Abstract
Phospholipase D (PLD), a major source of lipid second messengers (phosphatidic acid, diglycerides) in many cell types, is tightly regulated by protein kinases, but only a few of them have been identified. We show here that protein kinase B (AKT) is a novel major signaling effector of PLD activity induced by the formylpeptide f-Met-Leu-Phe (fMLP) in human neutrophil-like HL-60 cells (dHL-60 cells). AKT inhibition with the selective antagonist AKTib1/2 almost completely prevented fMLP-mediated activity of PLD, its upstream effector ERK1/2, but not p38 MAPK. Immunoprecipitation studies show that phosphorylated AKT, ERK, and PLD2 form a complex induced by fMLP, which can be prevented by AKTib1/2. In cell-free systems, AKT1 stimulated PLD activity via activation of ERK. AKT1 actually phosphorylated ERK2 as a substrate (Km 1 μm). Blocking AKT activation with AKTib1/2 also prevented fMLP- but not phorbol 12-myristate 13-acetate-mediated NADPH oxidase activation (respiratory burst, RB) of dHL-60 cells. Impaired RB was associated with defective membrane translocation of NADPH oxidase components p67phox and p47phox, ERK, AKT1, AKT2, but not AKT3. Depletion of AKT1 or AKT2 with antisense oligonucleotides further indicates a partial contribution of both isoforms in fMLP-induced activation of ERK, PLD, and RB, with a predominant role of AKT1. Thus, formylpeptides induce sequential activation of AKT, ERK1/2, and PLD, which represents a novel signaling pathway. A major primarily role of this AKT signaling pathway also emerges in membrane recruitment of NOX2 components p47phox, p67phox, and ERK, which may contribute to assembly and activation of the RB motor system, NADPH oxidase.
Keywords: ERK, Neutrophil, Phospholipase D, Protein Kinases, Respiratory Burst, Signal Transduction
Introduction
Phospholipase D (PLD)2 is a major source of lipid mediators in many cell types and has emerged as a key regulator of various physiological responses, such as cytoskeleton rearrangement, endocytosis, vesicle trafficking, cell migration, as well pathological processes (1). PLD cleaves phosphatidylcholine to produce free choline and a lipid second messenger, phosphatidic acid (PA). This latter is rapidly dephosphorylated into diglycerides (diacylglycerol, alkyl-acyl glycerol) which are potent PKC activators (2). Two distinct families of PLD, PLD1 and PLD2, have been cloned in mammalian cells and share ∼50% amino acid identity. Both PLDs are expressed ubiquitously and show a different intracellular distribution and are activated differently suggesting they regulate distinct functions (2, 3). PLD1 is located preferentially at the membrane of internal compartments and is activated by protein kinase C (PKC), small G proteins of the Rho and ADP-ribosylation factor families, Rac1, whereas PLD2, located at the plasma membrane, is not directly activated by these regulators (2). The biochemical regulation of PLD2 is poorly documented, although this isoform is apparently tightly regulated upon activation of various types of membrane receptors.
In a previous study with retinoic acid-differentiated human promyelocyte leukemia HL-60 cells (dHL-60 cells), we showed that the PLD2 isoform rather than PLD1 is mainly responsible for the PLD activity induced by the chemotactic peptide fMLP and regulates fMLP-mediated respiratory burst (RB), i.e. a robust production of reactive oxygen species by the NADPH oxidase complex NOX2 (4). Reactive oxygen species production by phagocytes is essential for killing bacteria and has been involved in various diseases (5, 6). We further showed that the fMLP-mediated PLD activity is mainly dependent on p44/42 MAPKs in human polymorphonuclear leukocytes, dHL-60 cells, and HEK 293 cells expressing the formylpeptide receptor fPR (4, 7). Other chemoattractants such as the chemokines MIP1-β or SDF1α also stimulate PLD activity via ERK1/2 activation in intact cells (8). We also showed that ERK is present in a signaling complex with PLD2 in resting cells and stimulates PLD2 phosphorylation and activity in cell-free systems (4). However, the molecular mechanisms by which chemoattractant receptors stimulate ERK and PLD pathways are not elucidated.
Protein kinase B (also called AKT), the human homologue of the viral oncogene v-AKT, is a serine/threonine kinase of the “AGC” superfamily, which is important in a variety of biological responses such as cell survival and proliferation (9). AKT possesses a pleckstrin homology domain that binds to lipids produced by phosphoinositide 3′-kinase (PI3-kinase) (10). Among these, phosphatidylinositol 3,4,5-trisphosphate, a bioactive lipid, exerts a critical function in mediating translocation at the plasma membrane of a number of effectors via their pleckstrin homology domains (11). Once recruited at the plasma membranes, AKT can be activated by phosphorylation at two sites, Thr308 by the 3-phosphoinositide-dependent protein kinase 1 PDK1(12), and Ser473 by PDK2 (13). In turn, AKT phosphorylates a number of proteins associated with cell survival/death pathways (Bad, procaspase-9, Forkhead family of transcription factors, Foxo1, CREB, IκB kinase). In human neutrophils, PI3-kinase has been shown to mediate the PLD activity induced by chemoattractants (14–16). Chemoattractants stimulate AKT phosphorylation in neutrophils (4, 17, 18). However, a role of AKT in up-regulating signaling pathways toward ERK1/2 and PLD is not known.
In this study, we have addressed whether AKT is required for chemoattractant-mediated ERK activation, PLD activity, and RB parameters. To this purpose, we have combined pharmacological, biochemical, and molecular biology approaches to manipulate AKT activation in differentiated HL-60 cells (dHL-60 cells). With this neutrophilic model, we provide evidence that AKT plays a crucial role in PLD activity and RB stimulated by fMLP. Interestingly, the PLD activity induced by AKT was mediated mainly through activation of ERK1/2, which we identified here as a novel AKT1 substrate. Antisense oligonucleotides to AKT and pharmacological inhibition of AKT further indicate that AKT1 and AKT2 contribute to fMLP- but not phorbol ester-mediated RB of dHL-60 cells, although phorbol esters stimulate a potent AKT activation. AKT signaling was primarily involved in the membrane translocation of the NOX2 components p47phox and p67phox and contributed to the p47phox phosphorylation by MAPKs.
EXPERIMENTAL PROCEDURES
Materials
Cell culture media, FBS, and Lipofectamine were from Invitrogen. Amplex Red was from Molecular Probes. Antibodies used were from Cell Signaling (AKT1, AKT2, AKT3, phospho-ERK1/2(Thr202/Tyr204), phospho-AKT1/2(Ser473), phospho-p38MAPK(Thr180/Tyr182), phospho-MEK1/2(Ser217/Ser221), from Santa Cruz (anti-ERK1, anti-MEK1/2, and peroxidase-conjugated antibodies), and Roche Applied Science (anti-HA (12CA5)). The AKT inhibitor VIII (AKTib1/2) and constitutively activated AKT1 were from Calbiochem. Anti-p47phox and anti-phospho-p47phox were produced as described (19). The mouse monoclonal anti-phospho-Ser/Thr-Pro, MPM-2 was from Millipore. Thiolated oligodeoxynucleotides, sense and antisense, to AKT1 and AKT2 were synthesized by Sigma, i.e. AKT1 antisense (5′-cac gtc gct cat ggt gcc-3′), AKT2 antisense (5′-aca cct cat tca tgg tgg-3′), and sense (5′-cat gct gtc act gca tcg-3′). SDS-PAGE and Western blotting reagents were from Bio-Rad. [γ-32P]ATP was from PerkinElmer Life Sciences. All other reagents were from Sigma.
