-induced phosphorylation of p38. C: representative Western blots. D: densitometric summary of 3 independent experiments. Conversely, IL-10 completely blocked the IL-1β-induced increase in JNK phosphorylation, as demonstrated by representative Western blots (E) and a quantitative summary (F) of 5 independent experiments. G: similarly, IL-10 suppressed IL-1β-induced MKK7 phosphorylation; representative Western blot of 3 independent experiments. *P < 0.05; **P < 0.01.
Fig. 5.
IL-1β-induced expression of IL-6 is inhibited by an ERK1/2 but not by a JNK antagonist. C2C12 myoblasts were incubated with PD98059 and SP600125 (10 µM) at a concentration that inhibits ERK1/2 and JNK activation, respectively (10 µM, data not shown), 1 h before treatment with IL-1β (1 ng/ml). IL-1β increased expression of IL-6 (n = 3). This IL-1β-induced increase in IL-6 concentration was significantly impaired by preincubation with the ERK1/2 inhibitor PD98059 but not by the JNK inhibitor SP600125. **P < 0.01.
IL-10 acts like a JNK peptide inhibitor to overcome IL-1β-induced inhibition of myoblast differentiation
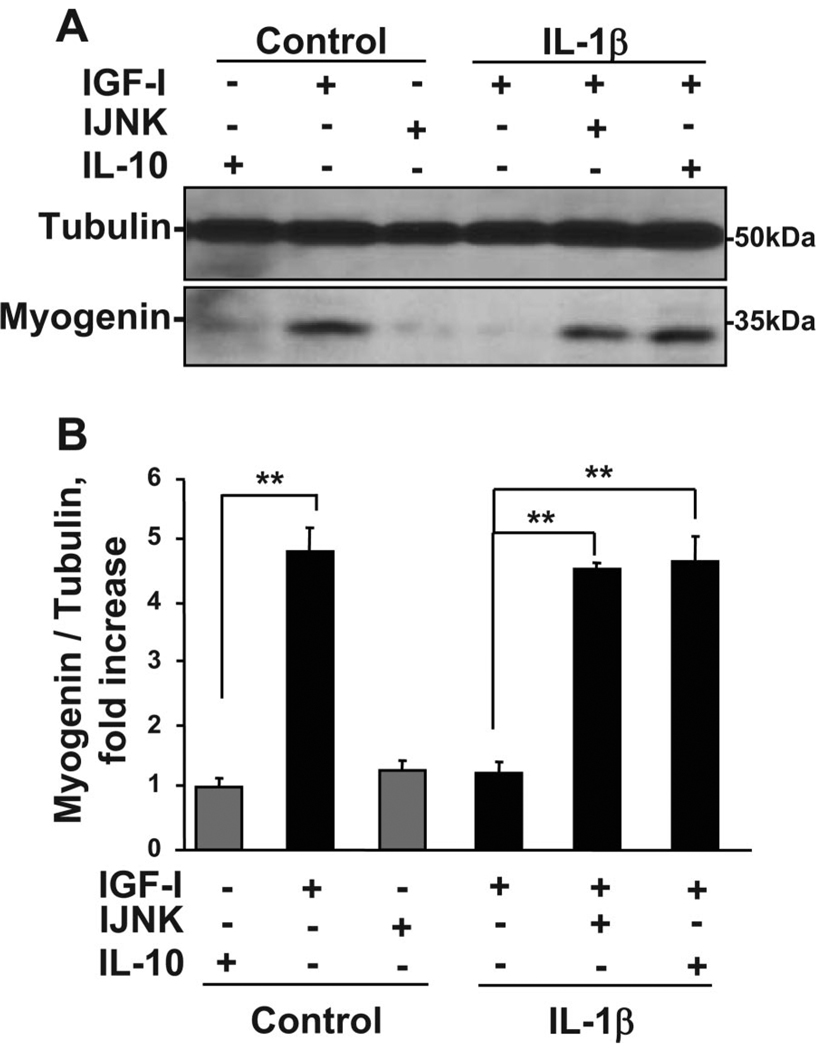
Here we tested the important hypothesis that JNK is required for IL-1β inhibitory activity in muscle progenitors. C2C12 myoblasts were pretreated with a specific peptide inhibitor of JNK (I-JNK, 2 µM) or IL-10 (10 ng/ml) for 1 h before treatment with IL-1β (1 ng/ml) for an additional hour. Cells were then incubated in the presence or absence of IGF-I for 24 h. Proteins from whole cell lysates were separated on SDS polyacrylamide gels, and membranes were blotted with a murine myogenin-specific antibody. Representative Western blots (Fig. 6A) and a densitometric summary of four independent experiments (Fig. 6B) demonstrate that JNK is required for IL-1β to inhibit myogenin expression and that the protective role of IL-10 in muscle progenitors is likely mediated by targeting JNK. Consistent with results described above, IL-1β completely inhibited the ability of IGF-I to promote expression of myogenin (P < 0.01; Fig. 6). More importantly, the cell-permeable peptide inhibitor of JNK, I-JNK, completely restored IGF-I-induced myogenin expression that was inhibited by IL-1β (P < 0.01; Fig. 6). These data are consistent with the ability of IL-10 to inhibit JNK phosphorylation (Fig. 4, A and B) and alleviate IL-1β inhibition of myogenin expression (P < 0.01; Fig. 6). Collectively, these new findings demonstrate that the protective biological activity of IL-10 in muscle progenitors is mediated by targeting the JNK kinase pathway and establish JNK as an important downstream mediator of proinflammatory cytokine activity in these cells.
