Abstract
A lysine residue in the putative proton uptake pathway of the ATP synthase a-subunit is found only in alkaliphilic Bacillus species and is proposed to play roles in proton capture, retention and passage to the synthase rotor. Here, Lys-180 was replaced with alanine (Ala), glycine (Gly), cysteine (Cys), arginine (Arg), or histidine (His) in the chromosome of alkaliphilic Bacillus pseudofirmus OF4. All mutants exhibited octylglucoside-stimulated ATPase activity and β-subunit levels at least as high as wild-type. Purified mutant F1F0-ATP synthases all contained substantial a-subunit levels. The mutants exhibited diverse patterns of native (no octylglucoside) ATPase activity and a range of defects in malate growth and in vitro ATP synthesis at pH 10.5. ATP synthesis by the Ala, Gly, and His mutants was also impaired at pH 7.5 in the presence of a protonophoric uncoupler. Thus Lys-180 plays a role when the protonmotive force is reduced at near neutral, not just at high pH. The Arg mutant exhibited no ATP synthesis activity in the alkaliphile setting although activity was reported for a K180R mutant of a thermoalkaliphile synthase (McMillan, D. G., Keis, S., Dimroth, P., and Cook, G. M. (2007) J. Biol. Chem. 282, 17395–17404). The hypothesis that a-subunits from extreme alkaliphiles and the thermoalkaliphile represent distinct variants was supported by demonstration of the importance of additional alkaliphile-specific a-subunit residues, not found in the thermoalkaliphile, for malate growth of B. pseudofirmus OF4. Finally, a mutant B. pseudofirmus OF4 synthase with switched positions of Lys-180 (helix 4) and Gly-212 (helix 5) retained significant coupled synthase activity accompanied by proton leakiness.
Keywords: ATP Synthase, Bacterial Metabolism, Bioenergetics, Energy Metabolism, F1F0-ATPase, Bacillus, Oxidative Phosphorylation, Alkaliphile
Introduction
Proton-coupled F1F0-ATP synthases are centrally important for non-fermentative cells that energize ATP synthesis using the energy of an electrochemical proton gradient, the PMF,3 across the cytoplasmic or thylakoid membrane (bacteria) and across the mitochondrial or chloroplast thylakoid membrane (eukaryotes) (1–3). ATP synthases are composed of two domains, with bacterial synthases having simpler structures than eukaryotic homologues. The cytoplasmically located, soluble F1 domain encompasses three catalytic α- and β-subunit pairs and single γ-, δ-, and ϵ-subunits. The membrane-associated F0 domain is composed of a single a-subunit, two b-subunits, and multiple c-subunits (2, 4–6). ATP synthases function as rotary nano-machines, in which inward translocation of protons through the F0 domain leads to rotation of a membrane-embedded ring-like rotor (2, 3, 7–10). The rotor is formed from 10–15 hairpin-like c-subunits, depending upon the organism (11–16). Essential steps in coupling of ATP synthesis to the PMF include the protonation of successive c-subunits of the rotor and, after full rotation of a protonated c-subunit, de-protonation of that subunit through interactions of c-subunits of the rotor with the a-subunit stator component (4, 5, 7). No high resolution structural data are yet available for the a-subunit, but extensive biochemical and genetic evidence indicates that this ATP synthase subunit plays roles in providing the proton path from outside the membrane surface to the carboxylates of interacting c-subunits of the rotor (4, 17–24). An essential, conserved arginine in TMH4 (Arg-210 in Escherichia coli) is proposed to prevent proton short-cutting to the cytoplasm without rotation (25) and to cause a shift in the pKa of the essential carboxylate so that the proton that has completed rotation dissociates and enters the proton exit pathway leading to the cytoplasm. That proton exit pathway is also likely to be within the a-subunit (4, 5, 18, 23, 26–28).
Valuable insights into the mechanism of ATP synthase have been obtained from studies of bacterial synthases because of the ease of introducing and analyzing effects of mutations (8, 14, 15, 29–31). Our own studies have focused on the ATP synthase of alkaliphilic Bacillus species. The model extreme alkaliphile for bioenergetic studies is Bacillus pseudofirmus OF4, which is genetically accessible and grows non-fermentatively on malate, using proton-coupled oxidative phosphorylation, at external pH values from 7.5 to >11 (30, 32–34). B. pseudofirmus OF4 and other alkaliphilic Bacillus species share sequence motifs in the a- and c-subunits of their proton-coupled ATP synthases (30, 33, 35). Initial mutagenesis work showed that several of these sequence deviations from the neutralophilic Bacillus consensus have indispensible roles in the synthetic function of the enzyme and non-fermentative growth on malate at high pH, but are not required for hydrolytic ATPase activity or non-fermentative growth of B. pseudofirmus OF4 near neutral pH (36, 37). Recent structural studies on the B. pseudofirmus OF4 tri-decameric c-rotor have begun to provide insights into how the major motifs of the alkaliphile c-subunits impact the structure and support the function of the rotor at high pH (31).
The current study focuses attention on alkaliphile-specific residues in the a-subunit. The impetus for the study was a set of conflicting observations on a particular “alkaliphile-specific” residue, Lys-180, that is found both in extremely alkaliphilic Bacillus species, e.g. B. pseudofirmus OF4, Bacillus halodurans C-125, and Bacillus alcalophilus, and in more temperate alkaliphiles such as Bacillus clausii and thermoalkaliphilic Caldalkalibacillus thermarum TA2.A1 (formerly called Bacillus sp. TA2.A1). This residue is located in the TMH4 in the putative proton uptake pathway of the a-subunit. TMH4 also contains the conserved, functionally critical arginine (Arg-172 in B. pseudofirmus OF4) in the proton pathway through the a-subunit (17, 18, 22). The unusual Lys-180 substitutes for a glycine residue typically found at this position in non-alkaliphiles (Fig. 1A). Mutagenesis studies of Lys-180 of B. pseudofirmus OF4 showed that its mutation to the consensus glycine of non-alkaliphiles resulted in a major deficit in ATP synthesis at pH 10.5, while significant capacity for ATP synthesis was retained at near neutral pH (37). We hypothesized that, at high pH, Lys-180 is a participant of the proton uptake path that is required for capture of entering protons and passage of the protons to the interface of the c-ring with the a-subunit. Subsequently, McMillan et al. (38) expressed the full hexahistidine-tagged atp operon from C. thermarum TA2.A1 in an E. coli mutant from which the native atp operon was deleted. They found that this thermoalkaliphile enzyme failed to support non-fermentative growth of E. coli or to carry out in vitro ATP synthesis below pH 8.0. Robust support of ATP synthesis at pH 7.0–7.5 was conferred upon the enzyme when histidine or glycine replaced the lysine, whereas replacement of Lys-180 with arginine resulted in robust synthase activity only at pH ≥8.5. They proposed that a synthase with Lys-180 might be unable to synthesize ATP at near neutral or neutral pH (38). The subsequent finding that C. thermarum TA2.A1 could not grow non-fermentatively below pH 8.0 was consistent with that proposal (39). However, the proposal was at odds with our observation of robust non-fermentative growth and ATP synthesis at pH 7.5 exhibited by wild-type B. pseudofirmus OF4, which utilizes a Lys-180-containing synthase over its broad pH range (37). Alignments of the a-subunits from diverse mesophilic Bacillus species and thermoalkaliphilic C. thermarum TA2.A1 revealed that there were many instances in which the thermoalkaliphile sequence departed from the consensus sequence for extremely alkaliphilic or from all mesophilic Bacillus strains, whether alkaliphilic or neutralophilic. Moreover, those residues were in functionally important regions for proton translocation, e.g. see position 184 and 205 in the TMH4–5 region (Fig. 1A). We hypothesized that the a-subunits from thermoalkaliphile and extreme alkaliphiles, which are not also thermophiles, are distinct variants. Presumably, the thermoalkaliphile variant reflects adaptations to the dual challenges of elevated temperature and pH, whereas the extreme alkaliphile variant reflects adaptations that underpin function in a more highly alkaline range. If so, it was likely that, if the Lys-180 of B. pseudofirmus OF4 was replaced with the same residues that had been introduced into the thermoalkaliphile synthase, their effects would be very different because of the distinct scaffold into which they were introduced. Here, we studied mutants with changes in the Lys-180 of B. pseudofirmus OF4 that were made in the native atp locus of the alkaliphile chromosome to either alanine, glycine, cysteine, arginine, or histidine, a somewhat larger panel from those made in the C. thermarum TA2.A1 synthase (38). In addition, we constructed a double mutant in which the position of Lys-180 in TMH4 was switched with that of the alkaliphile-specific Gly-212 of TMH5 with which Lys-180 apparently interacts (37, 40). The double mutant provided an opportunity to examine whether synthetic capacity and the proposed role for this pair of residues in preventing proton leakage (37) are dependent upon the native positioning of Lys-180 and Gly-212. Finally, the observations made in this study on the single mutants in B. pseudofirmus OF4 Lys-180 led us to examine an expanded panel of mutations in other residues to directly test the idea that the extreme alkaliphile and thermoalkaliphile a-subunits are distinct variants with multiple adaptations that are reflected in their sequences.
