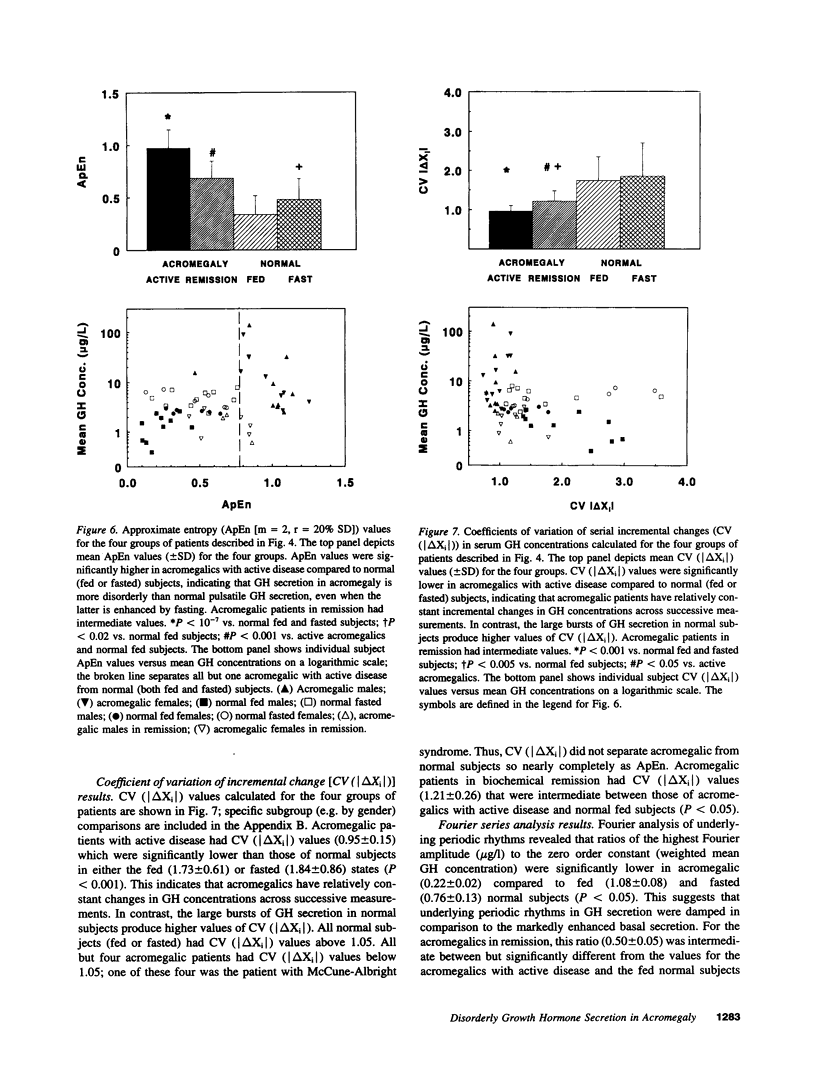
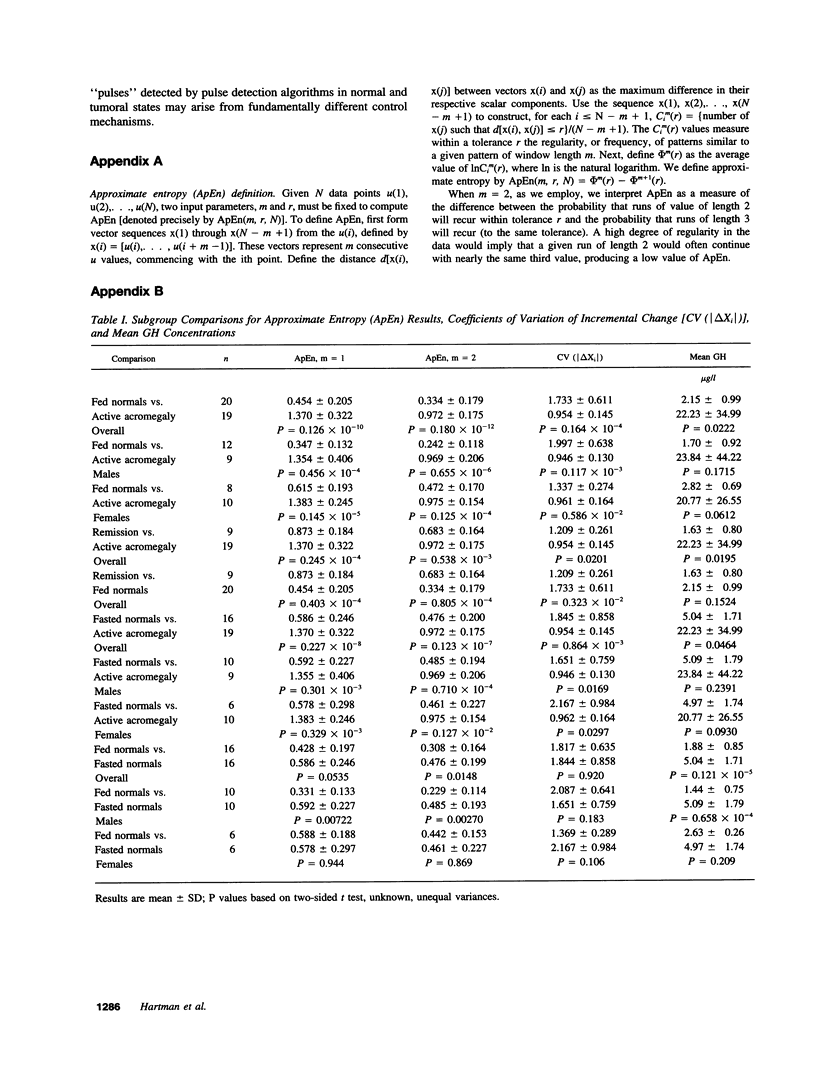
Abstract
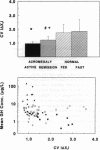
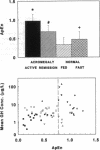
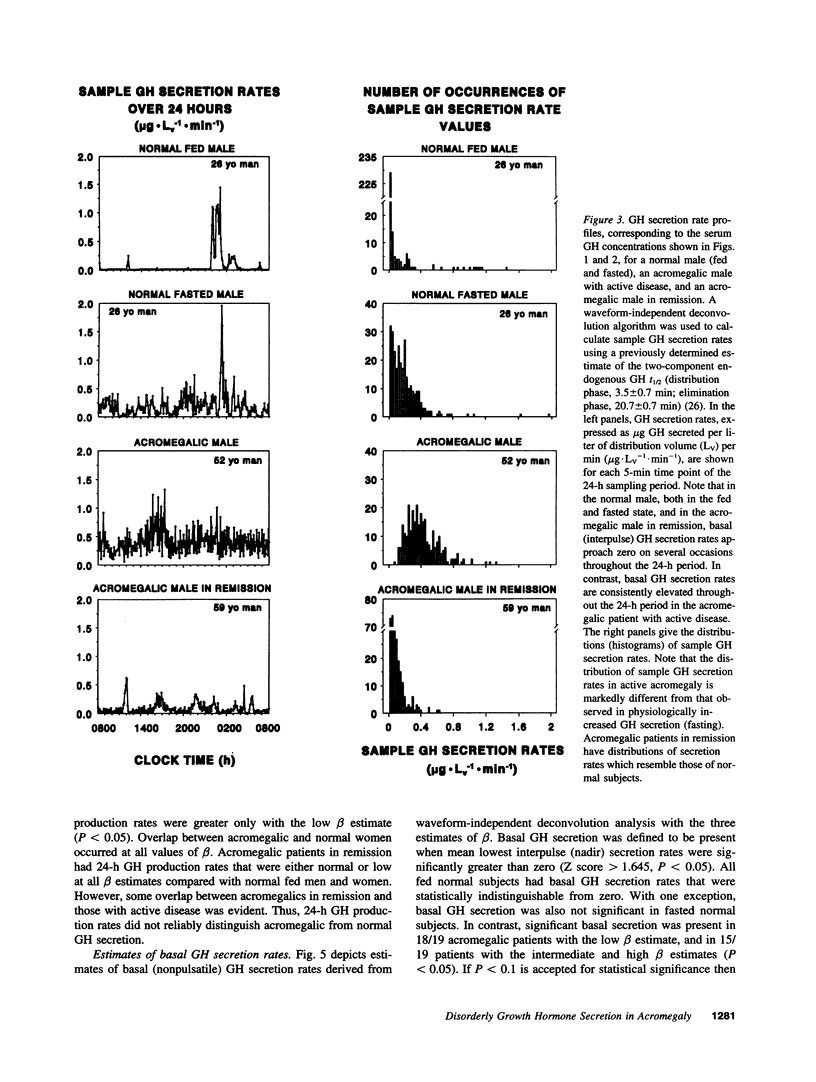
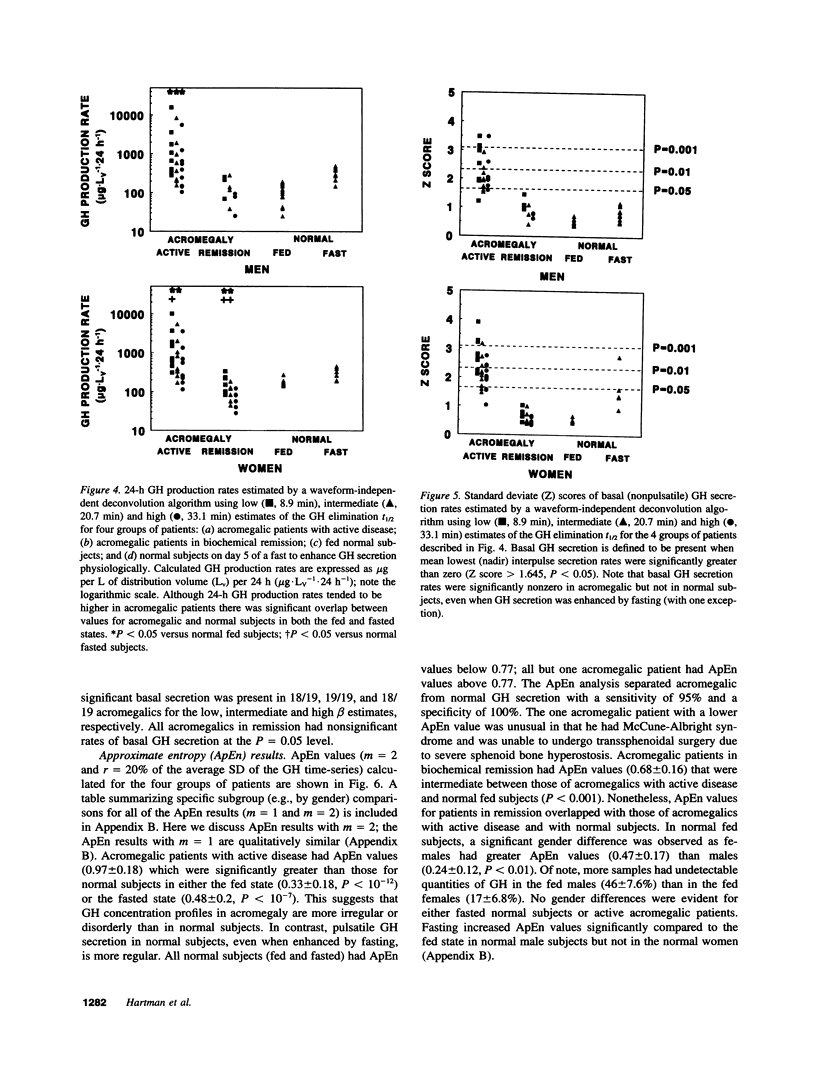
Pulses of growth hormone (GH) release in acromegaly may arise from hypothalamic regulation or from random events intrinsic to adenomatous tissue. To distinguish between these possibilities, serum GH concentrations were measured at 5-min intervals for 24 h in acromegalic men and women with active (n = 19) and inactive (n = 9) disease and in normal young adults in the fed (n = 20) and fasted (n = 16) states. Daily GH secretion rates, calculated by deconvolution analysis, were greater in patients with active acromegaly than in fed (P < 0.05) but not fasted normal subjects. Significant basal (nonpulsatile) GH secretion was present in virtually all active acromegalics but not those in remission or in fed and fasted normal subjects. A recently introduced scale- and model-independent statistic, approximate entropy (ApEn), was used to test for regularity (orderliness) in the GH data. All but one acromegalic had ApEn values greater than the absolute range in normal subjects, indicating reduced orderliness of GH release; ApEn distinguished acromegalic from normal GH secretion (fed, P < 10(-12); fasted, P < 10(-7)) with high sensitivity (95%) and specificity (100%). Acromegalics in remission had ApEn scores larger than those of normal subjects (P < 0.0001) but smaller than those of active acromegalics (P < 0.001). The coefficient of variation of successive incremental changes in GH concentrations was significantly lower in acromegalics than in normal subjects (P < 0.001). Fourier analysis in acromegalics revealed reduced fractional amplitudes compared to normal subjects (P < 0.05). We conclude that GH secretion in acromegaly is highly irregular with disorderly release accompanying significant basal secretion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. F., Buchfelder M., Hüttner A., Moreth S., Fahlbusch R. Recent advances in the molecular biology of growth-hormone secreting human pituitary tumours. Exp Clin Endocrinol. 1993;101(1):12–16. doi: 10.1055/s-0029-1211202. [DOI] [PubMed] [Google Scholar]