Fig. 6.
Both IL-10 and I-JNK suppress the ability of IL-1β to inhibit myogenin expression. C2C12 myoblasts were pretreated with an inhibitor of JNK, I-JNK (2 µM), or IL-10 (10 ng/ml) for 1 h before treatment with IL-1β (1 ng/ml) for another 1 h. Cells were than treated with IGF-I (50 ng/ml) for an additional 24 h to induce myogenin expression, which was determined by Western blotting with a monoclonal myogenin-specific antibody. Representative Western blots (A) and a densitometric summary (B) of 4 independent experiments demonstrated that IL-10 and I-JNK completely restored myogenin expression by suppressing IL-1β-induced inhibition of IGF-I biological activity. Neither IL-10 nor I-JNK altered myogenin expression in the absence of IL-1β. These results indicate that the protective biological activity of IL-10 in skeletal muscle development is expressed by inhibition of JNK. **P < 0.01.
DISCUSSION
Myoblasts are stem cells that function as skeletal muscle precursors. They are being intensively investigated for their potential applications in gene therapy for diseases ranging from heart disease to urinary incontinence (1, 32, 48). Severity of disease and tissue damage are often exacerbated by unrestrained chronic expression of inflammatory mediators (39, 40, 51). IL-10 was initially characterized as an anti-inflammatory cytokine that is secreted by TH-2 cells and acts to inhibit activation of TH-1 cells (17). Here we demonstrate for the first time that IL-10 plays a protective role in development of skeletal muscle progenitors by specifically inhibiting one aspect of IL-1R1 signaling and restoring IGF-I-induced myogenin expression. The mechanism of action of IL-10 does not occur by inhibition of cytokine synthesis (Fig. 2) or blocking of global activity of IL-1β receptors (Fig. 4), as assessed by phosphorylation of either ERK1/2 or p38. Similarly, IL-10 most likely does not globally affect IL-1β signaling by reducing the number of IL-1Rs or inducing the IL-1R antagonist. IL-10 alone also does not induce the phosphorylation of ERK1/2, p38, or JNK (Fig. 4). Instead, IL-10 specifically impairs the ability of IL-1β to induce JNK phosphorylation.
Recent studies with novel peptide inhibitors of JNK demonstrate that inhibition of JNK activity can ameliorate severity of disease in animal models of Alzheimer’s disease, stroke, and diabetes (7, 8, 34). Here we demonstrate that similar approaches might be applied to reversing the actions of JNK in muscle progenitor cells. The potent, multifaceted activity of JNK can be partly attributed to its ability to induce resistance to several growth factors, including growth hormone (30), insulin (22), IGF-I (53), and cortisol (42, 58). Consistent with this concept, we recently showed that a peptide inhibitor of JNK abrogates IGF-I resistance and therefore suppresses TNFα- and C2-ceramide-induced inhibition of myogenin expression (53). These data are consistent with the hypothesis that IL-1β and TNFα exert their actions on muscle progenitors by inducing phosphorylation of downstream transcription factors that disrupt the biological activity of growth factors such as IGF-I.