Cell Culture and Transfection
HL-60 cells were grown in RPMI 1640 medium with 10% FBS and 100 μg/ml penicillin and streptomycin. Myeloid differentiation was induced by incubating HL-60 cells (0.5 × 106/ml) with 1 μm all-trans-retinoic acid for 5 days (4). Cells were starved overnight, washed twice, and suspended in HBSS, pH 7.4, for signaling studies. In some experiments, cells were differentiated with 1 μm all-trans-retinoic acid for 1 day and then transfected with 8 μm AKT antisense oligonucleotides using Oligofectamine (4). Cells were harvested 3 days later in HBSS, pH 7.4, for signaling and functional studies.
PLD Activity and RB
PLD activity was measured using the fluorometric Amplex Red assay for mass choline quantification in homogenates of stimulated cells, as described (4). Briefly, 2 × 106 dHL-60 cells/300 μl of HBSS were pretreated with cytochalasin B (5 μg/ml/5min) before stimulation with fMLP or PMA, as described in the figure legends. Stimulation was stopped with cold HBSS, and cells were sonicated at 4 °C in 200 μl of 100 mm Na2HPO4, pH 8. The amount of choline was quantified at 37 °C using calibration curves obtained with choline chloride (4).
RB of d-HL-60 cells was monitored continuously using the cytochrome c reduction assay (20). Aliquots of 2 × 106 cells/ml of HBSS were stimulated under conditions used for the PLD assay. In some experiments, cells were pretreated with pharmacological inhibitors or dimethyl sulfoxide (up to 0.2%) for 15 min before stimulation. Superoxide production is expressed as a percentage of control values.
In Vitro PLD Activity and Phosphorylation of ERK by AKT1
Membrane and cytosolic fractions of resting cells were prepared by centrifugation of postnuclear supernatants (1000 × g, 2 min, 4 °C) over 41% sucrose cushion (120,000 × g, 50 min, 4 °C) as described (4). Membranes were washed and suspended in homogenization buffer. For PLD activity (21), 100 μg of membrane proteins were preincubated at 30 °C for 10 min in 100 μl of 50 mm Tris-HCl, pH 7.5, containing 10 mm MgCl2, 1 mm EGTA, 0.01% Brij 35, and 10 mm cold ATP, without (buffer) or with 50 units of recombinant AKT1 or 10 μm GTPγS. Then, a cytosolic fraction (100 μg of proteins) was added to the reaction mixture, and aliquots were collected at various times for choline assay.
In some experiments, particulate or cytosolic fraction alone (100 μg of proteins) was treated in the absence or presence of AKTib1/2 or U0126 for 10 min, then with 50 units of AKT1 for 15 min. Proteins were electrophoresed and blotted on nitrocellulose membrane for the detection of phosphoproteins by Western blotting.
Immunoprecipitation of AKT, ERK1/2, and HA-PLD2
Resting and stimulated HEK 293T cells expressing HA-tagged PLD2 were suspended (3 × 106 cells/0.3 ml) in buffer A (10 mm Tris-HCl, pH 7.4, 100 mm NaCl, 10% glycerol, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mm EGTA, 1 mm EDTA, 1 mm NaF, 1 mm NaVO4, 1 mm benzamidine, 1 mm PMSF, and a mixture of antiproteases) and sonicated at 4 °C (three pulses of 5 s) before incubation for 2 h at 4 °C with gentle agitation. Cell lysates were centrifuged (10,000 × g, 30 min), and the supernatant was diluted four times in buffer B (i.e. buffer A without detergent). Aliquots of 300 μg of proteins in 500 μl of buffer B were used to immunoprecipitate AKT, HA-PLD2, or ERK1/2 with 1 μg of antibody. After 1-h incubation at 4 °C, the immune complexes were incubated overnight at 4 °C with 50 μl of protein G-Sepharose beads. Beads were washed three times with buffer B, and proteins were heat-denatured and electrophoresed.
Electrophoresis and Western Blot Analysis
Stimulated cells (3 × 106 cells) were lysed in 200 μl of 50 mm Tris-HCl, pH 6.8, containing 2.5 mm orthovanadate, 2.5 mm EDTA, a mixture of antiproteases (CompleteTM; Roche Applied Science) and 1× Laemmli sample buffer. Proteins were denatured (5 min, 95 °C), separated by 10% SDS-PAGE, and transferred onto nitrocellulose membrane. Immunoblotting experiments were performed under standard conditions using primary phosphoantibodies and ECL for the detection of HRP-conjugated secondary antibody. The quantification of phosphorylated proteins was performed with ImageJ 1.62 software (National Institutes of Health).
In Vitro Phosphorylation of GST-ERK2 and p47phox by AKT1
Recombinant GST-ERK2 expressed in Escherichia coli from the pGEX plasmid (gift from Dr. M. Cobb, University of Texas) or GST p47phox (5 μg) was subjected to phosphorylation with a constitutively activated AKT1 in a 40 μl of 35 mm Tris-HCl buffer, pH 7.5, containing 50 mm glycero 2-phosphate, 0.4 mm EGTA, 10 mm magnesium acetate, and 10 unit/ml recombinant activated AKT. The phosphorylation reaction was initiated with 50 μm [γ-32P]ATP (specific radioactivity 150–300 Ci/mmol) and carried out at 30 °C for 30 min. Aliquots of the reaction mixture (20 μl) were subjected to 10% SDS-PAGE and then blotted for autoradiography at −70 °C.
In Vitro Phosphorylation of p47phox and MBP by ERK2
Recombinant p47phox or MBP (5 μg each) was phosphorylated by active AKT1 (Calbiochem) or ERK2 (New England Biolabs) in a reaction mixture containing 40 mm Hepes, pH 7.5, 10 mm MgCl2, 1 mm DTT, and 50 mm ATP (3 μCi of [γ-32P]ATP/assay) in a total volume of 100 μl at 30 °C for 30 min. The reaction was stopped by adding hot 2× Laemmli sample buffer. In some assays, 10 μm U0126 was incubated with AKT1 or ERK2 for 10 min prior starting the reaction with [γ-32P]ATP. The samples were separated on 13% polyacrylamide gels, and separated proteins were transferred to nitrocellulose following Towbin's procedure. MBP and p47phox phosphorylation were detected with a PhosphorImager and autoradiography.
Statistical Analysis
Unless otherwise stated, data represent means ± S.E. of at least three experiments. Statistically significant differences between means were calculated using the Student's paired t test with a threshold of p < 0.05 designated by *.
