Abstract
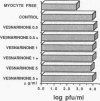
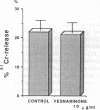
Controversy still exists concerning the therapy for viral myocarditis which manifests a wide variety of clinical symptoms. Vesnarinone, a quinolinone derivative that was developed as a positive inotropic agent with complex actions, including phosphodiesterase inhibition and cation channel modification, has recently been confirmed to improve the prognosis of patients with chronic heart failure. However, the precise mechanism of this beneficial effect is not yet clearly understood. In this study, using a murine model of acute viral myocarditis resulting from encephalomyocarditis virus infection, survival and myocardial damage were markedly improved by treatment with vesnarinone. In contrast, survival was not improved by treatment with amrinone, a phosphodiesterase inhibitor. Although vesnarinone did not inhibit viral replication or protect myocytes from viral direct cell injury, it did inhibit the increase in natural killer cell activity after viral infection. On the other hand, amrinone failed to inhibit natural killer cell activity. Both vesnarinone and amrinone suppressed the production of tumor necrosis factor-alpha. Therefore, we postulate that vesnarinone exerted its beneficial effects through an inhibition of natural killer cell activity, and that it serves as an immunomodulator providing new therapeutic possibilities for the treatment of viral myocarditis and/or immunological disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelmann W. H. Virus and the heart. Circulation. 1971 Nov;44(5):950–956. doi: 10.1161/01.cir.44.5.950. [DOI] [PubMed] [Google Scholar]
- Asanoi H., Sasayama S., Iuchi K., Kameyama T. Acute hemodynamic effects of a new inotropic agent (OPC-8212) in patients with congestive heart failure. J Am Coll Cardiol. 1987 Apr;9(4):865–871. doi: 10.1016/s0735-1097(87)80243-7. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J. S., Chavin K. D., Kunkel S. L. Anti-tumor necrosis factor antibodies suppress cell-mediated immunity in vivo. J Immunol. 1992 Jun 1;148(11):3412–3417. [PubMed] [Google Scholar]
- Busch F. W., Tillmann A., Becker E. W., Owsianowski M., Berg P. A. The inhibitory effects of a positive inotropic quinolinone derivative, 3,4-dihydro-6-[4-(3,4-dimethoxybenzoyl)-1-piperazinyl]-2(1H)- quinolinone (OPC-8212), on bone-marrow progenitor cells and peripheral lymphocytes. Eur J Clin Pharmacol. 1992;42(6):629–633. doi: 10.1007/BF00265927. [DOI] [PubMed] [Google Scholar]
- Chodera A., Feller K. Some aspects of pharmacokinetic and biotransformation differences in humans and mammal animals. Int J Clin Pharmacol Biopharm. 1978 Aug;16(8):357–360. [PubMed] [Google Scholar]
- Davies M. J., Ward D. E. How can myocarditis be diagnosed and should it be treated? Br Heart J. 1992 Oct;68(4):346–347. doi: 10.1136/hrt.68.10.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domke-Opitz I., Kirchner H. Stimulation of macrophages by endotoxin results in the reactivation of a persistent herpes simplex virus infection. Scand J Immunol. 1990 Aug;32(2):69–75. doi: 10.1111/j.1365-3083.1990.tb02895.x. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Baughman K. L., Lee W. K., Gottlieb S. H., Weiss J. L., Becker L. C., Strobeck J. E. Usefulness of OPC-8212, a quinolinone derivative, for chronic congestive heart failure in patients with ischemic heart disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1991 Nov 1;68(11):1203–1210. doi: 10.1016/0002-9149(91)90194-p. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Becker L. C., Llewellyn M. P., Baughman K. L. Evaluation of a new inotropic agent, OPC-8212, in patients with dilated cardiomyopathy and heart failure. Am Heart J. 1988 Sep;116(3):771–777. doi: 10.1016/0002-8703(88)90336-5. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Bristow M. R., Parmley W. W., Carson P. E., Pepine C. J., Gilbert E. M., Strobeck J. E., Hendrix G. H., Powers E. R., Bain R. P. Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone Study Group. N Engl J Med. 1993 Jul 15;329(3):149–155. doi: 10.1056/NEJM199307153290301. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. In situ immune autoradiographic identification of cells in heart tissues of mice with coxsackievirus B3-induced myocarditis. Am J Pathol. 1987 Nov;129(2):267–276. [PMC free article] [PubMed] [Google Scholar]
- Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Henke A., Mohr C., Sprenger H., Graebner C., Stelzner A., Nain M., Gemsa D. Coxsackievirus B3-induced production of tumor necrosis factor-alpha, IL-1 beta, and IL-6 in human monocytes. J Immunol. 1992 Apr 1;148(7):2270–2277. [PubMed] [Google Scholar]