Although JNK has been demonstrated to mediate TNFα inhibition of myogenin expression (53), this possibility has not been tested for other cytokines. In this study, we clearly show that a low concentration of IL-1β (1 ng/ml) induces ERK1/2, p38, and JNK phosphorylation in C2C12 myoblasts (Figs. 3 and 4). High concentrations of TNFα and IL-1β (40 ng/ml) have been reported to induce IL-6 synthesis by C2C12 cells within 2 h of treatment, and this increase in IL-6 synthesis could be inhibited by high concentrations of SP600125 (100 µM) (19). However, when a 40-fold lower more physiological concentration of IL-1β (1 ng/ml) is used to induce IL-6 production over 24 h, a low concentration of SP600125 (10 µM) that suppresses JNK phosphorylation (data not shown), does not inhibit the ability of IL-1β to induce IL-6 synthesis (Fig. 5). Therefore, data in this report clearly demonstrate that ERK1/2, but not JNK, is likely to mediate the ability of this low concentration of IL-1β to induce synthesis of IL-6 (Fig. 5). Because we previously found that SP600125 can be toxic to C2C12 cells over long incubation periods, we confirmed these inhibition experiments with another inhibitor. This inhibitor, I-JNK, blocks JNK activity via a different mechanism than SP600125. Importantly, JNK, but not ERK1/2 or p38, is responsible for inducing IL-1β-induced IGF-I myogenin protein expression, as shown by the data demonstrating that I-JNK fully blocks IL-1β-induced suppression of myoblast differentiation driven by IGF-I (Fig. 6). These data are consistent with the idea that JNK may serve as a universal downstream target for treatment of wasting disorders.
It is generally accepted that IL-10 is a classic anti-inflammatory cytokine that acts primarily by inhibiting the synthesis and expression of inflammatory mediators, including proinflammatory cytokines, cytokine receptors, co-stimulatory molecules and chemokines (16, 40). Here we establish that the mechanism of action of IL-10 on muscle progenitor cells is not dependent on its ability to inhibit the synthesis of proinflammatory cytokines or reduce the sensitivity of IL-1R1 on myoblasts. Instead, the mechanism of IL-10 action depends on its ability to prevent IL-1β from inducing JNK phosphorylation. Recent evidence suggests that the suppressor of cytokine signaling (SOCS) family of proteins is likely to be involved in even earlier upstream signaling events that are activated by the IL-10 receptors, which have been shown to be expressed on myoblasts. SOCS proteins, such as SOCS-2 and SOCS-3, are induced by IL-10 and are closely involved in its immunosuppressive responses (reviewed in Ref. 55). These proteins are responsible for the ability of IL-10 to suppress activation of janus kinase (JAK) and signal transducer and activator of transcription (STAT) proteins and for inhibition of proinflammatory cytokine synthesis (49, 50, 55, 56). These findings were recently extended to JNK with the discovery that SOCS-3 inhibits JNK phosphorylation by binding to TNF receptor-associated factor-6 (TRAF6). This SOCS-3/TRAF6 protein interaction blocks receptor recruitment of other downstream signaling components that lead to activation of JNK (18). Our results in myoblasts are consistent with the idea that IL-10 inhibits JNK phosphorylation indirectly by targeting a specific upstream mediator of JNK. Indeed, our results demonstrate that IL-10 inhibits the IL-1β-induced phosphorylation of an upstream activator of JNK, MKK7 (Fig. 4G).
Findings from these experiments provide clear support for three novel and important concepts regarding immune and endocrine interactions in muscle cells. First, these data extend the emerging concept that a single cell integrates multiple signals from diverse surface receptors to induce a specific response (23). We show that the proinflammatory cytokine, IL-1β, suppresses the biological activity of an endocrine growth hormone, IGF-I, and that an anti-inflammatory cytokine, IL-10, restores IGF-I-induced myogenin and MHC protein expression. This restoration does not occur by the classic IL-10 action of impairing synthesis of proinflammatory cytokines. Furthermore, IL-10 most likely did not globally affect IL-1β signaling by reducing the number of IL-1Rs or inducing IL-1R antagonist. Instead, the mechanism of IL-10 action in these cells occurs by specific inhibition of JNK activation downstream of IL-1R1. This is a true interaction because neither exogenous IL-1β nor IL-10 alters myogenin or MHC expression in the absence of IGF-I. Second, coupled with our previous findings, these new results demonstrate that key downstream effector proteins such as JNK play a central role in mediating specific physiological responses to a variety of proinflammatory cytokines and should be considered as a potential target for therapeutic treatment of inflammatory disorders. Third, the data establish a novel activity of IL-10 in muscle progenitor cells: IL-10 overcomes IL-1β-inhibition of IGF-I-induced myogenin and MHC expression by directly inhibiting exogenous IL-1β receptor signaling at concentrations that are lower than required for inhibition of cytokine synthesis. This new knowledge of IL-10 action may help improve strategies for clinical intervention of inflammatory disorders in muscle.
Acknowledgments
GRANTS
This research was supported by grants from the National Institutes of Health to K. W. Kelley (AI-50442, MH-51569, and AG-029573), R. W. Johnson (AG-16710 and AG-023580), and R. Dantzer (MH-071349) and the United States Department of Agriculture to R. H. McCusker (2004-35206-14144).