RESULTS
Inhibition of AKT Abrogates fMLP-mediated Activation of PLD, ERK1/2, but not p38 MAP Kinase
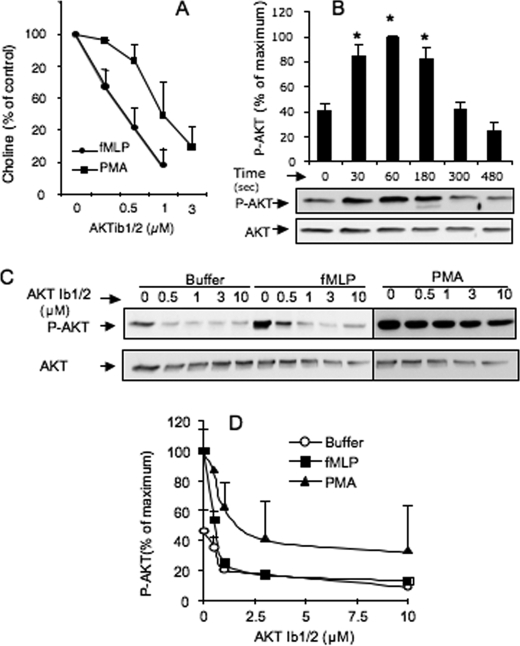
The contribution of AKT signaling pathway to PLD activation was examined in stimulated dHL-60 cells using AKTib1/2, a selective antagonist of the AKT isoforms (22). Under optimal conditions of PLD stimulation by fMLP (4), 0.25–1 μm AKTib1/2 induced a concentration-dependent inhibitory effect with an IC50 of ∼0.40 μm (Fig. 1A). At a concentration of 1 μm, the AKTib1/2 inhibitory effect reached ∼80%, suggesting a crucial role of AKT in chemoattractant-mediated PLD activation. PLD activity can be stimulated via PKC, which is an important part of the signaling network induced by fMLP in human neutrophils (23, 24). PKC also potently stimulates AKT phosphorylation in neutrophils (17). However, the contribution of AKT to the PKC-dependent PLD activity is not known and was explored here. Cell pretreatment with 0.5–3 μm AKTi1/2 also impaired the PLD activity induced by PMA in a concentration-dependent manner (IC50 value of ∼1 μm; Fig. 1A). These results suggest an important role of AKT in fMLP- and PMA-induced PLD activation.
FIGURE 1.
PLD activity induced by fMLP or PMA in dHL-60 cells is abrogated by the AKT antagonist, AKTib1/2. A, PLD activity in dHL-60 cells pretreated without (control) or with 0.25–3 μm AKTib1/2 for 15 min before stimulation with 1 μm fMLP or 0.5 μm PMA for 3 min. The stimulated production of choline by PLD is expressed as a percentage of control values (n = 5 experiments). B, time course of AKT phosphorylation induced by 1 μm fMLP in dHL-60 cells and its densitometric quantification (*, p < 0.05). C, AKT phosphorylation in dHL-60 cells pretreated without (control) or with 0.5–10 μm AKTib1/2 for 15 min before cell stimulation with 1 μm fMLP for 1 min or 0.5 μm PMA for 3 min. A representative Western blot of phospho-AKT (Ser473) (P-AKT) is shown in C, and the densitometric quantification (D) is expressed as percentage of maximal response (n = 4 experiments).
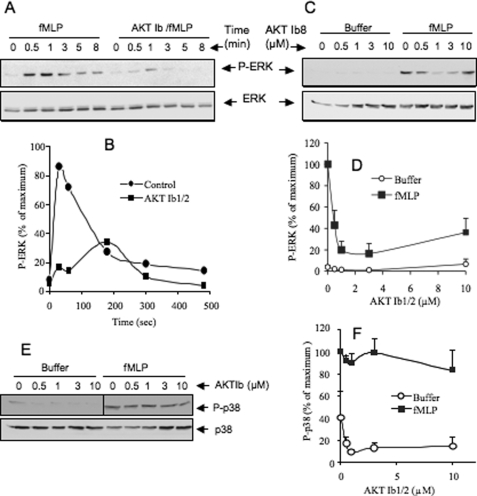
We previously showed that the PLD activity induced by fMLP in dHL-60 cells is mainly regulated by p44/42 MAPK (ERK1/2) (4). To determine a possible relationship between AKT and ERK activation, the active form of these kinases was examined by Western blotting. Treatment of dHL-60 cells with fMLP induced a rapid and transient phosphorylation of AKT (Fig. 1B) peaking at approximately 1 min of cell stimulation and then returning to basal values within 5 min, as in primary human neutrophils (20). Cell treatment with 0.5–10 μm AKTib1/2 reduced basal phosphorylation of AKT and abrogated fMLP-induced AKT phosphorylation (Fig. 1, C and D). This inhibition was almost complete with 1–2 μm AKTib1/2, a drug concentration close to the IC50 values for the three AKT isoforms (22). The fMLP-mediated rapid and transient AKT phosphorylation correlated with the phosphorylation of ERK1/2 (Fig. 2, A and B). However, a role of AKT in stimulating chemoattractant-mediated ERK activation is not known. AKTib1/2 (3 μm) was found to block the transient stimulation of ERK (1 min) induced by fMLP (Fig. 2, A and B). Concentration-response curve studies (Fig. 2, C and D) confirmed that ERK phosphorylation was strongly inhibited by low concentrations of AKTib1/2 (IC50 of 0.5 μm). Taking advantage of the selective inhibitory effect of AKTib1/2, we examined whether another MAPK stimulated by fMLP, p38 MAPK (20), was regulated by AKT. In contrast to ERK, the rapid and transient phosphorylation of p38 MAPK induced by fMLP was not altered by AKTib1/2 (Fig. 2, E and F), suggesting that this MAPK is activated independently of AKT.
FIGURE 2.
AKT mediates fMLP-induced activation of ERK but not p38 MAPK in dHL-60 cells. A and B, time course of ERK phosphorylation induced by 1 μm fMLP in dHL-60 cells that were pretreated without (control) or with 3 μm AKTib1/2 for 15 min. C–F, concentration-dependent effects of 0.5–10 μm AKTib1/2 on the phosphorylation of ERK1/2 (C and D) and p38 MAPK (E and F) induced by fMLP (1 μm, 1 min) in dHL-60 cells. Representative Western blots are shown in A, C, and E, and densitometric quantification is expressed as a percentage of the respective control in B, D, and F (n = 4 experiments).