- Asa S. L., Kovacs K., Stefaneanu L., Horvath E., Billestrup N., Gonzalez-Manchon C., Vale W. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinology. 1992 Nov;131(5):2083–2089. doi: 10.1210/endo.131.5.1425411. [DOI] [PubMed] [Google Scholar]
- Asa S. L., Scheithauer B. W., Bilbao J. M., Horvath E., Ryan N., Kovacs K., Randall R. V., Laws E. R., Jr, Singer W., Linfoot J. A. A case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factor. J Clin Endocrinol Metab. 1984 May;58(5):796–803. doi: 10.1210/jcem-58-5-796. [DOI] [PubMed] [Google Scholar]
- Barkan A. L., Stred S. E., Reno K., Markovs M., Hopwood N. J., Kelch R. P., Beitins I. Z. Increased growth hormone pulse frequency in acromegaly. J Clin Endocrinol Metab. 1989 Dec;69(6):1225–1233. doi: 10.1210/jcem-69-6-1225. [DOI] [PubMed] [Google Scholar]
- Beckers A., Stevenaert A., Foidart J. M., Hennen G., Frankenne F. Placental and pituitary growth hormone secretion during pregnancy in acromegalic women. J Clin Endocrinol Metab. 1990 Sep;71(3):725–731. doi: 10.1210/jcem-71-3-725. [DOI] [PubMed] [Google Scholar]
- Drews R. T., Gravel R. A., Collu R. Identification of G protein alpha subunit mutations in human growth hormone (GH)- and GH/prolactin-secreting pituitary tumors by single-strand conformation polymorphism (SSCP) analysis. Mol Cell Endocrinol. 1992 Sep;87(1-3):125–129. doi: 10.1016/0303-7207(92)90240-7. [DOI] [PubMed] [Google Scholar]
- Dufy-Barbe L., Bresson L., Sartor P., Odessa M. F., Dufy B. Calcium homeostasis in growth hormone (GH)-secreting adenoma cells: effect of GH-releasing factor. Endocrinology. 1992 Sep;131(3):1436–1444. doi: 10.1210/endo.131.3.1505473. [DOI] [PubMed] [Google Scholar]
- Faria A. C., Veldhuis J. D., Thorner M. O., Vance M. L. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab. 1989 Mar;68(3):535–541. doi: 10.1210/jcem-68-3-535. [DOI] [PubMed] [Google Scholar]
- Faunt L. M., Johnson M. L. Analysis of discrete, time-sampled data using Fourier series method. Methods Enzymol. 1992;210:340–356. doi: 10.1016/0076-6879(92)10017-8. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Holl R. W., Martha P., Jr, Bauler G., Hellman P., Wills M. R., Thorner M. O. Influence of matrix on concentrations of somatotropin measured in serum with commercial immunoradiometric assays. Clin Chem. 1989 Jul;35(7):1423–1426. [PubMed] [Google Scholar]