FIGURE 1.
Alignment of the a-subunit putative transmembrane helices 4 and 5 and a diagrammatic representation of all the mutants in the study. A, putative TMH4 and TMH5 are shown for the following organisms, with their accession numbers in parentheses. Alkaliphiles are shaded. They are the extreme alkaliphiles B. pseudofirmus OF4 (AAG48358), B. halodurans C-125 (NP_244625.1) and B. alcalophilus (P25965), the more moderately alkaliphilic B. clausii KSM-K16 (Q5WB72), B. selenitireducens MLS10 (CP001791), and thermoalkaliphile C. thermarum TA2.A1 (AAQ10084). The non-alkaliphilic Bacillus species are B. licheniformis (YP_093439), B. megaterium (AAA82520), B. subtilis 168 (NP_391568), and G. kaustophilus HTA42 (YP_149217); also included is the model Gram-negative E. coli K12 (YP_001732559). Residue numbers above the alignment are those for the B. pseudofirmus OF4 protein; the numbers in parentheses below the alignment refer to the E. coli protein. Gray shading highlights the Lys-180 and putatively interacting Gly-212 that are both found exclusively in alkaliphiles, although not in B. clausii or B. selenitireducens MLS10. Two additional deviations from the consensus sequence for non-alkaliphilic Bacillus species that are found in all of the alkaliphiles except for thermoalkaliphile C. thermarum TA2.A1 are boxed for alkaliphiles (Met-171 and Met-184) and underlined for the thermoalkaliphile. Some residues only found in most alkaliphiles but not all alkaliphiles are also boxed (Val-177, Ile-185, and Leu-205). B, a topological representation of the putative five-transmembrane helix structure of the B. pseudofirmus OF4 a-subunit with the location of the residues in TMH4 and -5 that were mutated as well as selected alkaliphile-specific residues of interest in other regions that are not conserved in the a-subunit from thermoalkaliphile C. thermarum; the replacements made in each location are indicated. Residues mutated to the residue found in C. thermarum at that position have a white background while other mutations have a black background.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Genetic Manipulations, and Growth Conditions
The E. coli strains used in this study were DH5αTM-T1R (Invitrogen) for cloning genes into the low copy vector pMW118 (Nippon Gene, Tokyo, Japan) and XL-1 blue MRF′ (Promega, Madison, WI) for cloning genes into pG+host4 (Appligene, Pleasanton, CA). Antibiotics were used at 100 μg/ml for ampicillin or 300 μg/ml for erythromycin. Alkaliphilic B. pseudofirmus OF4 strain F010, which has an additional EcoRI marker in the atpB gene of a methionine auxotroph strain 811M (37), was used for all experiments as a wild-type in this study. Mutants constructed in this study were K180A, K180C, K180G, K180H, K180R, K180G/G212K, E98S, M171L, V177I, M184L, I185G, I185S, and L205M in the a-subunit of the F1F0-ATP synthase encoded by atpB gene in the operon. Although the K180G mutant had been made before (37), it was newly constructed, because the earlier mutant was subsequently found to contain a silent mutation (changing the codon for Ala-179 from GCG to GCC). The mutants were made by recombination of the ΔF0, which carries an atpB-F deletion, with temperature-sensitive plasmid pG+host4 possessing the mutated F0 region as described previously (37). Details about the primers used are available upon request. For the K180G/G212K double mutant, the G212K mutation was first introduced in pMW118-F0. After confirming the sequence of the plasmid, the Lys-180 codon was also mutated to a glycine codon by the same method. The mutated F0 regions were then transferred into pG+host4 using the BamHI and KpnI sites. Transformation was conducted as described previously (41) except that 0.6 μg/ml erythromycin was used for the selection. The sequences of the F0 region of the plasmids and the entire atp operon of the double crossover mutants were verified by sequence analyses performed by the DNA Sequencing Core Facility at Mount Sinai School of Medicine, or by GENEWIZ (South Plainfield, NJ). Growth properties of the double crossover mutants were initially characterized for several colonies, which were from independent transformants of mutated pG+host4-F0 plasmids, to be sure there was a consistent phenotype. The five single mutations of Lys-180 were also introduced into a strain that, in addition to having the ΔF0 background, contained a six-codon addition encoding 6-His just after the N-terminal methionine of the β-subunit. These constructs facilitated purification of the ATP synthases from this mutant panel. All phenotypic measurements were done in the wild-type β-subunit background. Growth experiments were carried out as previously described in at least two independent experiments, each of which had duplicate cultures containing 2 ml of a semi-defined glucose or malate media that also contained 0.1% yeast extract (41). The pre-cultures were grown in glucose-containing medium at pH 7.5, and the cultures for the growth experiments were conducted at pH 7.5 and 10.5 and included controls with 0.1% yeast extract as the sole carbon source. Growth was measured as the A600 after shaking for 14 h at 30 °C, and those values were corrected as follows. The net growth on glucose was calculated by subtracting the growth on yeast extract alone. The net growth on malate was calculated by subtracting the small amount of growth observed with the ΔF0 mutant on malate.
Isolation of Everted Membrane Vesicles
The procedures for growth and isolation of everted membrane vesicles were modified versions of those used in earlier studies (36, 37). Overnight cultures were used to inoculate flasks containing 1liter of pre-warmed (37 °C) pH 10.5-buffered glucose-containing media that were then subjected to vigorous shaking in baffled flasks for 4–6 h at 37 °C until mid-to-late log phase. Harvested cultures were washed with 50 mm Tris-HCl, pH 8.0, 5 mm MgCl2 and stored as pellets at −20 °C. Two liters of a strain was generally used to generate an everted vesicle preparation. The cells were suspended in 10% glycerol, 50 mm Tris-HCl, pH 8.0, 5 mm MgCl2, 0.5 protease inhibitor tablets (Roche Applied Science), 1 mm freshly prepared phenylmethylsulfonyl fluoride, and 0.5 mg of DNase and passed once through a pre-cooled French Pressure cell at 16–18,000 p.s.i. Unbroken cells and debris were removed first by a low speed spin at 12,000 × g for 15 min at 4 °C, followed by a short ultracentrifugation at 4 °C, in which the run was terminated once the speed reached 160,000 × g (∼8 min). The supernatant was removed, and the everted vesicles were pelleted by centrifugation at 250,000 × g for 90 min at 4 °C. After careful removal of the supernatant, the vesicles were suspended in 10% glycerol, 10 mm Tris-HCl, pH 8.0, and 5 mm MgCl2 to a final concentration of 20.0 mg/ml and dispensed in aliquots, frozen in liquid N2, and stored at −80 °C. The vesicle protein concentration was measured by the Lowry method (42), using bovine serum albumin as a standard.
ATPase Assays and DCCD Inhibition
Octylglucoside (OG)-stimulated ATPase activities were determined by measuring the inorganic phosphate released in a 3-min assay at 37 °C, using 20–50 μg of membrane protein in 0.5 ml of assay mixture (36, 37). Assays were conducted in duplicate and on independent everted vesicle preparations. The native ATPase activities (i.e. unstimulated, no OG) of enzymes from wild-type and mutant strains and their inhibition by DCCD were determined as follows. Vesicles were diluted to 2 mg/ml in 50 mm MOPS-NaOH, pH 7.0, 5 mm MgCl2 in a final volume of 800 μl, and 300 μl of the suspension was put into each of two tubes. To one tube (−DCCD), 3 μl of methanol was added and to the other tube 3 μl of 10 mm DCCD (in methanol) was added and the incubation continued for 30 min at room temperature. At the end of the incubation, aliquots of 20 μl were pipetted into 0.48-ml reactions, pre-warmed to 37 °C, which contained 50 mm Tricine-NaOH, pH 8.0, 5 mm MgCl2, and 5 mm Na2ATP. Reactions were terminated at 10 min by injecting 200 μl of the reaction into 800 μl of malachite green reagent at room temperature (43–45). Color formation was stopped at 75 s by addition of 100 μl of 34% sodium citrate. These reactions were read at 620 nm in comparison to a Pi standard curve run in parallel to the sample reactions. The more sensitive malachite green Pi assay was required, because the amount of Pi formed in these reactions was ∼3% of that obtained in the OG ATPase assay. pH curves of the native hydrolytic activity (i.e. without OG) were determined using 40 μg of membrane protein in assays of 0.5-ml reaction mixtures at 37 °C containing 50 mm Bis-Tris propane (BTP) at the desired pH, 5 mm MgCl2, and 5 mm Na2ATP. The reactions were carried out for 10 min and terminated as described above.