AKT Stimulates PLD Activity, ERK, and PLD2 Phosphorylation in Cell-free Systems
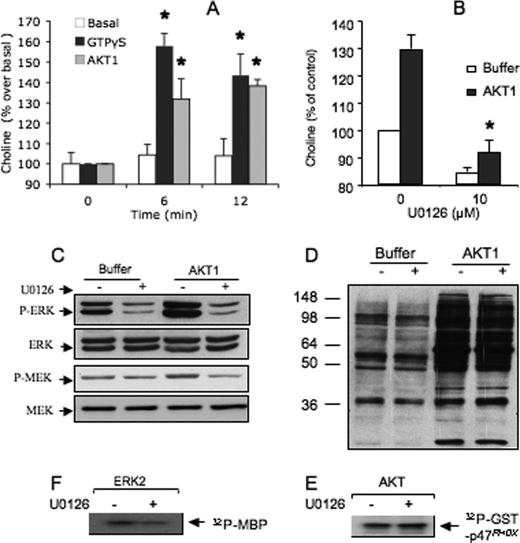
PLD activity can be stimulated in cell-free systems reconstituted with plasma membranes, cytosolic fractions, and ATP (21). We previously took advantage of this model to show that ERK2 stimulates PLD activity in plasma membranes of HEK293T cells (4). In this model, recombinant AKT1 also triggered a rapid and transient PLD activity compared with the basal response obtained in the absence of AKT1 but in the presence of ATP (Fig. 3A). This PLD activity was similar to that induced by GPTγS used here as a positive control (21). Interestingly, the pretreatment of plasma membranes and cytosolic fractions with the specific MEK antagonist, U0126 (10 μm), strongly inhibited AKT1-induced PLD activity, indicating that it was mediated by ERK signaling (Fig. 3B). Consistent with this possibility, AKT1 strongly stimulates ERK phosphorylation in cytosolic fractions (∼80% above basal values) (Fig. 3C). As expected, this phosphorylation of ERK1/2 was prevented when cytosolic fractions were pretreated with U0126. AKT1 also increased moderately the phosphorylation state of MEK1/2 in the cytosolic fraction (∼40% above basal values), and this MEK phosphorylation was also prevented by U0126 (Fig. 3C), suggesting that a role of MEK1/2 in AKT1-induced activation of ERK. This U0126 inhibitory effect was not due to a nonspecific inhibition of AKT1. This is supported by the observation that pretreatment of particulate fractions with U0126 before incubation with AKT1 did not alter the phosphorylation of various proteins (molecular mass ranging from 36 to 250 kDa) induced by AKT1 and revealed with a specific phospho-AKT substrate antibody (Fig. 3D). In agreement with this result, treatment of recombinant AKT1 with 10 μm U0126 has no effect on the phosphorylation of GST-p47phox (Fig. 3E). By contrast, we found that U0126 inhibits ERK2 activity. This is supported by the observation that the phosphorylation of MBP by recombinant ERK2 in vitro was inhibited by pretreatment of ERK2 with 10 μm U0126 (∼40% of control values, p < 0.05, Fig. 3F). This property of U0126 was unexpected because U0126 is known to target MEK1/2 specifically.
FIGURE 3.
AKT1 stimulates PLD activity and phosphorylation of cytosolic ERK1/2 in cell-free systems. A, PLD activity in cell membrane fractions incubated in the presence of ATP without (buffer) or with recombinant AKT1 added at time zero. The production of choline induced by AKT1 for 6 and 12 min is expressed as a percentage of basal values at time zero set at 100% (mean of three experiments). B, PLD activity measured in the same way for 6 min, except that cell fractions were pretreated without (control) or with 10 μm U0126 for 10 min. PLD activity is expressed as a percentage of basal control values (*, p < 0.05). C, cytosolic fraction pretreated without or with 10 μm U0126 for 10 min, then with AKT1 and ATP for 15 min. Results show a representative Western blot of ERK1/2 and MEK1/2 and their phosphorylation state (P-ERK, P-MEK). D, particulate fraction purified from dHL-60 cells pretreated with 10 μm U0126 for 10 min before incubation with ATP in the absence or presence of AKT1 for 30 min. Proteins were resolved by SDS-PAGE and detected by Western blotting with an antibody against AKT phosphorylation sites. E, recombinant ERK2 treated in the absence or presence of 10 μm U0126 for 10 min, then incubated with MBP and [γ-32P]ATP for 30 min. Data show the autoradiography of phosphorylated MBP. F, recombinant AKT1 treated with the absence or presence of 10 μm U0126 for 10 min, then incubated with GST-p47phox and [γ-32P]ATP for 30 min. Data show the autoradiography of phosphorylated p47phox.
ERK2 Is a Substrate of AKT
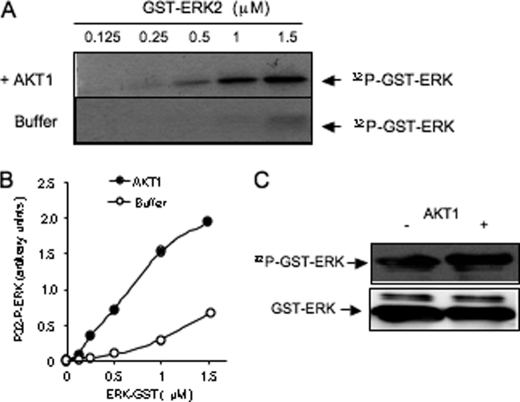
The observation that AKT1 more potently stimulated the phosphorylation of ERK compared with MEK (Fig. 3C) raises the possibility that AKT could phosphorylate ERK directly. To clarify this point, 0.125–1.5 μm recombinant GST-ERK2 was treated with [γ32-P]ATP in the absence (control) or presence of AKT1. This treatment stimulated the phosphorylation of GST-ERK2 (Fig. 4A). In this experiment, GST alone (1.5 μm) was not phosphorylated by AKT1 (data not shown), in agreement with previous report (25). The basal phosphorylation of GST-ERK2 measured in the absence of AKT1 (ERK autophosphorylation) was low. The estimated amount of phosphorylation due to AKT (total minus basal ERK phosphorylation) was dependent on the concentration of ERK2 (Fig. 4B). The apparent Km value of AKT1 for ERK2, calculated from a Lineweaver-Burk representation of the data (not shown), was low (∼1 μm), which suggests that ERK2 is a good substrate of AKT. In addition, the phosphorylated GST-ERK2 was detected in Western blot experiments using a phospho-ERK antibody that recognizes the active form of ERK (Thr202/Tyr204) (Fig. 4C). Compared with basal values, AKT1 significantly increased phosphorylation of ERK2 (∼40%), suggesting that it may contribute to the ERK activation state.
FIGURE 4.
AKT1 phosphorylates GST-ERK2 in vitro. A, 0.125–1.5 μm recombinant GST-ERK2 incubated for 30 min with [γ-32P]ATP in the absence of AKT1 (buffer) or in the presence of AKT1. Proteins were electrophoresed and blotted for autoradiography analysis (P32-GST-ERK). B, densitometric quantification of [32P]-GST-ERK of D. C, GST-ERK was treated with cold ATP without or with AKT1 for 30 min, electrophoresed on SDS-PAGE, and detected by Western blotting with anti-phospho-ERK(Thr202/Tyr204). Data are representative of three experiments.