- Furlanetto R. W., Marino J. M. Radioimmunoassay of somatomedin C/insulin-like growth factor I. Methods Enzymol. 1987;146:216–226. doi: 10.1016/s0076-6879(87)46023-0. [DOI] [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., LONERGAN E. T. SURVIVAL OF ENDOGENOUS HUMAN GROWTH HORMONE IN PLASMA. J Clin Endocrinol Metab. 1964 Jun;24:501–505. doi: 10.1210/jcem-24-6-501. [DOI] [PubMed] [Google Scholar]
- Gelato M. C., Merriam G. R., Vance M. L., Goldman J. A., Webb C., Evans W. S., Rock J., Oldfield E. H., Molitch M. E., Rivier J. Effects of growth hormone-releasing factor on growth hormone secretion in acromegaly. J Clin Endocrinol Metab. 1985 Feb;60(2):251–257. doi: 10.1210/jcem-60-2-251. [DOI] [PubMed] [Google Scholar]
- Gelato M. C., Oldfield E., Loriaux D. L., Merriam G. R. Pulsatile growth hormone secretion in patients with acromegaly and normal men: the effects of growth hormone-releasing hormone infusion. J Clin Endocrinol Metab. 1990 Sep;71(3):585–590. doi: 10.1210/jcem-71-3-585. [DOI] [PubMed] [Google Scholar]
- Hartman M. L., Clayton P. E., Johnson M. L., Celniker A., Perlman A. J., Alberti K. G., Thorner M. O. A low dose euglycemic infusion of recombinant human insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. J Clin Invest. 1993 Jun;91(6):2453–2462. doi: 10.1172/JCI116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M. L., Faria A. C., Vance M. L., Johnson M. L., Thorner M. O., Veldhuis J. D. Temporal structure of in vivo growth hormone secretory events in humans. Am J Physiol. 1991 Jan;260(1 Pt 1):E101–E110. doi: 10.1152/ajpendo.1991.260.1.E101. [DOI] [PubMed] [Google Scholar]
- Hartman M. L., Veldhuis J. D., Johnson M. L., Lee M. M., Alberti K. G., Samojlik E., Thorner M. O. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J Clin Endocrinol Metab. 1992 Apr;74(4):757–765. doi: 10.1210/jcem.74.4.1548337. [DOI] [PubMed] [Google Scholar]