SDS-PAGE and Immunoblot Analyses
Tris-Tricine gels containing 11% acrylamide (46) were run in the Criterion gel apparatus (Bio-Rad), with a size of ∼7 × 13 cm. This size gel was required to resolve the δ- and a-subunits, as well as optimizing the separation between the α/β-subunits and the c-ring. Samples were dissolved in a pH 7.0 sample buffer with final concentrations of 12% glycerol, 50 mm Tris, 50 mm dithiothreitol, 2% SDS, 0.01% bromphenol blue, for ∼30 min at room temperature prior to electrophoresis. Piperazine diacrylamide (Bio-Rad) was substituted for Bis as the cross-linker. Gels were silver-stained according to Nesterenko et al. (47). An acidified chloroform/methanol extraction of a- and c-subunits from the purified ATP synthase was carried out according to Vorberger et al. (48).
For immunoblots, samples were denatured at 65 °C for 10 min and 0.1 μg to 3 μg of everted membrane vesicles were run on Tris-Tricine 10% SDS-PAGE minigels (46). The polypeptides were then transferred electrophoretically to a 0.2-μm pore size nitrocellulose membrane (Bio-Rad). The blots were probed with a monoclonal antibody against the E. coli β-subunit (Molecular Probes) as a primary antibody and goat or rabbit anti-mouse peroxidase-conjugate (Sigma or Bio-Rad) as a secondary antibody to detect the β-subunit of the F1F0-ATP synthase from B. pseudofirmus OF4 strains. The blots were developed using ECL Western blotting Substrate (Pierce). Pictures were scanned, and the images were analyzed using ImageJ 1.40v software (National Institutes of Health). The bands were quantified and compared with the signal from wild-type by using blots with sub-saturating signal levels.
Assays of ATP Synthesis in ADP + Pi-loaded Membrane Vesicles
Measurements of ATP synthesis in right-side-out (RSO) membrane vesicles were performed as in earlier studies (37). The ATP synthesized was measured by luminometry (49). Background ATP levels without energization were subtracted from the values obtained with energization to yield the ATP synthesized during the reaction. As described before (36), this protocol used an intravesicular pH of 8.3 that corresponded to a typical cytoplasmic pH of alkaliphile cells growing at pH 10.5. Thus vesicles shifted to pH 7.5 for assay had an imposed pH gradient in the chemiosmotically favorable direction, and those shifted to pH 10.5 had an imposed pH gradient in the chemiosmotically unfavorable direction. The average value for wild-type ATP synthesis in these assays was 4.7 ± 1.1 and 2.3 ± 0.6 nmol/mg of vesicle protein/10 s at pH 7.5 and at 10.5, respectively. In experiments assessing the CCCP sensitivity of ATP synthesis by wild-type versus the K180H, K180G, and K180A mutants, ADP + Pi-loaded RSO membrane vesicles prepared from each of the strains were preincubated in the reaction buffer with a range of concentrations of the uncoupler, dissolved in 95% ethanol, for 1 min. Synthesis was then initiated by addition of ascorbate-phenazine methosulfate. All reactions contained 0.1% of the appropriate stock CCCP concentration or 0.1% ethanol for controls lacking CCCP.
Measurement of Intracellular pH after pH Shift from 8.5 to 10.5
Intracellular pH was calculated from the transmembrane pH gradient 10 min after a pH up-shift from 8.5 to 10.5 as described in detail elsewhere (36). The wild-type and mutant cells were grown at pH 8.5 on semi-defined glucose medium for assays of the K180R and at pH 8.5 on semi-defined malate medium for assays of the double K180G/G212K mutant.
Purification of the F1F0-ATP Synthase from Wild-type and Mutant Strains
Wild-type and mutant strains harboring a 6-His tag in the β-subunit were grown as described above for everted vesicle preparation except that the pH 10.5-buffered glucose medium also contained 10% Luria broth (1 g of Tryptone, 0.5 g of yeast extract, and 1 g of NaCl per liter). Everted vesicles were prepared similarly, except that that the cells were subjected to two passages through the French Pressure cell at 20,000 p.s.i. The resulting vesicles were suspended in 20% glycerol, 50 mm Tris-HCl, pH 8.0, 5 mm MgCl2, 0.2 mm dithiothreitol, and 0.2 mm phenylmethylsulfonyl fluoride. All of the following steps were conducted at 0–4 °C. While the suspended vesicles were gently stirred, dodecyl maltoside (Anatrace, Maumee, OH) and soybean polar lipid (Avanti, Alabaster, AL) were added from stock solutions in a dropwise manner to final concentrations of 1% and 2 mg/ml, respectively, and allowed to stir for 1 h. The final concentration of vesicles in this suspension was set at 10 mg protein/ml. Ultracentrifugation at 250,000 × g for 100 min separated the dodecyl maltoside extract (supernatant), which was removed by pipette, from a small pellet of unextracted material. NaCl and imidazole were added to the extract from stock solutions to final concentrations of 0.1 m and 10 mm, respectively. The extract was loaded onto a Ni-NTA column (Qiagen) that was pre-equilibrated in Ni-NTA buffer containing 10% glycerol, 50 mm Tris-HCl, pH 8.0, 5 mm MgCl2, 0.1 m NaCl, 10 mm imidazole, 0.2 mm dithiothreitol, and 0.05% dodecyl maltoside. The column was then washed with 10 column volumes of Ni-NTA buffer, 2 column volumes of Ni-NTA buffer with 20 mm imidazole, and the ATP synthase was eluted with Ni-NTA buffer containing 150 mm imidazole. Fractions were assayed for OG ATPase activity, and peak fractions were pooled, dispensed in aliquots, frozen in liquid N2, and stored at −80 °C.
