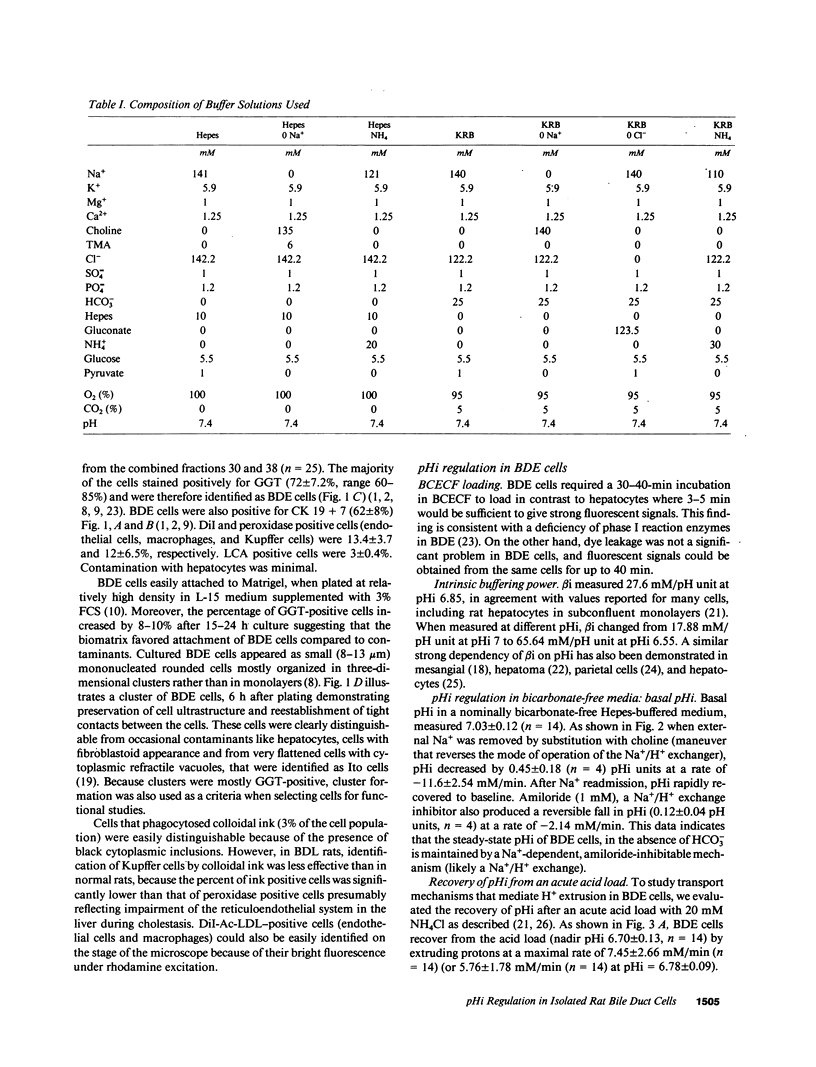
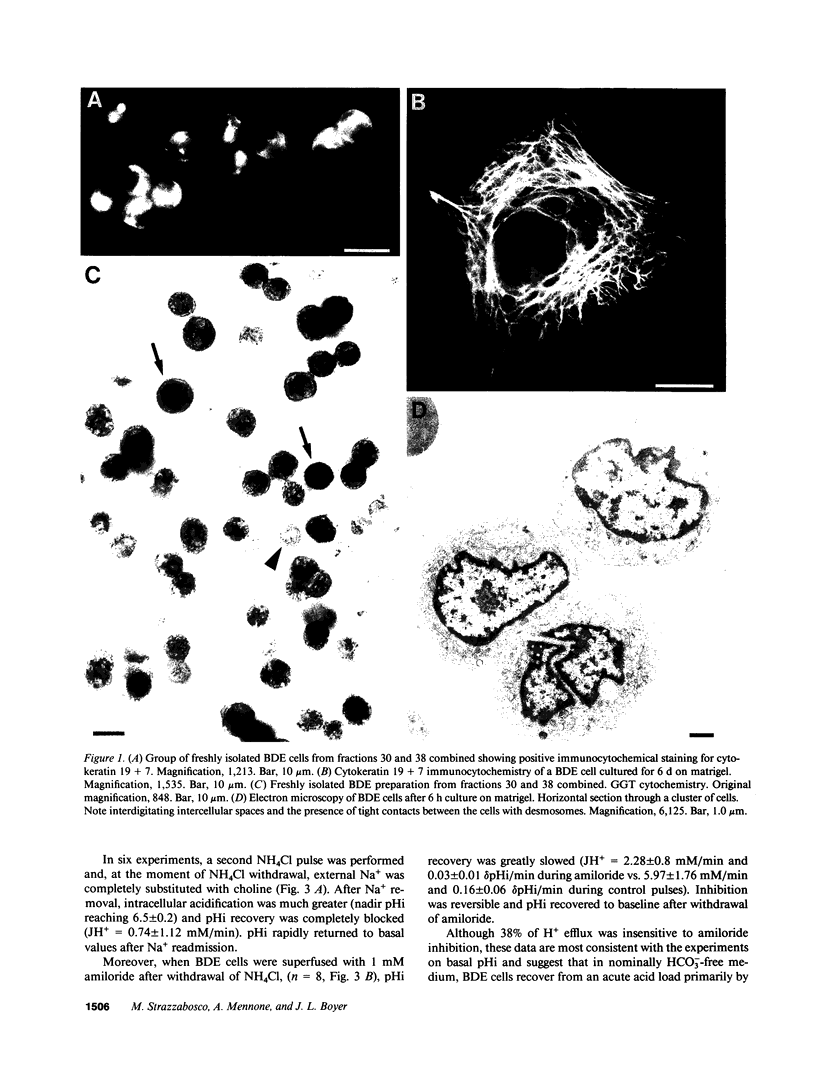
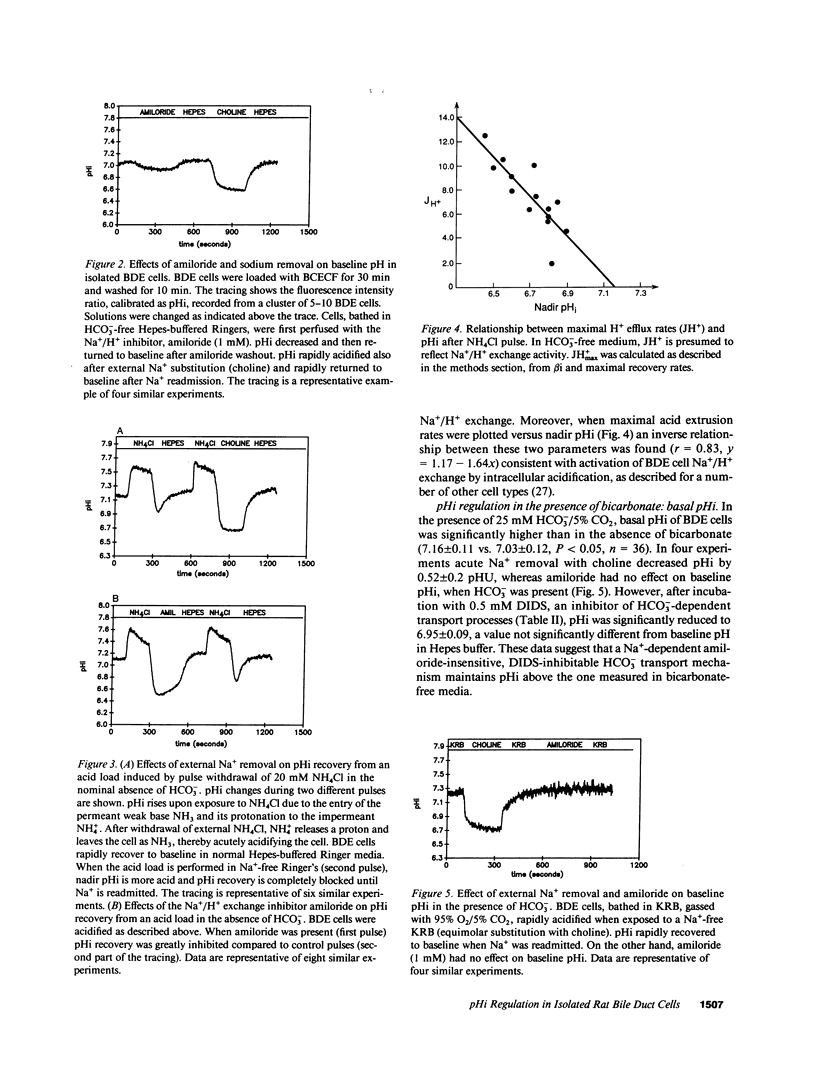
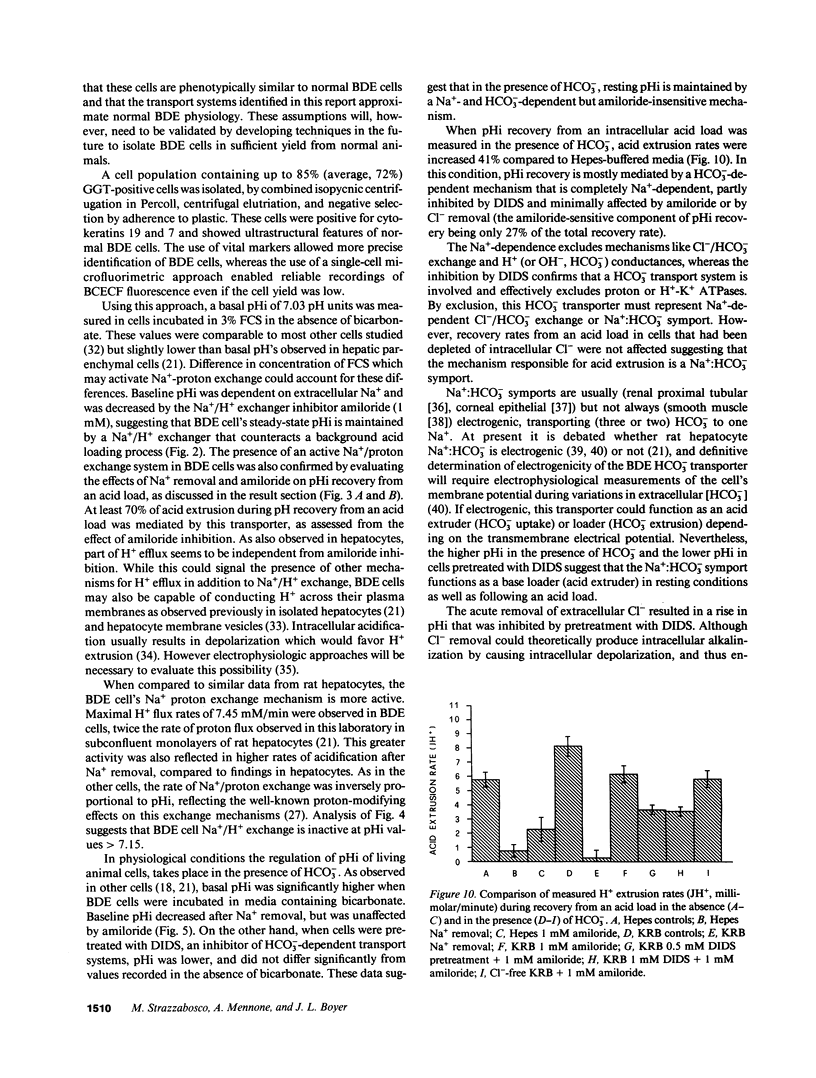
Abstract
To evaluate ion transport mechanisms in bile duct epithelium (BDE), BDE cells were isolated from bile duct-ligated rats. After short-term culture pHi was measured with a single cell microfluorimetric set-up using the fluorescent pHi indicator BCECF, and calibrated with nigericin in high K+ concentration buffer. Major contaminants were identified using vital markers. In HCO3(-)-free media, baseline pHi (7.03 +/- 0.12) decreased by 0.45 +/- 0.18 pH units after Na+ removal and by 0.12 +/- .04 after amiloride administration (1 mM). After acid loading (20 mM NH4Cl) pHi recovery was inhibited by both Na+ removal and amiloride (JH+ = 0.74 +/- 1.1, and JH+ = 2.28 +/- 0.8, respectively, vs. 5.47 +/- 1.97 and 5.97 +/- 1.76 mM/min, in controls, respectively). In HCO3- containing media baseline pHi was higher (7.16 +/- 0.1, n = 36, P less than 0.05) and was decreased by Na+ substitution but not by amiloride. Na+ removal inhibited pHi recovery after an intracellular acid load (0.27 +/- 0.26, vs. 7.7 +/- 4.1 mM/min, in controls), whereas amiloride reduced JH+ only by 27%. pH recovery was inhibited by DIDS (0.5-1 mM), but not by Cl- depletion. Finally, acute Cl- removal increased pHi by 0.18 pH units in the absence but not presence of DIDS. These data indicate that BDE cells possess mechanisms for Na+/H+ exchange, Na+:HCO3- symport and Cl-/HCO3 exchange. Therefore BDE may be capable of transepithelial H+/HCO3- transport.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Intracellular pH regulation by vertebrate muscle. Annu Rev Physiol. 