- Hartman M. L., Veldhuis J. D., Vance M. L., Faria A. C., Furlanetto R. W., Thorner M. O. Somatotropin pulse frequency and basal concentrations are increased in acromegaly and are reduced by successful therapy. J Clin Endocrinol Metab. 1990 May;70(5):1375–1384. doi: 10.1210/jcem-70-5-1375. [DOI] [PubMed] [Google Scholar]
- Herman V., Fagin J., Gonsky R., Kovacs K., Melmed S. Clonal origin of pituitary adenomas. J Clin Endocrinol Metab. 1990 Dec;71(6):1427–1433. doi: 10.1210/jcem-71-6-1427. [DOI] [PubMed] [Google Scholar]
- Hindmarsh P. C., Matthews D. R., Brain C. E., Pringle P. J., di Silvio L., Kurtz A. B., Brook C. G. The half-life of exogenous growth hormone after suppression of endogenous growth hormone secretion with somatostatin. Clin Endocrinol (Oxf) 1989 Apr;30(4):443–450. doi: 10.1111/j.1365-2265.1989.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Ho K. Y., Veldhuis J. D., Johnson M. L., Furlanetto R., Evans W. S., Alberti K. G., Thorner M. O. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988 Apr;81(4):968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. J., Friberg R. D., Barkan A. L. Regulation of pulsatile growth hormone secretion by fasting in normal subjects and patients with acromegaly. J Clin Endocrinol Metab. 1992 Sep;75(3):812–819. doi: 10.1210/jcem.75.3.1517371. [DOI] [PubMed] [Google Scholar]
- Hofland L. J., van Koetsveld P. M., van Vroonhoven C. C., Stefanko S. Z., Lamberts S. W. Heterogeneity of growth hormone (GH) release by individual pituitary adenoma cells from acromegalic patients, as determined by the reverse hemolytic plaque assay: effects of SMS 201-995, GH-releasing hormone and thyrotropin-releasing hormone. J Clin Endocrinol Metab. 1989 Mar;68(3):613–620. doi: 10.1210/jcem-68-3-613. [DOI] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Mandell G. L., Sullivan J. A., Sinha Y. N., Leong D. A. Spontaneous oscillations of intracellular calcium and growth hormone secretion. J Biol Chem. 1988 Jul 15;263(20):9682–9685. [PubMed] [Google Scholar]
- Iranmanesh A., Grisso B., Veldhuis J. D. Low basal and persistent pulsatile growth hormone secretion are revealed in normal and hyposomatotropic men studied with a new ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1994 Mar;78(3):526–535. doi: 10.1210/jcem.78.3.8126122. [DOI] [PubMed] [Google Scholar]