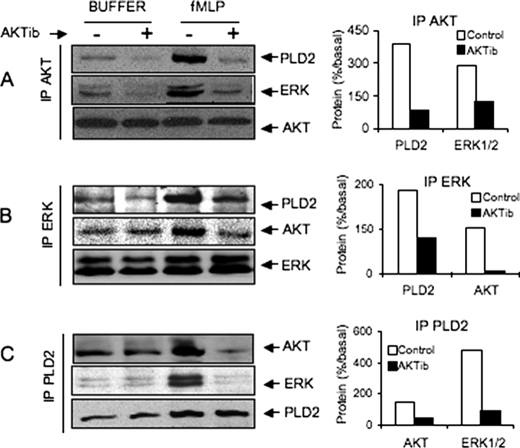
AKT, ERK, and PLD2 Form a Signaling Complex in Stimulated Cells
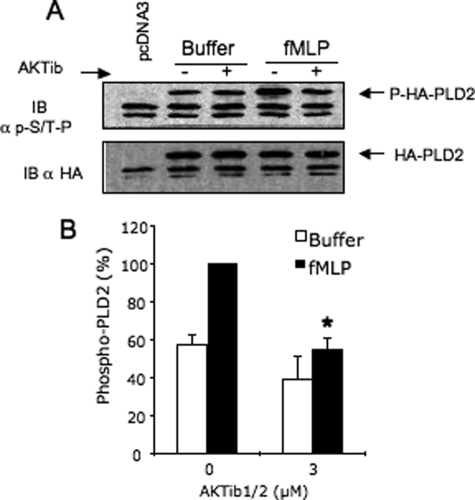
To examine a possible interaction among AKT, ERK1/2, or PLD in vivo, co-immunoprecipitation studies were performed with lysates of fMLP-stimulated cells which were pretreated or not with AKTib1/2. For this purpose, HEK cells transfected with HA-PLD2 were used because HL-60 cells express weak amounts of endogenous PLD. We showed previously that in both cell types, fMLP stimulated a similar time course of PLD activity which was dependent on ERK activation but not PKC (4). In these HEK cells stably expressing fPR, AKT co-immunoprecipitated with ERK in resting cells and reciprocally, whereas a greater amount of both protein kinases was obtained after cell stimulation with fMLP (Fig. 5, A and B). Blocking AKT activation with 3 μm AKTib1/2 strongly inhibited the formation of the immunocomplex, suggesting that activated AKT may be required for the recruitment of ERK. In addition, we confirmed that HA-PLD2 and ERK1/2 are present in the same signaling complex in resting cells (Fig. 5C). This complex also contained a substantial amount of AKT which increased after cell stimulation with fMLP. Blocking AKT activation with AKTib1/2 also inhibited the formation of the ERK·PLD2 complex. Consistent with these data, AKTib1/2 also prevented the phosphorylation of PLD2 induced by fMLP (Fig. 6). We previously reported that this phosphorylation was dependent mainly on ERK1/2 activation (4). Taken together, these data suggest an important role of AKT in the activation of PLD2 via ERK1/2.
FIGURE 5.
Co-immunoprecipitation of AKT, ERK1/2, and HA-PLD2 in resting and stimulated cells. HEK293 cells stably expressing fPR were transfected with empty vector (pcDNA3) or plasmid encoding wild-type HA-PLD2. Cells in suspension were treated in the absence or presence of 3 μm AKTib1/2 for 15 min before stimulation with 1 μm fMLP for 60 s. Immunoprecipitation (IP) of AKT (A), ERK1/2 (B), and HA-PLD (C) was performed with cell homogenates, and the presence of partners was revealed by Western blotting. For each immunoprecipitation experiment, the stimulating effect of fMLP was calculated and is expressed as a percentage of stimulated control values (right panels). Data are representative of three independent experiments.
FIGURE 6.
The phosphorylation of HA-PLD2 induced by fMLP in HEK cells is abrogated by the AKT antagonist AKTib1/2 prevents. HEK29 T cells stably expressing the fMLP receptor fPR were transiently transfected with an empty vector (pcDNA3) or with HA-PLD2. Cells were treated with AKTib1/2 (3 μm, 15 min) and then stimulated with fMLP (1 μm) for 2 min. HA-PLD2 was immunoprecipitated, Western-blotted (IB), and detected with the MPM-2 antibody against phosphorylated Ser/Thr-Pro and with an anti-HA antibody after membrane stripping. The amount of PLD2 phosphorylation was quantified and expressed as a percentage of maximal values (mean of four experiments; *, p < 0.05).
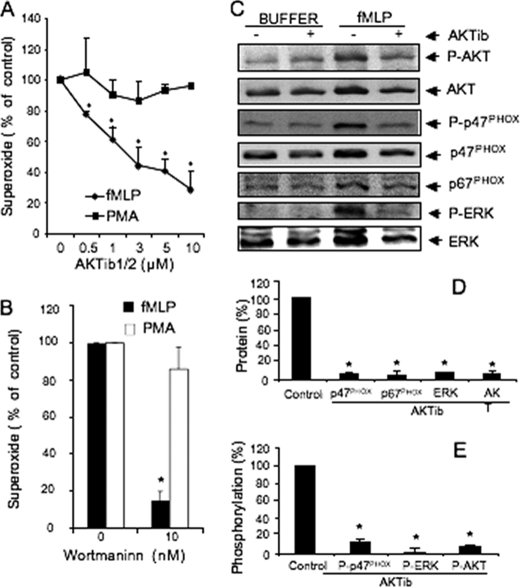
Blocking AKT Stimulation Prevents fMLP- but Not PMA-mediated RB of dHL-60 Cells
AKT has been proposed to stimulate neutrophil RB due to its ability to phosphorylate the NADPH oxidase component p47phox (26), although controversial studies have been reported (20, 27). We took advantage of the potent AKTib1/2-blocking effects to examine further the contribution of AKT in RB. Treatment of dHL-60 cells with 0.5–10 μm AKTib1/2 dramatically inhibited fMLP-induced RB with an IC50 value of 1 μm. In contrast, the PMA-induced RB was not altered (Fig. 7A). Thus, although AKT is potently activated in PMA-stimulated cells, it does not mediate PKC-mediated NOX2 activation. Consistent with this hypothesis, the PI3-kinase antagonist wortmannin failed to inhibit PMA-induced RB of dHL-60 cells, whereas fMLP-mediated RB was almost completely inhibited (Fig. 7B), in agreement with previous studies (28).
FIGURE 7.
AKT activation is required for fMLP-mediated RB and translocation of NOX components but not for PMA-induced RB. A and B, dHL-60 cells pretreated without (control) or with 0.25–3 μm AKTib1/2 (A) or with 10 nm wortmannin (B) for 15 min before stimulation with 1 μm fMLP or 0.5 μm PMA for 5 min. Superoxide production is expressed as a percentage of control values (five experiments). C, representative Western blot of p47phox, p67phox, ERK, AKT, phospho-p47phox (P-p47phox), phospho-ERK1/2 (P-ERK), and phospho-AKT (P-AKT) in the membrane fractions of dHL-60 cells that were pretreated in the absence or presence of AKTib1/2 before stimulation with 1 μm fMLP for 2 min. D, amount of proteins translocated at the membrane in fMLP-stimulated cells quantified and expressed as a percentage of stimulated control values (n = 3 experiments). E, phosphorylated form of p47phox, ERK, and AKT quantified and expressed as percentage of stimulated control values (n = 3 experiments). *, p < 0.05.
Activation of the NADPH oxidase is dependent on the phosphorylation of the NOX2 components (p47phox and p67phox) and their translocation to the plasma membranes where they assemble with the cytochome b558 to form an active complex (29, 30). As shown in Fig. 7C, fMLP did stimulate the translocation of p47phox and p67phox at the plasma membranes in dHL-60 cells. Treatment of dHL-60 cells with AKTib1/2 prevented the membrane translocation of AKT induced by fMLP. This treatment also blocked the membrane translocation of ERK1/2, p47phox, and p67phox induced by fMLP and inhibited the state of p47phox phosphorylation (Fig. 7C). This phosphorylation of p47phox was detected using an antibody directed against MAPK consensus sites (19). A role of ERK is likely predominant because the fMLP-mediated RB in dHL-60 cells was also blocked by U0126 (4). Taken together, these results indicate that AKT may primarily regulate the membrane translocation of NOX2 components and the phosphorylation of p47phox by MAPKs.