RESULTS
The Levels of Membrane ATP Synthase Protein and ATPase Activities, and the Subunit Composition of ATP Synthase Preparations of Wild-type and Mutant Strains
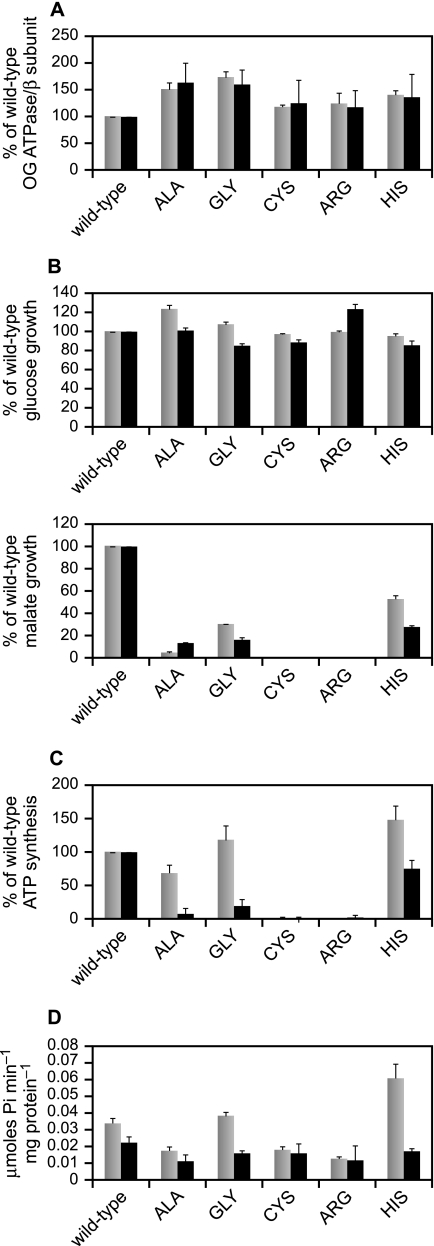
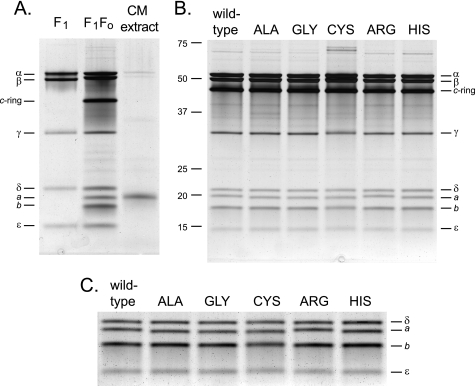
Assessment of the total hydrolytic ATPase activity of the alkaliphile synthase, which is quite latent (30), requires addition of effectors such as OG to the reaction mix. The OG ATPase activities of everted vesicles from the single Lys-180 mutants are shown in comparison with that of wild-type membranes in Fig. 2A, where we also show the quantitation of the β-subunit content of the vesicles by immunoblot. These results indicate that the mutations had between 117 and 173% of the wild-type amount of enzyme or OG-stimulated ATPase activity. That is, mutation of Lys-180 did not preclude normal or supranormal expression of the ATP synthase. Because there was a correlation between the OG ATPase and the β-subunit content (relative to the wild-type), it appeared that the specific catalytic ATPase activity of the mutant forms was similar to the wild-type. This made it unlikely that there was a major deficiency in a-subunit content but to directly check this, the subunit compositions of affinity-purified His-tagged mutant enzymes (see “Experimental Procedures”) were directly compared with the wild-type enzyme (50). Except for the K180C mutant preparation, the yields and specific activities of the preparations were similar. From 4 liters of culture, 1.4–2.4 mg of ATP synthase protein was isolated (1.8 mg of wild-type), with specific activities ranging from 30.3 to 35.2 units/mg (wild-type was 33.6). The K180C ATP synthase was notable for its low specific activity, 10.7 units/mg, and lowest yield of all the strains at 1.2 mg. As shown in Fig. 3A, the wild-type ATP synthase on SDS-PAGE is resolved into eight bands. By co-migration of the bands of purified F1 with the bands of purified F1F0, the α, β, γ, δ, and ϵ bands were identified (Fig. 3A, left and middle lanes). The c-subunit migrates as an intact c-ring (31). To determine which of the bands corresponded to the a-subunit, we used the acidified chloroform/methanol extraction method, which has been shown to extract both a- and c-subunits (48). The chloroform/methanol extract in the right lane in Fig. 3A shows that the a-subunit is the band migrating just below δ. The band identified in the Fig. as the b-subunit was verified as such by mass spectrometry (data not shown). The gels in Fig. 3 were run for an extended period to optimally separate the δ- and a-subunits and as a consequence, any c-monomer present was run off the gel. In gels not shown, there were only minor amounts of monomer in the ATP synthase preparations but a large amount of c-monomer in the chloroform/methanol extract. The purified ATP synthase from each of the mutant strains showed all eight bands, as shown in Fig. 3B. The K180C synthase exhibited two closely spaced large molecular weight proteins migrating at ∼67 kDa that were absent from other preparations and were not identified (Fig. 3B). To enhance visualization of the a-subunits from the different samples, the section of the gel in Fig. 3B containing the small subunits was cropped out and subjected to auto levels in Adobe Photoshop in Fig. 3C. All the mutant strains exhibited the presence of a significant level of a-subunit, including K180C, with some small differences in the migration of the a-subunits noted for the different strains.
FIGURE 2.
Functional characterization of the single Lys-180 mutants in comparison with wild-type. A, OG-stimulated ATPase activity and β-subunit content of everted vesicles isolated from each of the mutants. The mutants were grown in the semi-defined medium supplemented with 50 mm glucose at pH 10.5. The values for the mutants are given as percentages of wild-type with the wild-type set at 100%. Gray bars, OG ATPase; black bars, β-subunit content. B, growth of wild-type and mutant strains as a function of pH and carbon source. Top, growth on glucose; bottom, growth on malate. Gray bars, pH 7.5; black bars, pH 10.5. C, ATP synthesis by ADP + Pi-loaded RSO membrane vesicles at pH 7.5 and 10.5. The vesicles were prepared from cells grown in the semi-defined medium supplemented with 50 mm glucose at pH 10.5. Gray bars, pH 7.5; black bars, pH 10.5. D, the native ATPase activity of everted vesicles was determined with and without DCCD (100 μm) as detailed under “Experimental Procedures.” Gray bars, no DCCD; black bars, plus DCCD. The results in A and B are the mean values of duplicate assays conducted in two independent experiments, in C from duplicate samples in 2–6 independent vesicle preparations, and in D from at least 3 independent everted vesicle preparations. The values shown are the mean values with ±S.D. as error bars.
FIGURE 3.
SDS-PAGE analysis of purified ATP synthase preparations from the wild-type and single Lys-180 mutant strains. A, the identity of the a-subunit band was determined on a wild-type ATP synthase sample by an acidified chloroform/methanol extraction, which has been shown to extract both a- and c-subunits (48). The extract is shown in the right lane; for comparison, the purified B. pseudofirmus OF4 F1 is shown on the left, and the purified F1F0 is in the middle lane. B, patterns for each of the mutant enzymes side-by-side with the wild-type preparation. The ATP synthases were purified as detailed under “Experimental Procedures.” The samples (2 μg) were resolved on 11% gels and silver stained. C, to facilitate comparison of the a-subunit bands, the region of the small subunits from the gel in B was cropped out, subjected to auto levels in Adobe Photoshop to intensify the bands, and enlarged. The bromphenol blue front was run off the gels in A and B to optimize resolution of the δ- and a-subunits; as a consequence, trace amounts of c-monomer in samples in B and the substantial amount of c-monomer in the chloroform/methanol extract in A were not visualized. The F1 preparation in A is not His-tagged, and therefore its β-subunit migrated more rapidly than its counterpart in the F1F0 preparation.
Different Patterns of Adverse Functional Effects and of Native ATPase Activity Patterns Result from Different Single Amino Acid Substitutions of Lys-180 of the a-Subunit of the B. pseudofirmus OF4 F1F0-ATP Synthase
Growth of all the single Lys-180 mutants on glucose was comparable to that of the wild-type at both pH 7.5 and 10.5 (Fig. 2B, top), so none of the mutant enzymes disrupted cell functions affecting growth under fermentative conditions. By contrast, significant deficits were observed for growth of all the single Lys-180 mutants under non-fermentative conditions, on malate, relative to the wild-type alkaliphile (Fig. 2B, bottom). Under a slightly different protocol than that used in the earlier study (37), both the original and newly constructed K180G mutants showed a greater deficit than observed earlier for growth at pH 7.5, with growth at 30% of wild-type (shown for the new construct in Fig. 2B). The growth at pH 10.5 was even worse (16% of wild-type), as observed before (37). Among the new mutations in this study, the K180H mutant supported the best malate growth, although its growth was still highly defective relative to that of wild-type, 53 and 28% of wild-type growth at pH 7.5 and pH 10.5, respectively. Of the remaining three mutants, K180A, K180C, and K180R, only the K180A mutant exhibited any growth on malate. Malate growth of K180A was even poorer than that of the K180G mutant at both pH 7.5 and 10.5, so that the higher growth at pH 10.5 than 7.5 is unlikely to be significant (Fig. 2B, bottom).
Assays of ADP + Pi-loaded vesicles from the K180G and K180A mutants showed that both mutants lacked significant ATP synthesis capacity at pH 10.5 (Fig. 2C). This was consistent with their growth phenotypes on malate (Fig. 2B) and with the hypothesis that the positive charge of Lys-180 is essential for ATP synthesis at highly alkaline pH (37, 38). By contrast, the K180G and the K180H mutants, and to a lesser extent the K180A mutant, exhibited much more ATP synthesis in the vesicle assay at pH 7.5 than expected from their growth phenotype on malate at pH 7.5 (Fig. 2, compare C with B, bottom). Synthesis by the glycine and histidine replacement mutants was comparable to that of wild-type at pH 7.5, not lower. ATP synthesis by the K180H mutant at pH 10.5 was also higher (75% of wild-type synthesis) (Fig. 2C) than that expected from the relative difference in the malate growth of the two strains at pH 10.5 (Fig. 2B, bottom).