- Jaffe C. A., Friberg R. D., Barkan A. L. Suppression of growth hormone (GH) secretion by a selective GH-releasing hormone (GHRH) antagonist. Direct evidence for involvement of endogenous GHRH in the generation of GH pulses. J Clin Invest. 1993 Aug;92(2):695–701. doi: 10.1172/JCI116639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs K., Horvath E., Thorner M. O., Rogol A. D. Mammosomatotroph hyperplasia associated with acromegaly and hyperprolactinemia in a patient with the McCune-Albright syndrome. A histologic, immunocytologic and ultrastructural study of the surgically-removed adenohypophysis. Virchows Arch A Pathol Anat Histopathol. 1984;403(1):77–86. doi: 10.1007/BF00689340. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Verleun T., Oosterom R. The interrelationship between the effects of somatostatin and human pancreatic growth hormone-releasing factor on growth hormone release by cultured pituitary tumor cells from patients with acromegaly. J Clin Endocrinol Metab. 1984 Feb;58(2):250–254. doi: 10.1210/jcem-58-2-250. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Harsh G., Lyons J., Davis R. L., McCormick F., Bourne H. R. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990 Dec;71(6):1416–1420. doi: 10.1210/jcem-71-6-1416. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Lüdecke D., Kautzky R., Saeger W., Schrader D. Selective removal of hypersecreting pituitary adenomas? An analysis of endocrine function, operative and microscopical findings in 101 cases. Acta Neurochir (Wien) 1976;35(1-3):27–42. doi: 10.1007/BF01405930. [DOI] [PubMed] [Google Scholar]
- MacGillivray M. H., Frohman L. A., Doe J. Metabolic clearance and production rates of human growth hormone in subjects with normal and abnormal growth. J Clin Endocrinol Metab. 1970 May;30(5):632–638. doi: 10.1210/jcem-30-5-632. [DOI] [PubMed] [Google Scholar]
- Pincus S. M. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. M., Cummins T. R., Haddad G. G. Heart rate control in normal and aborted-SIDS infants. Am J Physiol. 1993 Mar;264(3 Pt 2):R638–R646. doi: 10.1152/ajpregu.1993.264.3.R638. [DOI] [PubMed] [Google Scholar]