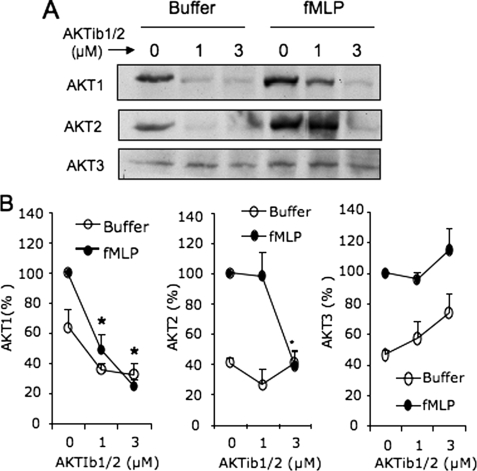
Contribution of AKT Isoforms to fMLP-mediated Phosphorylation of ERK, PLD Activity, and RB of dHL-60 Cells
The concentrations of AKTib1/2 used here to inhibit fMLP-mediated RB (Fig. 7) and signaling events, ERK phosphorylation (Fig. 2), and PLD activity (Fig. 1A), are in the range of the AKTib1/2 IC50 values for AKT1, AKT2, and AKT3, i.e. ∼0.06, 0.2, and 2 μm, respectively (22). To determine the functional contribution of AKT isoforms, we first examined their ability to translocate at the membranes upon cell stimulation with fMLP. All three AKT isoforms are present in dHL-60 cells (Fig. 8) and human neutrophils (data not shown), although AKT3 expression was weaker. All three isoforms translocated at the membranes upon stimulation of dHL-60 cells by fMLP (Fig. 8). Interestingly, AKTib1/2 (1 and 3 μm) strongly inhibited the translocation of AKT1 (IC50 of 1 μm). The translocation of AKT2 was inhibited with 3 μm AKTib1/2 only, whereas AKT3 translocation was not altered. Based on these AKTIb1/2 inhibitory concentrations, these results strongly suggest that AKT1 and AKT2 are potential candidates regulating fMLP-mediated signaling through ERK/PLD and RB. To investigate further the functional contribution of these two AKT isoforms, antisense oligonucleotides were used to deplete AKT1 or AKT2 in dHL-60 cells.
FIGURE 8.
Membrane translocation of AKT isoforms induced by fMLP in control and AKTIb1/2-treated dHL-60 cells. Cells were pretreated for 15 min with 1 and 3 μm AKTib1/2 and stimulated with 1 μm fMLP (2 min). A, cells were disrupted by sonication, and the membrane fractions were prepared and Western-blotted. B, the amount of AKT1, AKT2, and AKT3 was quantified and expressed as a percentage of the maximal control values obtained with fMLP (mean ± S.E., n = 3 experiments; *, p < 0.05).
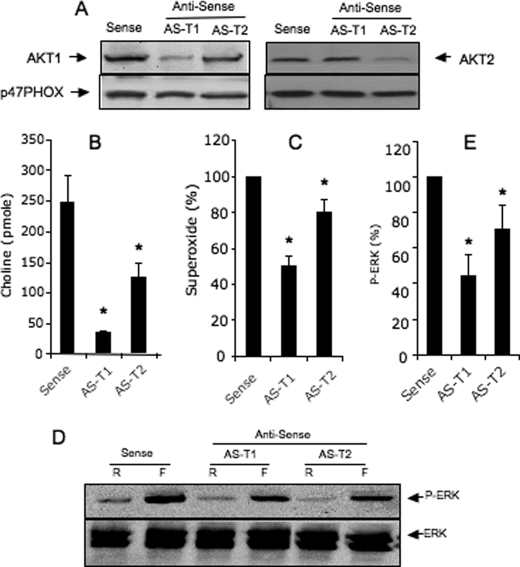
The depletion of AKT1 (∼60-70 % versus control (p < 0.05) did not alter the expression of AKT2, and inversely (Fig. 9A), the depletion of both AKTs also did not alter the expression of p47phox, a main component of the NADPH oxidase complex. AKT1 and AKT2 antisense oligonucleotides inhibited fMLP-mediated PLD activity by ∼80 and 40%, respectively; p < 0.05 (Fig. 9B). The state of ERK phosphorylation induced by fMLP was also reduced by AKT1 and AKT2 antisense oligonucleotides (∼55 and 35% respectively; p < 0.05) (Fig. 6D). The fMLP-induced RB was also significantly reduced by the AKT1 and AKT2 antisense (∼50 and 20%, respectively; p < 0.05). By contrast, the depletion of both AKTs did not alter PMA-mediated RB (data not shown).
FIGURE 9.
Effect of antisense oligonucleotides on AKT1 and AKT2 on fMLP-mediated PLD activity, ERK phosphorylation, and RB in dHL-60 cells. Cells were transfected with 8 μm antisense oligonucleotides with AKT1 (AS-T1) or AKT2 (AS-T2) or sense (control). A, expression of AKT1, AKT2, and p47phox in transfected dHL-60 cells, revealed by Western blotting. B–E, RB (B), PLD activity (C), and phosphorylation of ERK (D and E) were stimulated with 1 μm fMLP. Significant differences between control and antisense-treated cells are indicated by * p < 0.05. Blots are representative of three experiments.
DISCUSSION
This study provides new insights to the signaling network of chemoattractant receptors coupled to G proteins. Our results first indicate that the activation of AKT triggered by fMLP in dHL-60 cells is a major early event leading to stimulation of ERK1/2, PLD activity, and production of oxidants by the NADPH oxidase NOX2. We confirmed these results with primary human neutrophils. However, human neutrophils were found to be more sensitive to AKTib1/2 inhibitory effects than dHL-60 cells (data not shown). These conclusions are supported by two different approaches: (i) the use of AKTib1/2, which provides advantages of quickly and potently preventing intracellular redistribution of both AKT1 and AKT2 (Fig. 8); and (ii) the depletion of these two AKT isoforms by antisense oligonucleotides, although the respective contribution of AKT1 and AKT2 was partial (Fig. 9) compared with AKTIb1/2. Our data further show that AKT1 utilizes ERK2 as a substrate in vitro and promotes PLD activity via ERK1/2 in cell-free systems. We showed previously that ERK1/2 phosphorylates PLD2, the main PLD isoform responsible for fMLP-induced PLD activity and RB of dHL-60 cells. ERK2 also stimulated PLD activity in cell-free systems (4). We show here that AKT is required for the phosphorylation of PLD2 via ERK induced by fMLP in dHL-60 cells. Thus, AKT is a crucial intermediate linking PI3-kinase activation to the ERK/PLD pathway.
The ability of AKT to stimulate the activation of ERK induced by chemoattractants has not been described previously. In contrast, a report by Zimmermann and Moelling using HEK cells showed that AKT inhibits signaling via the ERK module as a consequence of AKT phosphorylation of c-Raf on a highly conserved serine (Ser259) (25). The reasons for these discrepancies are unclear and may be related to the cell type and stimulus used. Our conclusions are supported by different approaches. First, in vitro, AKT directly phosphorylated ERK (Fig. 4, A and B) and increased its activation state (Fig. 4C). Second, in a cytosolic fraction of dHL-60 cells, AKT1 strongly stimulates the phosphorylation of ERK1/2 (Fig. 3C). At least two processes may account for this activation. The first mechanism may involve the activation of ERK by MEK1/2 because this latter was weakly activated in the presence of AKT1, and its inhibition by U0126 prevented ERK phosphorylation (Fig. 3C). It is not known whether MEK is directly or indirectly activated by AKT. The second mechanism may involve a direct phosphorylation of ERK by AKT as shown here in vitro (Fig. 4). In intact cells, an interaction of AKT and ERK is suggested by the observation that fMLP increases the amount of AKT co-immunoprecipitated with ERK, and reciprocally (Fig. 5). Interestingly, the ability of the AKTib1/2 to prevent ERK recruitment suggests that the activated form of AKT is required for interaction with ERK. In contrast to ERK, p38MAPK does not appear to be regulated by AKT because the AKT antagonist AKTib1/2 has no impact on the activation of p38 MAP kinase induced by fMLP (Fig. 2, E and F). By contrast, AKT can be phosphorylated by the p38 MAPK substrate MAPKAPK-2 in neutrophils (31).