Native ATPase Activity and Inhibition by DCCD
The native ATPase activity is the activity measured in the absence of stimulating effectors; it is the proton-coupled and DCCD-sensitive hydrolytic activity that is ∼30-fold lower than the OG-stimulated activity in the wild-type B. pseudofirmus OF4 enzyme (51). The native ATPase activity of the Ala, Cys, and Arg mutants was lower than that of wild type, and these mutant synthases were inhibited by DCCD to a much lower extent than the wild-type enzyme (Fig. 2D). Both the K180G and K180H mutants, especially the latter, exhibited elevated native ATPase activity relative to the wild-type enzyme, and both of these mutant enzymes were markedly more sensitive to DCCD than the wild-type enzyme, 59 and 71% inhibition for K180G and K180H, respectively, as compared with 33% inhibition of the wild-type enzyme by DCCD.
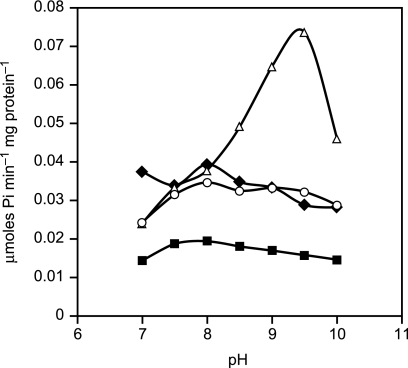
Cain and Simoni (52) reported a shift in the pH dependence of ATP hydrolysis by a mutant in the E. coli a-subunit, E219L, that led to greater activity at elevated pH than observed in the wild-type E. coli enzyme. We conducted assays to ascertain whether the native ATPase activity of the wild-type or mutant enzymes that retained a basic amino acid at position 180 or at position 212 exhibited marked pH dependence (Fig. 4). Using BTP as the buffer for these reactions (instead of the usual Tricine buffer), we found that the native hydrolytic activity of the wild-type enzyme was relatively insensitive to pH over the pH range from 7 to 10, with a modest peak at pH 8.0. The K180R mutant enzyme behaved like the wild-type enzyme in the flatness of response to pH. In contrast, the K180H mutant enzyme showed a sharp pH optimum at pH 9.5 that was dramatically higher than the other strains tested.
FIGURE 4.
pH dependence of the native ATPase activity in everted vesicles of the wild-type, K180R, K180H, and K180G/G212K mutant strains. The ATPase activity was determined as detailed under “Experimental Procedures” in a BTP buffer at the indicated pH values. The average values were obtained from 3–6 independent everted vesicle preparations. For clarity, the standard deviations were left out. The standard deviations were less than 27% of the mean. Use of BTP buffer, instead of the standard Tricine buffer used in the pH 8.0 assays in Fig. 2D, changed the ratio of aK180H to wild-type activity at pH 8.0 from about 1.8 to 1.0. Diamonds, wild type; circles, double mutant aK180G/aG212K; squares, aK180R; and triangles, aK180H.
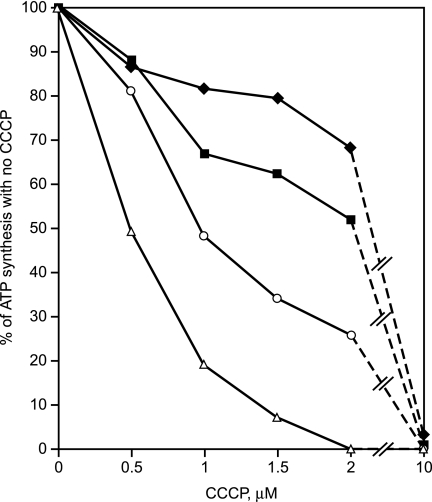
ATP Synthesis by the K180H, K180G, and K180A Mutants Is More Sensitive Than the Wild-Type to Low Concentrations of the Protonophore CCCP
The mutant enzymes that functioned well in ATP synthase assays at pH 7.5 but were suboptimal at pH 10.5 apparently required Lys-180 to function as robustly as the wild type at pH 10.5, at which the bulk PMF is low (30). If so, reduction of the PMF by CCCP treatment at pH 7.5 might also reduce ATP synthesis by the mutants more than by the wild-type in the vesicle assays, especially mutants K180G and K180A. Indeed, the sensitivities of ATP synthesis to inhibition by CCCP was wild-type < K180H < K180G < K180A, as shown in Fig. 5. In Fig. 5, synthesis data are presented as percentages of each strain's own capacity to synthesize ATP in the absence of CCCP.
FIGURE 5.
CCCP sensitivity of ATP synthesis by ADP + Pi-loaded RSO vesicles from the wild-type, K180H, K180G, and K180A strains. For each of the four strains, the amount of synthesis during the 10-s reaction in the absence of added CCCP is taken as 100% for that strain. Subsequent points showing synthesis at increasing [CCCP] for each strain are the percentage of their individual no-CCCP values. The experiments were conducted at pH 7.5 as described under “Experimental Procedures.” The vesicles were prepared from cells grown in the semi-defined medium supplemented with 50 mm glucose at pH 10.5. The results shown are the mean of triplicate determinations from five or six independent vesicle preparations of each strain. For clarity, the error bars are not shown. The ±S.D. for these determinations were below 25% of the mean, except for the 1 μm CCCP point for K180H, which was 50%, and the 1.5 and 2 μm CCCP points for K180G, which were 42 and 35%, respectively. Diamonds, wild-type; squares, K180H; circles, K180G; triangles, K180A.
Exchange of the Lysine and Glycine Residues at Positions 180 and 212
In the E. coli ATP synthase, mutation of Gly-218, which is in the position corresponding to alkaliphile Lys-180 of TMH4 (Fig. 1), to either aspartate (found in chloroplasts) or lysine (as found in alkaliphiles) resulted in poor or no non-fermentative growth. A second site suppressor mutation in E. coli His-245, in the equivalent position of alkaliphile Gly-212 of TMH5, restored non-fermentative growth (40). Both of these residues are predicted to be part of the proton uptake path of a-subunit (17, 18, 22). Here we switched Lys-180 with Gly-212 in B. pseudofirmus OF4 to produce the double mutant K180G/G212K. The switched mutant had levels of the ATP synthase protein and OG-stimulated ATPase activity that were ∼80% of the wild-type levels (Fig. 6A). Its native ATPase activity was slightly elevated (112%) and more sensitive to DCCD than the wild-type (52% versus 33%) (Fig. 6D), but had the same pH profile as the wild-type (Fig. 4). The mutant exhibited compromised growth relative to the wild-type at both pH 7.5 and 10.5, especially at pH 10.5, and the deficit was observed on both malate and glucose (Fig. 6B). The double mutant exhibited almost wild-type levels of ATP synthesis at pH 7.5. At pH 10.5, the double mutant exhibited a somewhat variable deficit in ATP synthesis that was similar to the magnitude of the growth defects at pH 10.5 (Fig. 6C). However, it was clear that the enzyme retained significant synthetic function at both pH 7.5 and 10.5 when the Lys-180 was relocated in exchange for Gly.
FIGURE 6.
Functional characterization of the K180G/G212K double mutant in comparison with wild-type. A, β-subunit content and OG-stimulated ATPase activity of everted vesicles isolated from the wild-type and mutant strains. The strains were grown in the semi-defined medium supplemented with 50 mm glucose at pH 10.5. The values for the mutants are given as % of wild-type with the wild-type set at 100%. Gray bars, OG ATPase; black bars, β-subunit content. B, growth of wild-type and double mutant strains as a function of pH and carbon source. Top graph, glucose; bottom graph, malate; gray bars, pH 7.5; black bars, pH 10.5. C, ATP synthesis by ADP + Pi-loaded RSO membrane vesicles at pH 7.5 and 10.5. The vesicles were prepared from cells grown in the semi-defined medium supplemented with 50 mm glucose at pH 10.5. Gray bars, pH 7.5; black bars, pH 10.5. D, the native ATPase activity with and without 100 μm DCCD was assayed as described under “Experimental Procedures.” Gray bars, no DCCD; black bars, plus DCCD. E, determination of the cytoplasmic pH of cells grown on 50 mm malate at pH 8.5 and subsequently subjected to a shift in the external pH from 8.5 to 10.5. See “Experimental Procedures” for details. The results shown in all panels are the mean results from duplicate determinations from 2–4 independent experiments for panels A–C and 6 independent experiments for panel D. The error bars show ±S.D.