- Pincus S. M., Gladstone I. M., Ehrenkranz R. A. A regularity statistic for medical data analysis. J Clin Monit. 1991 Oct;7(4):335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- Pincus S. M., Keefe D. L. Quantification of hormone pulsatility via an approximate entropy algorithm. Am J Physiol. 1992 May;262(5 Pt 1):E741–E754. doi: 10.1152/ajpendo.1992.262.5.E741. [DOI] [PubMed] [Google Scholar]
- Ralph M. R., Foster R. G., Davis F. C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990 Feb 23;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Sönksen P. H. Disappearance rate of endogenous and exogenous human growth hormone in man. J Clin Endocrinol Metab. 1970 Mar;30(3):386–392. doi: 10.1210/jcem-30-3-386. [DOI] [PubMed] [Google Scholar]
- Riedel M., Günther T., von zur Mühlen A., Brabant G. The pulsatile GH secretion in acromegaly: hypothalamic or pituitary origin? Clin Endocrinol (Oxf) 1992 Sep;37(3):233–239. doi: 10.1111/j.1365-2265.1992.tb02316.x. [DOI] [PubMed] [Google Scholar]
- Roelfsema F., de Boer H., Frölich M. The influence of octreotide treatment on pulsatile growth hormone release in acromegaly. Clin Endocrinol (Oxf) 1990 Aug;33(2):297–306. doi: 10.1111/j.1365-2265.1990.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Roelfsema F., van der Heide D., Lowry P. J., van Dulken H., Schröder-van der Elst J. Plasma growth hormone half-life after selective removal of adenoma in acromegalics determines the outcome of surgery. Clin Endocrinol (Oxf) 1988 Jan;28(1):51–59. doi: 10.1111/j.1365-2265.1988.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Spada A., Arosio M., Bochicchio D., Bazzoni N., Vallar L., Bassetti M., Faglia G. Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase. J Clin Endocrinol Metab. 1990 Dec;71(6):1421–1426. doi: 10.1210/jcem-71-6-1421. [DOI] [PubMed] [Google Scholar]
- Stewart J. K., Clifton D. K., Koerker D. J., Rogol A. D., Jaffe T., Goodner C. J. Pulsatile release of growth hormone and prolactin from the primate pituitary in vitro. Endocrinology. 1985 Jan;116(1):1–5. doi: 10.1210/endo-116-1-1. [DOI] [PubMed] [Google Scholar]