- Iijima T., Taira N. Membrane current changes responsible for the positive inotropic effect of OPC-8212, a new positive inotropic agent, in single ventricular cells of the guinea pig heart. J Pharmacol Exp Ther. 1987 Feb;240(2):657–662. [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Kereiakes D. J., Parmley W. W. Myocarditis and cardiomyopathy. Am Heart J. 1984 Nov;108(5):1318–1326. doi: 10.1016/0002-8703(84)90760-9. [DOI] [PubMed] [Google Scholar]
- Lee M. D., Zentella A., Vine W., Pekala P. H., Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987 May;84(9):2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Kalman J., Mayer L., Fillit H. M., Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990 Jul 26;323(4):236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Boltz R. C., Blake J. T., Nguyen M., Talento A., Fischer P. A., Springer M. S., Sigal N. H., Slaughter R. S., Garcia M. L. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med. 1993 Mar 1;177(3):637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori A., Kawai C. An animal model of congestive (dilated) cardiomyopathy: dilatation and hypertrophy of the heart in the chronic stage in DBA/2 mice with myocarditis caused by encephalomyocarditis virus. Circulation. 1982 Aug;66(2):355–360. doi: 10.1161/01.cir.66.2.355. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Kawai C. An experimental model for congestive heart failure after encephalomyocarditis virus myocarditis in mice. Circulation. 1982 Jun;65(6):1230–1235. doi: 10.1161/01.cir.65.6.1230. [DOI] [PubMed] [Google Scholar]
- Monrad E. S., Matsumori A., Murphy J. C., Fox J. G., Crumpacker C. S., Abelmann W. H. Therapy with cyclosporine in experimental murine myocarditis with encephalomyocarditis virus. Circulation. 1986 May;73(5):1058–1064. doi: 10.1161/01.cir.73.5.1058. [DOI] [PubMed] [Google Scholar]
- O'Connell J. B., Reap E. A., Robinson J. A. The effects of cyclosporine on acute murine Coxsackie B3 myocarditis. Circulation. 1986 Feb;73(2):353–359. doi: 10.1161/01.cir.73.2.353. [DOI] [PubMed] [Google Scholar]
- Ohnishi A., Ishizaki T. Pharmacokinetic profile of OPC-8212 in humans: a new, nonglycosidic, inotropic agent. J Clin Pharmacol. 1988 Aug;28(8):719–726. doi: 10.1002/j.1552-4604.1988.tb03206.x. [DOI] [PubMed] [Google Scholar]
- Ostensen M. E., Thiele D. L., Lipsky P. E. Tumor necrosis factor-alpha enhances cytolytic activity of human natural killer cells. J Immunol. 1987 Jun 15;138(12):4185–4191. [PubMed] [Google Scholar]
- Packer M. The search for the ideal positive inotropic agent. N Engl J Med. 1993 Jul 15;329(3):201–202. doi: 10.1056/NEJM199307153290310. [DOI] [PubMed] [Google Scholar]
- Packer M. Treatment of chronic heart failure. Lancet. 1992 Jul 11;340(8811):92–95. doi: 10.1016/0140-6736(92)90406-s. [DOI] [PubMed] [Google Scholar]
- Poli G., Kinter A., Justement J. S., Kehrl J. H., Bressler P., Stanley S., Fauci A. S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Santoli D., Koprowski H. Mechanisms of activation of human natural killer cells against tumor and virus-infected cells. Immunol Rev. 1979;44:125–163. doi: 10.1111/j.1600-065x.1979.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Sasayama S., Inoue M., Asanoi H., Kodama K., Hori M., Sakurai T., Kawai C. Acute hemodynamic effects of a new inotropic agent, OPC-8212, on severe congestive heart failure. Heart Vessels. 1986;2(1):23–28. doi: 10.1007/BF02060240. [DOI] [PubMed] [Google Scholar]
- Sasayama S. What do the newer inotropic drugs have to offer? Cardiovasc Drugs Ther. 1992 Feb;6(1):15–18. doi: 10.1007/BF00050911. [DOI] [PubMed] [Google Scholar]
- Seko Y., Shinkai Y., Kawasaki A., Yagita H., Okumura K., Takaku F., Yazaki Y. Expression of perforin in infiltrating cells in murine hearts with acute myocarditis caused by coxsackievirus B3. Circulation. 1991 Aug;84(2):788–795. doi: 10.1161/01.cir.84.2.788. [DOI] [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Sherry B., Schoen F. J., Wenske E., Fields B. N. Derivation and characterization of an efficiently myocarditic reovirus variant. J Virol. 1989 Nov;63(11):4840–4849. doi: 10.1128/jvi.63.11.4840-4849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka N., Kishimoto C., Matsumori A., Kawai C. Effects of prednisolone on acute viral myocarditis in mice. J Am Coll Cardiol. 1986 Apr;7(4):868–872. doi: 10.1016/s0735-1097(86)80349-7. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]