1986;48:349–361. doi: 10.1146/annurev.ph.48.030186.002025. [DOI] [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988 Feb;81(2):569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Zhai W. R., Liu M. H., Slott P. A., Paronetto F., Tavoloni N. Isolation of a nonparenchymal liver cell fraction enriched in cells with biliary epithelial phenotypes. Gastroenterology. 1989 Nov;97(5):1248–1260. doi: 10.1016/0016-5085(89)91696-x. [DOI] [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Zhai W. R., Slott P. A., Liu M. H., Sarkozi L., Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989 Jul;257(1 Pt 1):G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Nolan K., Hardison W. G. Role of bicarbonate in biliary excretion of diisothiocyanostilbene disulfonate. Am J Physiol. 1988 Dec;255(6 Pt 1):G713–G722. doi: 10.1152/ajpgi.1988.255.6.G713. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Liver sinusoidal cells. Identification of a subpopulation for erythrocyte catabolism. J Cell Biol. 1972 Jul;54(1):107–119. doi: 10.1083/jcb.54.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Chenderovitch J. Secretory function of the rabbit common bile duct. Am J Physiol. 1972 Sep;223(3):695–706. doi: 10.1152/ajplegacy.1972.223.3.695. [DOI] [PubMed] [Google Scholar]
- Desmet V. J. Cholangiopathies: past, present, and future. Semin Liver Dis. 1987 May;7(2):67–76. doi: 10.1055/s-2008-1040566. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Persico M., Scharschmidt B. F. Electrophysiological evidence for Na+-coupled bicarbonate transport in cultured rat hepatocytes. Am J Physiol. 1989 Mar;256(3 Pt 1):G491–G500. doi: 10.1152/ajpgi.1989.256.3.G491. [DOI] [PubMed] [Google Scholar]
- Fricker G., Landmann L., Meier P. J. Extrahepatic obstructive cholestasis reverses the bile salt secretory polarity of rat hepatocytes. J Clin Invest. 1989 Sep;84(3):876–885. doi: 10.1172/JCI114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Boyer J. L. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987 Mar-Apr;7(2):216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Gleeson D., Smith N. D., Boyer J. L. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989 Jul;84(1):312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Graf J., Boyer J. L. Na-H exchange regulates intracellular pH in isolated rat hepatocyte couplets. Am J Physiol. 1987 Jan;252(1 Pt 1):G109–G113. doi: 10.1152/ajpgi.1987.252.1.G109. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989 Nov;97(5):1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Pretlow T. P., Fausto N., Pitts A. M., Pretlow T. G., 2nd Separation of two populations of cells with gamma-glutamyl transpeptidase from carcinogen-treated rat liver. J Natl Cancer Inst. 1981 May;66(5):967–973. [PubMed] [Google Scholar]
- Jentsch T. J., Keller S. K., Koch M., Wiederholt M. Evidence for coupled transport of bicarbonate and sodium in cultured bovine corneal endothelial cells. J Membr Biol. 1984;81(3):189–204. doi: 10.1007/BF01868713. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Lambert A. Chloride-bicarbonate exchange and related transport processes. Biochim Biophys Acta. 1982 Dec;694(4):353–374. doi: 10.1016/0304-4157(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Maher J. J., Bissell D. M., Friedman S. L., Roll F. J. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., D'Amico P., Gengo T. F., Sirica A. E. Enzyme profile of rat bile ductular epithelial cells in reference to the resistance phenotype in hepatocarcinogenesis. Hepatology. 1989 Mar;9(3):477–485. doi: 10.1002/hep.1840090323. [DOI] [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., Sirica A. E. Biochemical characteristics of hyperplastic rat bile ductular epithelial cells cultured "on top" and "inside" different extracellular matrix substitutes. Cancer Res. 1988 Nov 1;48(21):6145–6153. [PubMed] [Google Scholar]