We showed previously that the transient PLD activity induced by fMLP in dHL-60 cells is mainly due to the PLD2 isoform. Moreover, PLD2 is phosphorylated by ERK2 in vitro and via ERK in fMLP-stimulated cells (4). The present study further indicates that AKT may activate the ERK/PLD2 signaling pathway. This is supported by the observation that AKTib1/2 prevented fMLP-mediated ERK activation (Fig. 2A) and PLD activity (Fig. 1A) and phosphorylation (Fig. 6) with the same efficiency (IC50 of 0.5–1 μm) and that AKT1 also stimulated PLD activity via ERK in cell-free systems (Fig. 3, A and B). However, we cannot exclude the possibility that AKT activates PLD2 directly. Indeed, AKT consensus phosphorylation sites are present in the PLD2 sequence (Thr175), and transfection experiments in COS cells indicate phosphorylation in PLD2-myc following AKT activation (32).
Stimulation of neutrophil RB is dependent on translocation of NOX2 components (p47phox, p67phox) at the plasma membrane which allows their interaction with the membrane-bound cytochome b558 (NOX2) to form an active complex able to reduce molecular oxygen (6). AKT has been proposed to stimulate neutrophil NADPH oxidase activity, based on a reconstituted cell-free system in which p47phox and p67phox were phosphorylated in vitro by AKT (26). A role of AKT in regulating RB was also suggested by the use of an AKT peptide antagonist and by co-immunoprecipitation studies of AKT with p47phox in vivo (18). However, a number of observations suggest that activated AKT by itself is not sufficient to activate NADPH oxidase of intact cells directly: (i) AKT phosphorylation is stimulated by neutrophil stimuli that were unable to stimulate RB such as GM-CSF (27) or SDF1α (20); (ii) in dHL-60 cells stimulated by PMA, AKT was strongly phosphorylated (Fig. 1) but does not appear to regulate RB (Fig. 6A) in agreement with other works (18); (iii) the two sites of p47phox phosphorylated by AKT (Ser304, Ser328) can also be phosphorylated by other kinases such as conventional PKC and PKCζ for Ser304 (33, 34) and by PKA for Ser328 (33). Taken together, these data suggest that AKT would primarily regulate NADPH oxidase assembly rather than directly stimulating the oxidase. This hypothesis is supported here by our observation that treatment of resting dHL-60 cells by AKTib1/2 primarily induced a reverse translocation of p47phox and p67phox and blocked further membrane translocation of these components induced by fMLP (Fig. 7C). AKT also regulated the fMLP-induced membrane translocation of ERK (Fig. 7) and its activation (Fig. 2). In a previous work, ERK was required to induce fMLP-mediated RB in dHL-60 cells (4). In vitro, ERK phosphorylates two MAPK consensus sites of p47phox (Ser345 and Ser348) which are distinct from PKC sites (33). The p47phox (Ser345) is also phosphorylated by MAPKs in neutrophils primed with GM-CSF, and TNFα and has been proposed to mediate the oxidase activation (19). ERK is also required for stimulation of PLD activity induced by chemoattactants (4). PLD generates large amounts of lipid second messengers and up-regulates RB (35). PA generated by PLD recruits p47phox at the plasma membrane (36) and mediates phosphorylation of p22phox, the small membrane-bound subunit of NOX2 (37). PA is then dephosphorylated in diglycerides which are potent activators of conventional and novel PKCs. However, in dHL-60 cells, fMLP-mediated RB was not sensitive to PKC inhibition by the antagonist GF109203X, although the PLD2 was strongly involved in RB (4). One possible interpretation for this regulation may be a predominant implication of PA-activated kinases. Among these, the atypical PKCζ is one potential candidate because it was found to be relatively insensitive to PKC inhibitors (38). PKCζ can be directly activated by PA (39). In addition, PKCζ phosphorylates p47phox and regulates fMLP-mediated RB of dHL-60 cells (40). Because the RB of dHL-60 cells is strongly regulated by ERK (4) and AKT (Figs. 7A and 9C), the novel signaling pathway AKT/ERK/PLD2 identified here may likely participate to different steps of NOX2 activation via PKCζ or other PA-activated protein kinases (41). The use of AKTib1/2 in this study revealed that blocking AKT activation did not alter the RB of dHL-60 cells in response to the direct activation of PKC by PMA (Fig. 7A). This observation was surprising because PMA strongly stimulated AKT (Fig. 1C) and PLD as well ERK (4). One possible interpretation of these data is that the PKCs that are directly activated by PMA may phosphorylate NOX2 components and promote NOX2 activation independently of AKT and ERK. Consistent with this, we found that the PMA-mediated RB of dHL-60 cells was also not inhibited in dHL-60 cells in which AKT1 or AKT2 was depleted with antisense oligonucleotides (data not shown). However, these results contrast with a recent report indicating that mouse bone marrow neutrophils lacking AKT2 exhibited impaired PMA-mediated RB compared with wild type (42). In this mouse model, AKT2 but not AKT1 was found to regulate fMLP-induced RB as well granule enzyme release, which also contrasts with our data. In this report, the mouse RB was studied using a chemiluminescence assay which is dependent on the release of granule peroxidase. It is thus not clear whether impaired RB is linked to defective enzyme release, alteration of NOX2 activity, or other biochemical differences between mouse and human neutrophils.
In conclusion, this study shows that AKT stimulation induced by fMLP is an important step in the activation of ERK1/2 and PLD activity and promotes NADPH oxidase activation parameters in dHL-60 cells. Moreover, the cell-free systems used indicate that AKT1 directly phosphorylates ERK promoting its activation state, as well PLD activity. Both AKT1 and AKT2 contribute partially to activation of ERK, PLD, and RB induced by fMLP. From these data and our previous studies, we propose that AKT, ERK, and PLD form a novel signaling pathway linking fPR stimulation to the assembly and activation of the NADPH oxidase. Considering the importance of these three major internal effectors in physiopathological processes, their sequential activation described here is of great interest not only for RB regulation and host defense mechanisms but for a variety of cellular responses mediated by chemoattractant receptors coupled to pertussis-sensitive G proteins.
Supplementary Material
Acknowledgments
We thank M. A. Frohman for PLD2 expression vector, M. Cobb for the GST-ERK expression vector, and A. M. Fraynal for technical assistance.
This work was supported by a grant from the Université Paris Descartes (S. P.) and l'Agence Nationale pour la Recherche.