REFERENCES
- 1.Adams GR. Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab. 2006;31:782–790. doi: 10.1139/h06-053. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez B, Quinn LS, Busquets S, Lopez-Soriano FJ, Argiles JM. TNF-alpha modulates cytokine and cytokine receptors in C2C12 myotubes. Cancer Lett. 2002;175:181–185. doi: 10.1016/s0304-3835(01)00717-0. [DOI] [PubMed] [Google Scholar]
- 3.Argiles JM, Busquets S, Lopez-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol. 2005;37:1609–1619. doi: 10.1016/j.biocel.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21:3104–3112. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbe MF, Barr AE. Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav Immun. 2006;20:423–429. doi: 10.1016/j.bbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett BL, Satoh Y, Lewis AJ. JNK: a new therapeutic target for diabetes. Curr Opin Pharmacol. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 8.Bonny C, Borsello T, Zine A. Targeting the JNK pathway as a therapeutic protective strategy for nervous system diseases. Rev Neurosci. 2005;16:57–67. doi: 10.1515/revneuro.2005.16.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Dantzer R, Kelley KW. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol. 2004;172:7713–7720. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- 10.Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Cytokine-hormone interactions: tumor necrosis factor alpha impairs biologic activity and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. Endocrinology. 2003;144:2988–2996. doi: 10.1210/en.2003-0087. [DOI] [PubMed] [Google Scholar]
- 11.Courtin D, Jamonneau V, Mathieu JF, Koffi M, Milet J, Yeminanga CS, Kumeso VK, Cuny G, Bilengue CM, Garcia A. Comparison of cytokine plasma levels in human African trypanosomiasis. Trop Med Int Health. 2006;11:647–653. doi: 10.1111/j.1365-3156.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- 12.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 13.de Kruif MD, Lemaire LC, Giebelen IA, van Zoelen MA, Pater JM, van den Pangaart PS, Groot AP, de Vos AF, Elliott PJ, Meijers JC, Levi M, van der Poll T. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845–1851. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]
- 14.Divangahi M, Demoule A, Danialou G, Yahiaoui L, Bao W, Xing Z, Petrof BJ. Impact of IL-10 on diaphragmatic cytokine expression and contractility during Pseudomonas infection. Am J Respir Cell Mol Biol. 2007;36:504–512. doi: 10.1165/rcmb.2006-0038OC. [DOI] [PubMed] [Google Scholar]
- 15.Duarte J, Deshpande P, Guiyedi V, Mecheri S, Fesel C, Cazenave PA, Mishra GC, Kombila M, Pied S. Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status. Malar J. 2007;6:1. doi: 10.1186/1475-2875-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 18.Frobose H, Ronn SG, Heding PE, Mendoza H, Cohen P, Mandrup-Poulsen T, Billestrup N. Suppressor of cytokine signaling-3 inhibits interleukin-1 signaling by targeting the TRAF-6/TAK1 complex. Mol Endocrinol. 2006;20:1587–1596. doi: 10.1210/me.2005-0301. [DOI] [PubMed] [Google Scholar]
- 19.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1153–R1164. doi: 10.1152/ajpregu.00164.2003. [DOI] [PubMed] [Google Scholar]
- 20.Gimeno MJ, Pascual G, Garcia-Honduvilla N, Prieto A, Alvarez de Mon M, Bellon JM, Bujan J. Modulatory role of IL10 in endothelial cell damage and platelet adhesion. Histol Histopathol. 2003;18:695–702. doi: 10.14670/HH-18.695. [DOI] [PubMed] [Google Scholar]
- 21.Grounds MD. Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology. 2002;3:19–24. doi: 10.1023/a:1015234709314. [DOI] [PubMed] [Google Scholar]
- 22.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 23.Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science. 2005;310:1646–1653. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]
- 24.Johannessen LN, Nilsen AM, Lovik M. The mycotoxins citrinin and gliotoxin differentially affect production of the pro-inflammatory cytokines tumour necrosis factor-alpha and interleukin-6, and the anti-inflammatory cytokine interleukin-10. Clin Exp Allergy. 2005;35:782–789. doi: 10.1111/j.1365-2222.2005.02249.x. [DOI] [PubMed] [Google Scholar]