- Thomas G. B., Cummins J. T., Francis H., Sudbury A. W., McCloud P. I., Clarke I. J. Effect of restricted feeding on the relationship between hypophysial portal concentrations of growth hormone (GH)-releasing factor and somatostatin, and jugular concentrations of GH in ovariectomized ewes. Endocrinology. 1991 Feb;128(2):1151–1158. doi: 10.1210/endo-128-2-1151. [DOI] [PubMed] [Google Scholar]
- Thompson R. G., Rodriguez A., Kowarski A., Blizzard R. M. Growth hormone: metabolic clearance rates, integrated concentrations, and production rates in normal adults and the effect of prednisone. J Clin Invest. 1972 Dec;51(12):3193–3199. doi: 10.1172/JCI107146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U H. S., Kelley P., Lee W. H. Abnormalities of the human growth hormone gene and protooncogenes in some human pituitary adenomas. Mol Endocrinol. 1988 Jan;2(1):85–89. doi: 10.1210/mend-2-1-85. [DOI] [PubMed] [Google Scholar]
- Vallar L., Spada A., Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987 Dec 10;330(6148):566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Johnson M. L. A novel general biophysical model for simulating episodic endocrine gland signaling. Am J Physiol. 1988 Dec;255(6 Pt 1):E749–E759. doi: 10.1152/ajpendo.1988.255.6.E749. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Johnson M. L. Deconvolution analysis of hormone data. Methods Enzymol. 1992;210:539–575. doi: 10.1016/0076-6879(92)10028-c. [DOI] [PubMed] [Google Scholar]
- Weinstein L. S., Shenker A., Gejman P. V., Merino M. J., Friedman E., Spiegel A. M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991 Dec 12;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Siler T. M., DeVane G. W. Effect of somatostatin in patients with acromegaly: suppression of growth hormone, prolactin, insulin and glucose levels. N Engl J Med. 1974 Apr 25;290(17):935–938. doi: 10.1056/NEJM197404252901704. [DOI] [PubMed] [Google Scholar]
- Zimmerman D., Young W. F., Jr, Ebersold M. J., Scheithauer B. W., Kovacs K., Horvath E., Whitaker M. D., Eberhardt N. L., Downs T. R., Frohman L. A. Congenital gigantism due to growth hormone-releasing hormone excess and pituitary hyperplasia with adenomatous transformation. J Clin Endocrinol Metab. 1993 Jan;76(1):216–222. doi: 10.1210/jcem.76.1.8421089. [DOI] [PubMed] [Google Scholar]