- PLD
- phospholipase D
- AKTib
- AKT inhibitor 8
- fMLP
- formyl-methionyl-leucine-phenylalanine
- fPR
- formylpeptide receptor
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- HBSS
- Hanks' balanced saline solution
- PA
- phosphatidic acid
- PI3-kinase
- phosphoinositide 3′-kinase
- PMA
- phorbol 12-myristate 13-acetate
- RB
- respiratory burst
- MBP
- myelin basic protein.
REFERENCES
- 1.Foster D. A., Xu L. (2003) Mol. Cancer Res. 1, 789–800 [PubMed] [Google Scholar]
- 2.Exton J. H. (2002) Rev. Physiol. Biochem. Pharmacol. 144, 1–94 [DOI] [PubMed] [Google Scholar]
- 3.Liscovitch M., Czarny M., Fiucci G., Tang X. (2000) Biochem. J. 345, 401–415 [PMC free article] [PubMed] [Google Scholar]
- 4.Paruch S., El-Benna J., Djerdjouri B., Marullo S., Périanin A. (2006) FASEB J. 20, 142–144 [DOI] [PubMed] [Google Scholar]
- 5.Babior B. M. (1978) N. Engl. J. Med. 298, 659–668 [DOI] [PubMed] [Google Scholar]
- 6.Babior B. M. (2000) Am. J. Med. 109, 33–44 [DOI] [PubMed] [Google Scholar]
- 7.Djerdjouri B., Lenoir M., Giroud J. P., Périanin A. (1999) Biochem. Biophys. Res. Commun. 264, 371–375 [DOI] [PubMed] [Google Scholar]
- 8.Paruch S., Heinis M., Lemay J., Hoeffel G., Marañón C., Hosmalin A., Périanin A. (2007) FASEB J. 21, 4038–4046 [DOI] [PubMed] [Google Scholar]
- 9.Vivanco I., Sawyers C. L. (2002) Nat. Rev. Cancer 2, 489–501 [DOI] [PubMed] [Google Scholar]
- 10.Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. (1995) Cell 81, 727–736 [DOI] [PubMed] [Google Scholar]
- 11.Lemmon M. A. (2003) Traffic 4, 201–213 [DOI] [PubMed] [Google Scholar]
- 12.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 13.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 14.Reinhold S. L., Prescott S. M., Zimmerman G. A., McIntyre T. M. (1990) FASEB J. 4, 208–214 [DOI] [PubMed] [Google Scholar]
- 15.Naccache P. H., Hamelin B., Gaudry M., Bourgoin S. (1991) Cell. Signal. 3, 635–644 [DOI] [PubMed] [Google Scholar]
- 16.Bonser R. W., Thompson N. T., Randall R. W., Tateson J. E., Spacey G. D., Hodson H. F., Garland L. G. (1991) Br. J. Pharmacol. 103, 1237–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C., Dinauer M. C. (2001) J. Immunol. 166, 1223–1232 [DOI] [PubMed] [Google Scholar]
- 18.Chen Q., Powell D. W., Rane M. J., Singh S., Butt W., Klein J. B., McLeish K. R. (2003) J. Immunol. 170, 5302–5308 [DOI] [PubMed] [Google Scholar]
- 19.Dang P. M., Stensballe A., Boussetta T., Raad H., Dewas C., Kroviarski Y., Hayem G., Jensen O. N., Gougerot-Pocidalo M. A., El-Benna J. (2006) J. Clin. Invest. 116, 2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenoir M., Djerdjouri B., Périanin A. (2004) J. Immunol. 172, 7136–7143 [DOI] [PubMed] [Google Scholar]
- 21.Olson S. C., Bowman E. P., Lambeth J. D. (1991) J. Biol. Chem. 266, 17236–17242 [PubMed] [Google Scholar]
- 22.Barnett S. F., Defeo-Jones D., Fu S., Hancock P. J., Haskell K. M., Jones R. E., Kahana J. A., Kral A. M., Leander K., Lee L. L., Malinowski J., McAvoy E. M., Nahas D. D., Robinson R. G., Huber H. E. (2005) Biochem. J. 385, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang P. M., Hakim J., Périanin A. (1994) FEBS Lett. 349, 338–342 [DOI] [PubMed] [Google Scholar]
- 24.Rabiet M. J., Huet E., Boulay F. (2007) Biochimie 89, 1089–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann S., Moelling K. (1999) Science 286, 1741–1744 [DOI] [PubMed] [Google Scholar]
- 26.Hoyal C. R., Gutierrez A., Young B. M., Catz S. D., Lin J. H., Tsichlis P. N., Babior B. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5130–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein J. B., Rane M. J., Scherzer J. A., Coxon P. Y., Kettritz R., Mathiesen J. M., Buridi A., McLeish K. R. (2000) J. Immunol. 164, 4286–4291 [DOI] [PubMed] [Google Scholar]
- 28.Vlahos C. J., Matter W. F., Brown R. F., Traynor-Kaplan A. E., Heyworth P. G., Prossnitz E. R., Ye R. D., Marder P., Schelm J. A., Rothfuss K. J., Serlin B., Simpson P. J. (1995) J. Immunol. 154, 2413–2422 [PubMed] [Google Scholar]
- 29.Bokoch G. M. (1995) Blood 86, 1649–1660 [PubMed] [Google Scholar]
- 30.Sheppard F. R., Kelher M. R., Moore E. E., McLaughlin N. J., Banerjee A., Silliman C. C. (2005) J. Leukocyte Biol. 78, 1025–1042 [DOI] [PubMed] [Google Scholar]
- 31.Rane M. J., Pan Y., Singh S., Powell D. W., Wu R., Cummins T., Chen Q., McLeish K. R., Klein J. B. (2003) J. Biol. Chem. 278, 27828–27835 [DOI] [PubMed] [Google Scholar]
- 32.Di Fulvio M., Frondorf K., Gomez-Cambronero J. (2008) Cell. Signal. 20, 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Benna J., Faust R. P., Johnson J. L., Babior B. M. (1996) J. Biol. Chem. 271, 6374–6378 [DOI] [PubMed] [Google Scholar]
- 34.Dang P. M., Cross A. R., Babior B. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 3001–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauldry S. A., Bass D. A., Cousart S. L., McCall C. E. (1991) J. Biol. Chem. 266, 4173–4179 [PubMed] [Google Scholar]
- 36.Karathanassis D., Stahelin R. V., Bravo J., Perisic O., Pacold C. M., Cho W., Williams R. L. (2002) EMBO J. 21, 5057–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regier D. S., Greene D. G., Sergeant S., Jesaitis A. J., McPhail L. C. (2000) J. Biol. Chem. 275, 28406–28412 [DOI] [PubMed] [Google Scholar]
- 38.Kochs G., Hummel R., Meyer D., Hug H., Marmé D., Sarre T. F. (1993) Eur. J. Biochem. 216, 597–606 [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi H., Exton J. H. (1992) J. Biol. Chem. 267, 16347–16354 [PubMed] [Google Scholar]
- 40.Dang P. M., Fontayne A., Hakim J., El-Benna J., Périanin A. (2001) J. Immunol. 166, 1206–1213 [DOI] [PubMed] [Google Scholar]
- 41.Regier D. S., Waite K. A., Wallin R., McPhail L. C. (1999) J. Biol. Chem. 274, 36601–36608 [DOI] [PubMed] [Google Scholar]
- 42.Chen J., Tang H., Hay N., Xu J., Ye R. D. (2010) Blood 115, 4237–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.