The double mutant exhibited a growth deficit on glucose that was most pronounced at pH 10.5 but was also evident at pH 7.5 (Fig. 6B). Such a deficit is rarely seen in F0 mutants of B. pseudofirmus OF4 (36, 37). It suggested that the double mutant might have a substantial proton leak. This was tested by an alkaline shift assay, measuring the cytoplasmic pH of cells that were grown on malate at pH 8.5, after a shift in external pH from 8.5 to 10.5. As shown in Fig. 6E, the malate-grown wild-type cells had a cytoplasmic pH of 8.4 after the shift, similar to previous measurements (36), whereas the cytoplasmic pH of the double mutant was 0.6 units higher, pH 9.0.
Mutations in Other Alkaliphile-specific a-subunit Residues in B. pseudofirmus OF4 That Are Absent from C. thermarum TA2.A1 Reduce Malate Growth at High pH
The complete inability of the B. pseudofirmus OF4 a-subunit mutant with the K180R substitution to support ATP synthesis or malate growth was in sharp contrast to the efficacy of a comparable mutant to support ATP synthesis at alkaline pH by the enzyme from thermoalkaliphilic C. thermarum (38). This increased the paradox noted in the introduction that is posed by the inability of the thermoalkaliphilic enzyme to function synthetically at pH 7.5 unless its native Lys-180 was replaced by glycine or histidine (38). To test the hypothesis that the a-subunits of the extremely alkaliphilic B. pseudofirmus OF4 and the thermoalkaliphilic C. thermarum TA2.A1 are distinctly adapted variants with broad differences, we tested the effects of mutations in six residues in the B. pseudofirmus OF4 a-subunit that are found in the mesophilic alkaliphile but not in the thermoalkaliphile. Five of these residues are in TMH4 or TMH5. These include Met-171 and Met-184 that are an example of a group of residues that are conserved in all the mesophilic alkaliphiles shown in Fig. 1A but are different in C. thermarum TA2.A1, which has Leu-171 (also found in non-alkaliphilic bacteria) and Ala-184 (found only in the thermoalkaliphile) in these positions. Ile-185, also in TMH4–5, is one of an amino acid family, Ile/Val/Leu, that is conserved in all the mesophilic alkaliphiles, whereas C. thermarum TA2.A1 and neutralophilic Bacillus species have Gly/Ser. Val-177 of TMH4–5 is conserved in the three most extreme alkaliphiles but is Ile in the less extreme alkaliphiles, C. thermarum TA2.A1 and neutralophilic Bacillus species. In helix 5, Leu-205 found in most alkaliphiles, except B. halodurans C-125, which has threonine at that position, was mutated to methionine that is found in the thermoalkaliphile and in Bacillus neutralophiles. The final mutation chosen for the expanded a-subunit mutant panel in B. pseudofirmus OF4 was a mutation of Glu-98 to Ser in a position predicted to be at the edge of the functionally important TMH2 (53) on the periplasmic side of the membrane (Fig. 1B). Ser or other neutral amino acids are found in this position in neutralophilic bacteria, whereas C. thermarum TA2.A1 has a Lys residue in this position. The topological diagram in Fig. 1B summarizes all the mutations made in this study.
The results of the extended mutant panel showed that mutations in each of the six of the B. pseudofirmus OF4 residues produced functional deficits (Table 1). Three of the mutants, E98S, M171L, and M184L, had approximately wild-type or somewhat elevated OG ATPase activity and β-subunit content. E98S and M171L grew normally on glucose but showed defective malate growth, especially at pH 10.5, whereas M184L exhibited defective growth on both glucose and malate, with the largest growth deficit on malate at pH 10.5. The V177I and L205M mutants had levels of OG ATPase activity and β-subunit content that were 80–87% of wild-type. The V177I mutation resulted in a substantial deficit in malate growth at both pH values, especially at pH 10.5 at which growth was 32% of wild-type. This mutant also had a substantial deficit in glucose growth at pH 10.5. The L205M mutant grew almost as well as wild-type on glucose but grew poorly on malate at both pH values. Finally, mutations of Ile-185 to either Gly (the residue in C. thermarum TA2.A1) or Ser resulted in reductions of greater than 35% in the OG ATPase activity and β-subunit content relative to wild-type. These reductions were accompanied by only a moderate defect in glucose growth or malate growth at pH 7.5 but a ∼50% deficit in malate growth at pH 10.5.
TABLE 1.
Phenotypes of mutants of the B. pseudofirmus OF4 a-subunit that are conserved in mesophilic alkaliphiles but not in C. thermarum TA2.A1
The growth results are from two independent experiments carried out in duplicate. The OG ATPase and β-subunit content assays were done on two independent everted membrane vesicle preparations. The values are percent of wild-type ± S.D.
| Strain | Glucose |
Malate |
OG ATPase | β-Subunit content | ||
|---|---|---|---|---|---|---|
| pH 7.5 | pH 10.5 | pH 7.5 | pH 10.5 | |||
| Wild-type | 100 | 100 | 100 | 100 | 100 | 100 |
| aE98S | 92.4 ± 0.8 | 95.0 ± 12.8 | 80.0 ± 0.7 | 44.1 ± 7.3 | 124.3 ± 4.4 | 114.4 ± 3.8 |
| aM171L | 91.7 ± 2.4 | 100.6 ± 10.2 | 78.0 ± 2.9 | 49.0 ± 8.6 | 97.3 ± 5.9 | 122.0 ± 12.7 |
| aV177I | 73.0 ± 3.9 | 52.8 ± 4.2 | 54.7 ± 3.2 | 31.9 ± 1.6 | 83.9 ± 10.2 | 83.0 ± 14.5 |
| aM184L | 78.5 ± 10.2 | 62.0 ± 13.3 | 29.8 ± 6.1 | 20.4 ± 9.2 | 100.7 ± 8.8 | 103.4 ± 10.5 |
| aI185G | 81.0 ± 8.8 | 74.9 ± 17.4 | 83.6 ± 4.6 | 48.6 ± 14.8 | 69.2 ± 1.9 | 57.8 ± 10.8 |
| aI185S | 73.4 ± 5.4 | 64.1 ± 14.6 | 71.8 ± 5.8 | 47.5 ± 10.9 | 64.8 ± 14.0 | 48.2 ± 6.5 |
| aL205M | 91.2 ± 8.0 | 85.8 ± 13.7 | 33.2 ± 6.4 | 29.6 ± 9.6 | 80.3 ± 6.2 | 87.0 ± 26.7 |
DISCUSSION
The defects resulting from different single replacements of the Lys-180 of B. pseudofirmus OF4 underscore the importance of this specific basic residue for malate growth at high pH and ATP synthesis capacity. All the mutants retained significant amounts of the a-subunit (Fig. 3C). There were small but reproducible mobility differences between the wild-type and some of the mutant a-subunits on SDS-PAGE gels. These differences may reflect structural changes that are not completely lost under the denaturing conditions of the electrophoresis and that modestly increase or decrease the binding of SDS. Such anomalous mobility effects have been characterized for small model membrane proteins as well as for the c-subunit from B. pseudofirmus OF4 (54, 55). Three mutants exhibited malate growth, K180A, K180G, and K180H. The latter two mutants, which retained more malate growth capacity than K180A, exhibited a more severe growth defect at pH 10.5 than at pH 7.5 relative to wild-type (Fig. 2B). One factor we consider likely to be involved in the deficit in malate growth of the K180H mutant at pH 10.5 is that the pKa of His-180 in the alkaliphile context is not as good a match as that of Lys-180 for proton capture and/or proton passage to the c-ring rotor.