- 25.Kelley KW. From hormones to immunity: the physiology of immunology. Brain Behav Immun. 2004;18:95–113. doi: 10.1016/j.bbi.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun. 2007;21:384–392. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi N, Nagumo H, Agematsu K. IL-10 enhances B-cell IgE synthesis by promoting differentiation into plasma cells, a process that is inhibited by CD27/CD70 interaction. Clin Exp Immunol. 2002;129:446–452. doi: 10.1046/j.1365-2249.2002.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang CH, Hong-Brown L, Frost RA. Cytokine inhibition of JAK-STAT signaling: a new mechanism of growth hormone resistance. Pediatr Nephrol. 2005;20:306–312. doi: 10.1007/s00467-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 31.Lecart S, Morel F, Noraz N, Pene J, Garcia M, Boniface K, Lecron JC, Yssel H. IL-22, in contrast to IL-10, does not induce Ig production, due to absence of a functional IL-22 receptor on activated human B cells. Int Immunol. 2002;14:1351–1356. doi: 10.1093/intimm/dxf096. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Wu Y, Chen BG. Myoblast therapy: from bench to bedside. Cell Transplant. 2006;15:455–462. doi: 10.3727/000000006783981710. [DOI] [PubMed] [Google Scholar]
- 33.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 34.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 35.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 36.Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol. 2004;96:1292–1298. doi: 10.1152/japplphysiol.01064.2003. [DOI] [PubMed] [Google Scholar]
- 37.Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- 38.Ogando D, Cella M, Ribeiro ML, Weissmann C, Aisemberg J, Franchi A. IL-10 inhibits nitric oxide synthesis in murine uterus. Neuroimmunomodulation. 2004;11:127–132. doi: 10.1159/000075322. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill LA. Signal transduction pathways activated by the IL-1 receptor/ toll-like receptor superfamily. Curr Top Microbiol Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- 40.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2000;RE1:2000. doi: 10.1126/stke.442000re1. [DOI] [PubMed] [Google Scholar]
- 41.Ozturk K, Demir B, Oke R, Durmaz H. Dose-related effects of recombinant human interleukin-10 on hypoxia-induced skeletal muscle injury in immature rats. J Orthop Sci. 2006;11:620–625. doi: 10.1007/s00776-006-1063-4. [DOI] [PubMed] [Google Scholar]
- 42.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18–31. [PubMed] [Google Scholar]
- 44.Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol. 2006;41:320–327. doi: 10.1016/j.exger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Saidenberg-Kermanac’h N, Bessis N, Deleuze V, Bloquel C, Bureau M, Scherman D, Boissier MC. Efficacy of interleukin-10 gene electrotransfer into skeletal muscle in mice with collagen-induced arthritis. J Gene Med. 2003;5:164–171. doi: 10.1002/jgm.321. [DOI] [PubMed] [Google Scholar]
- 46.SAS. User’s guide. 8.2 ed. Cary, NC: SAS Institute; 2003. [Google Scholar]
- 47.Schippers EF, van’t Veer C, van Voorden S, Martina CA, Huizinga TW, le Cessie S, van Dissel JT. IL-10 and toll-like receptor-4 polymorphisms and the in vivo and ex vivo response to endotoxin. Cytokine. 2005;29:215–228. doi: 10.1016/j.cyto.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 49.Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays. 1999;21:47–52. doi: 10.1002/(SICI)1521-1878(199901)21:1<47::AID-BIES6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 51.Steinke JW, Borish L. 3. Cytokines and chemokines. J Allergy Clin Immunol. 2006;117:S441–S445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- 53.Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology. 2006;147:4363–4373. doi: 10.1210/en.2005-1541. [DOI] [PubMed] [Google Scholar]
- 54.Strle K, MCcClusker RH, Tran L, King A, Johnson RW, Freund GG, Dantzer R, Kelley KW. Novel activity of an anti-inflammatory cytokine: IL-10 prevents TNFalpha-induced resistance to IGF-I in myoblasts. J Neuroimmunol. 2007;188:48–55. doi: 10.1016/j.jneuroim.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 56.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Wu H, Lakdawala VS, Hu F, Hanson ND, Miller AH. Inhibition of Jun N-terminal kinase (JNK) enhances glucocorticoid receptor-mediated function in mouse hippocampal HT22 cells. Neuropsychopharmacology. 2005;30:242–249. doi: 10.1038/sj.npp.1300606. [DOI] [PubMed] [Google Scholar]
- 59.Zemse SM, Hilgers RH, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am J Physiol Heart Circ Physiol. 2007;292:H3103–H3108. doi: 10.1152/ajpheart.00456.2006. [DOI] [PubMed] [Google Scholar]
- 60.Zhang ZL, Shen SX, Lin B, Yu LY, Zhu LH, Wang WP, Luo FH, Guo LH. Intramuscular injection of interleukin-10 plasmid DNA prevented autoimmune diabetes in mice. Acta Pharmacol Sin. 2003;24:751–756. [PubMed] [Google Scholar]