Strikingly, the ATP synthesis capacity of the K180G and K180H mutants at pH 7.5 was at least equal to the wild-type activity assayed in everted vesicles (Fig. 2C). Even the synthesis activity of the less active K180A mutant was high at pH 7.5 relative to the malate growth pattern (Fig. 2, compare B and C). We suggest that the large discrepancy between the robust ATP synthesis in the vesicle assay and the malate growth of these three mutants at least in part reflects a difference in proton delivery to the synthase during whole cell growth versus the energization of the synthase with ascorbate-phenazine methosulfate in the vesicle assays. The proton-pumping terminal oxidase of B. pseudofirmus OF4 is of special importance in supporting non-fermentative growth and oxidative phosphorylation in the alkaliphile and may interact specifically with the ATP synthase to achieve proton transfer from the respiratory chain (33, 56–58). The vesicle energization regimen supplies electrons from ascorbate-phenazine methosulfate directly to this site, whereas the whole respiratory chain that powers ATP synthesis during malate growth is likely to have rate-limiting steps upstream of the terminal oxidase. We hypothesize that this difference accounts for the robust ATP synthesis by these mutants at pH 7.5 in the in vitro assay, whereas malate growth at pH 7.5 shows a significant deficit relative to wild-type. The assays of the native (no OG) ATPase activity of the mutants indicate an additional property of the K180H and K180G mutants that may contribute to the discrepancy between their high retention of in vitro synthesis capacity relative to a larger reduction in malate growth compared with wild-type. These two mutant enzymes both displayed higher native ATPase activity and a greater sensitivity to DCCD than wild-type (Fig. 2D). The sensitivity to DCCD suggests that these mutant enzymes are not uncoupled but are catalyzing increased ATPase activity that is coupled to proton translocation. Another possible explanation for the enhanced DCCD inhibition is an increased reactivity of the c-subunit to DCCD resulting from the mutations. Moreover, the K180H showed a sharp and distinctive increase in native ATPase activity at high pH although even at its peak, the native activity is much lower than the total ATPase activity in the presence of OG (0.074 compared with 1.29 μmol of Pi−1 mg protein−1 for the His mutant). The very low native ATPase activity found in alkaliphiles, i.e. ATPase latency, is considered to be required for growth at very alkaline pH values where small dips in an already low bulk PMF could result in a reversal of the ATP synthase pump (29, 51, 59). The loss of cellular ATP and/or alkalinization of the cytoplasm that would result from outward proton pumping by the ATPase would be detrimental, especially at very high pH. Thus for both the K180H and the K180G mutants, the high native ATPase activity may be part of the explanation for a larger deficit in malate growth than in synthetic capacity at pH 10.5. An important finding of this study is the first evidence that Lys-180 of the a-subunit plays a role when the PMF is reduced at pH 7.5 by treatment of vesicles with low concentrations of CCCP. All three of the Lys-180 mutants that exhibited synthetic capacity also exhibited greater sensitivity of that capacity than the wild-type to inhibition by CCCP. The sensitivity of the three mutants correlated with the extent of synthetic function lost (Fig. 5). This indicates that the alkaliphile-specific Lys-180 in the putative proton-uptake pathway from the outer surface of the membrane has a role at low PMF even when the external conditions are not highly alkaline. The enzymes of the two remaining single Lys-180 mutants, K180C and the K180R, were catalytically inactive at both pH 7.5 and 10.5. The adverse effects of the cysteine replacement are not surprising, because this is a more dramatic replacement than the others. It was included in the panel because, if it had retained partial function, it might have formed the basis for experiments probing the proposed aqueous proton uptake path with sulfhydryl reagents.
The total inactivity of the K180R mutant was, on the other hand, unanticipated because a mutant enzyme of C. thermarum TA2.A1 with the same replacement was highly functional in synthesis at high pH (38). The possibility that the K180R mutant of B. pseudofirmus OF4 was so leaky to protons that growth was precluded at both pH 7.5 and 10.5 was examined in pH shift assays but no significant leakiness was found (data not shown). We hypothesized that the pKa of Arg may be too high in the B. pseudofirmus OF4 context but not in the C. thermarum TA2.A1 context, because the a-subunit of the thermoalkaliphile is a significantly different variant, molded by its need to adapt to the multiple stresses of alkali (from pH 8–10) and high temperature as opposed to the B. pseudofirmus OF4 adaptation to alkali (from pH 7.5 to >11) alone.
This hypothesis was supported by the diverse detrimental effects on malate growth, especially at high pH, of mutations that changed other residues of the B. pseudofirmus OF4 a-subunit that are found in mesophilic alkaliphiles but not in C. thermarum TA2.A1. Functional differences between these organisms related to the a-subunit include the following observations in B. pseudofirmus OF4 that are contrary to observations in C. thermarum TA2.A1 (38, 39): robust non-fermentative growth and ATP synthase activity of the native Lys-180 containing enzyme at pH < 8 and at alkaline pH values well above 10; total loss of malate growth and ATP synthesis by a K180R mutant; and a major deficit in malate growth by a K180G mutant at near neutral pH.
The differences between the a-subunits of the mesophile B. pseudofirmus OF4 and thermorphile C. thermarum TA2.A1 were further demonstrable by the effects observed on mutations in generally conserved alkaliphile residues not found in the thermoalkaliphile. The strains carrying the mutations described in Table 1 generally showed defective malate growth, especially at pH 10.5. Without a structure of the a-subunit, it is not possible to delineate the precise nature of the defect caused by each of these mutations. As shown by Angevine and Fillingame (60), aV177, equivalent to E. coli aM215, is likely to be in the proton uptake pathway (as is aK180). Thus, perturbation at this position could affect the ability of this putative aqueous channel to transport protons ultimately to the glutamate at position 54 in the c-subunit. E. coli a-subunit residue I223, which aligns with alkaliphile aI185, can be cross-linked to a residue in the C-terminal portion of the c-subunit (61). So although E. coli aI223 can be mutated to cysteine without evident effect (60), mutations at that position in the alkaliphile may interfere with the interaction between the a- and c-subunits, which are likely to be more constrained at high pH than at near neutral pH. The other residues Met-171 and Met-184 in helix 4, as well as Leu-205 in helix 5, which are equivalent to E. coli Leu-209, Phe-222, and Asn-238, respectively, can be mutated in E. coli to cysteine (17, 60) without noticeable effect on non-fermentative growth. Glu-98 putatively resides in a periplasmic loop between helix 2 and helix 3 but close to the end of helix 2. It should be noted that the significant effect of mutating Glu-98 on pH 10.5 malate growth contrasts with the conclusions of studies in E. coli that found no effect of substitution of this residue (17, 62), but which were carried out near neutral pH in E. coli.
We suggest that the transmembrane residues Val-171, Val-177, Met-184, Met-185, and Leu-205 help provide the appropriate scaffold for a functioning mesophilic alkaliphile a-subunit to interact with its partner c-ring. Mutations in these residues could subtly change the conformation of helices 4 and 5, resulting in a suboptimal proton uptake pathway and/or interaction with the c-subunits. The Glu-98 is putatively part of a periplasmic loop that we have suggested, based on the effects of replacement of the loop with the neutralophile B. megaterium sequence, may contribute to proton uptake.
The final finding of interest was the phenotype of the mutant, K180G/G212K, that switched the position of the Lys-180 with that of alkaliphile-specific Gly-212 (Fig. 6). The retention of synthetic function (Fig. 6) supported earlier evidence that the two residues are part of the proton-uptake pathway and function interactively in cytoplasmic proton retention (33). The enzyme also retains native ATPase and DCCD sensitivity profiles that are roughly comparable to wild-type. Thus the observed proton leakiness, which probably accounts both for the malate growth deficit and for the somewhat low and highly variable synthetic capacity at pH 10.5, is not the result of a completely disrupted structure. Rather, the re-positioned Lys-180 may be compromised in a direct role in preventing proton leaks (37) or may be compromised in a role in enhancing the ability of Arg-172 to prevent proton loss through short circuits during rotary function of the enzyme (25, 37). In alkaliphiles, specific adaptations of both the c- and a-subunits of the alkaliphile ATP synthase are critical to both the ATP synthesis that achieves this proton capture and to prevention of loss of cytoplasmic protons through the synthase (36, 37).
This work was supported, in whole or in part, by National Institutes of Health Grant GM28454 from NIGMS.
- PMF
- protonmotive force
- BTP
- Bis-Tris propane
- CCCP
- carbonyl m-chlorophenylhydrazone
- DCCD
- N,N′-dicyclohexylcarbodiimide
- OG
- octylglucoside
- RSO
- right-side out
- TMH
- transmembrane helix
- MOPS
- 4-morpholinepropanesulfonic acid
- NTA
- nitrilotriacetic acid.
REFERENCES
- 1.Mitchell P. (1961) Nature 191, 144–148 [DOI] [PubMed] [Google Scholar]
- 2.Stock D., Gibbons C., Arechaga I., Leslie A. G., Walker J. E. (2000) Curr. Opin. Struct. Biol. 10, 672–679 [DOI] [PubMed] [Google Scholar]
- 3.von Ballmoos C., Cook G. M., Dimroth P. (2008) Annu. Rev. Biophys. 37, 43–64 [DOI] [PubMed] [Google Scholar]
- 4.Fillingame R. H., Angevine C. M., Dmitriev O. Y. (2003) FEBS Lett. 555, 29–34 [DOI] [PubMed] [Google Scholar]
- 5.von Ballmoos C., Wiedenmann A., Dimroth P. (2009) Annu. Rev. Biochem. 78, 649–672 [DOI] [PubMed] [Google Scholar]
- 6.Yoshida M., Muneyuki E., Hisabori T. (2001) Nat. Rev. Mol. Cell Biol. 2, 669–677 [DOI] [PubMed] [Google Scholar]
- 7.Nakamoto R. K., Baylis Scanlon J. A., Al-Shawi M. K. (2008) Arch. Biochem. Biophys. 476, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanishi-Matsui M., Sekiya M., Nakamoto R. K., Futai M. (2010) Biochim. Biophys. Acta 1797, 1343–1352 [DOI] [PubMed] [Google Scholar]
- 9.Nishizaka T., Oiwa K., Noji H., Kimura S., Muneyuki E., Yoshida M., Kinosita K., Jr. (2004) Nat. Struct. Mol. Biol. 11, 142–148 [DOI] [PubMed] [Google Scholar]
- 10.Ueno H., Suzuki T., Kinosita K., Jr., Yoshida M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W., Hermolin J., Fillingame R. H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier T., Morgner N., Matthies D., Pogoryelov D., Keis S., Cook G. M., Dimroth P., Brutschy B. (2007) Mol. Microbiol. 65, 1181–1192 [DOI] [PubMed] [Google Scholar]
- 13.Meier T., Polzer P., Diederichs K., Welte W., Dimroth P. (2005) Science 308, 659–662 [DOI] [PubMed] [Google Scholar]
- 14.Pogoryelov D., Reichen C., Klyszejko A. L., Brunisholz R., Muller D. J., Dimroth P., Meier T. (2007) J. Bacteriol. 189, 5895–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogoryelov D., Yu J., Meier T., Vonck J., Dimroth P., Muller D. J. (2005) EMBO Rep. 6, 1040–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stock D., Leslie A. G., Walker J. E. (1999) Science 286, 1700–1705 [DOI] [PubMed] [Google Scholar]
- 17.Angevine C. M., Herold K. A., Fillingame R. H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13179–13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angevine C. M., Herold K. A., Vincent O. D., Fillingame R. H. (2007) J. Biol. Chem. 282, 9001–9007 [DOI] [PubMed] [Google Scholar]
- 19.Langemeyer L., Engelbrecht S. (2007) Biochim. Biophys. Acta 1767, 998–1005 [DOI] [PubMed] [Google Scholar]
- 20.Moore K. J., Angevine C. M., Vincent O. D., Schwem B. E., Fillingame R. H. (2008) J. Biol. Chem. 283, 13044–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore K. J., Fillingame R. H. (2008) J. Biol. Chem. 283, 31726–31735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwem B. E., Fillingame R. H. (2006) J. Biol. Chem. 281, 37861–37867 [DOI] [PubMed] [Google Scholar]
- 23.Steed P. R., Fillingame R. H. (2008) J. Biol. Chem. 283, 12365–12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steed P. R., Fillingame R. H. (2009) J. Biol. Chem. 284, 23243–23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitome N., Ono S., Sato H., Suzuki T., Sone N., Yoshida M. (2010) Biochem. J. 430, 171–177 [DOI] [PubMed] [Google Scholar]
- 26.Aksimentiev A., Balabin I. A., Fillingame R. H., Schulten K. (2004) Biophys. J. 86, 1332–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elston T., Wang H., Oster G. (1998) Nature 391, 510–513 [DOI] [PubMed] [Google Scholar]
- 28.Lau W. C., Rubinstein J. L. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimroth P., Cook G. M. (2004) Adv. Microb. Physiol. 49, 175–218 [DOI] [PubMed] [Google Scholar]
- 30.Hicks D. B., Liu J., Fujisawa M., Krulwich T. A. (2010) Biochim. Biophys. Acta 1797, 1362–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preiss L., Yildiz O., Hicks D. B., Krulwich T. A., Meier T. (2010) PLoS Biol. 8, e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krulwich T. A. (1995) Mol. Microbiol. 15, 403–410 [DOI] [PubMed] [Google Scholar]
- 33.Krulwich T. A., Hicks D. B., Swartz T. H., Ito M. (2007) in Physiology and Biochemistry of Extremophiles (Gerday C., Glansdorff N. eds) pp. 311–329, ASM Press, Washington, DC [Google Scholar]
- 34.Sturr M. G., Guffanti A. A., Krulwich T. A. (1994) J. Bacteriol. 176, 3111–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivey D. M., Krulwich T. A. (1992) Res. Microbiol. 143, 467–470 [DOI] [PubMed] [Google Scholar]
- 36.Liu J., Fujisawa M., Hicks D. B., Krulwich T. A. (2009) J. Biol. Chem. 284, 8714–8725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Hicks D. B., Guffanti A. A., Baldwin K., Krulwich T. A. (2004) J. Biol. Chem. 279, 26546–26554 [DOI] [PubMed] [Google Scholar]
- 38.McMillan D. G., Keis S., Dimroth P., Cook G. M. (2007) J. Biol. Chem. 282, 17395–17404 [DOI] [PubMed] [Google Scholar]
- 39.McMillan D. G., Keis S., Berney M., Cook G. M. (2009) Appl. Environ. Microbiol. 75, 7649–7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartzog P. E., Cain B. D. (1994) J. Biol. Chem. 269, 32313–32317 [PubMed] [Google Scholar]
- 41.Ito M., Guffanti A. A., Zemsky J., Ivey D. M., Krulwich T. A. (1997) J. Bacteriol. 179, 3851–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 43.Chan K. M., Delfert D., Junger K. D. (1986) Anal. Biochem. 157, 375–380 [DOI] [PubMed] [Google Scholar]
- 44.Maehama T., Taylor G. S., Slama J. T., Dixon J. E. (2000) Anal. Biochem. 279, 248–250 [DOI] [PubMed] [Google Scholar]
- 45.Rowlands M. G., Newbatt Y. M., Prodromou C., Pearl L. H., Workman P., Aherne W. (2004) Anal. Biochem. 327, 176–183 [DOI] [PubMed] [Google Scholar]
- 46.Schägger H., von Jagow G. (1987) Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 47.Nesterenko M. V., Tilley M., Upton S. J. (1994) J. Biochem. Biophys. Methods 28, 239–242 [DOI] [PubMed] [Google Scholar]
- 48.Vorburger T., Ebneter J. Z., Wiedenmann A., Morger D., Weber G., Diederichs K., Dimroth P., von Ballmoos C. (2008) FEBS J. 275, 2137–2150 [DOI] [PubMed] [Google Scholar]
- 49.Stanley P. E., Williams S. G. (1969) Anal. Biochem. 29, 381–392 [DOI] [PubMed] [Google Scholar]
- 50.Ono S., Sone N., Yoshida M., Suzuki T. (2004) J. Biol. Chem. 279, 33409–33412 [DOI] [PubMed] [Google Scholar]
- 51.Hicks D. B., Krulwich T. A. (1990) J. Biol. Chem. 265, 20547–20554 [PubMed] [Google Scholar]
- 52.Cain B. D., Simoni R. D. (1989) J. Biol. Chem. 264, 3292–3300 [PubMed] [Google Scholar]
- 53.DeLeon-Rangel J., Zhang D., Vik S. B. (2003) Arch. Biochem. Biophys. 418, 55–62 [DOI] [PubMed] [Google Scholar]
- 54.Liu J., Fackelmayer O., Preiss L., Meier T., Hicks D. B., Krulwich T. A. (2010) Biochim. Biophys. Acta 1797S, 35–3619751801 [Google Scholar]
- 55.Rath A., Glibowicka M., Nadeau V. G., Chen G., Deber C. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilmour R., Krulwich T. A. (1997) J. Bacteriol. 179, 863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quirk P. G., Guffanti A. A., Plass R. J., Clejan S., Krulwich T. A. (1991) Biochim. Biophys. Acta 1058, 131–140 [DOI] [PubMed] [Google Scholar]
- 58.Quirk P. G., Hicks D. B., Krulwich T. A. (1993) J. Biol. Chem. 268, 678–685 [PubMed] [Google Scholar]
- 59.Stocker A., Keis S., Vonck J., Cook G. M., Dimroth P. (2007) Structure 15, 904–914 [DOI] [PubMed] [Google Scholar]
- 60.Angevine C. M., Fillingame R. H. (2003) J. Biol. Chem. 278, 6066–6074 [DOI] [PubMed] [Google Scholar]
- 61.Jiang W., Fillingame R. H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6607–6612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vik S. B., Ishmukhametov R. R. (2005) J. Bioenerg. Biomembr. 37, 445–449 [DOI] [PubMed] [Google Scholar]