- Meier P. J., Knickelbein R., Moseley R. H., Dobbins J. W., Boyer J. L. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985 Apr;75(4):1256–1263. doi: 10.1172/JCI111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley R. H., Meier P. J., Aronson P. S., Boyer J. L. Na-H exchange in rat liver basolateral but not canalicular plasma membrane vesicles. Am J Physiol. 1986 Jan;250(1 Pt 1):G35–G43. doi: 10.1152/ajpgi.1986.250.1.G35. [DOI] [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Scharschmidt B. F., Zimmerli B., Meier P. J. Rat hepatocytes exhibit basolateral Na+/HCO3- cotransport. J Clin Invest. 1989 Apr;83(4):1225–1235. doi: 10.1172/JCI114005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Smith N. D., Boyer J. L. Permeability characteristics of bile duct in the rat. Am J Physiol. 1982 Jan;242(1):G52–G57. doi: 10.1152/ajpgi.1982.242.1.G52. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Machen T. E., Williams J. A. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G925–G930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Tavoloni N. Role of ductular bile water reabsorption in canine bile secretion. J Lab Clin Med. 1985 Aug;106(2):154–161. [PubMed] [Google Scholar]
- Tavoloni N. The intrahepatic biliary epithelium: an area of growing interest in hepatology. Semin Liver Dis. 1987 Nov;7(4):280–292. doi: 10.1055/s-2008-1040583. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Townsley M. C., Machen T. E. Na-HCO3 cotransport in rabbit parietal cells. Am J Physiol. 1989 Sep;257(3 Pt 1):G350–G356. doi: 10.1152/ajpgi.1989.257.3.G350. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub W. H., Machen T. E. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989 Sep;257(3 Pt 1):G317–G327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- Wenzl E., Machen T. E. Intracellular pH dependence of buffer capacity and anion exchange in the parietal cell. Am J Physiol. 1989 Nov;257(5 Pt 1):G741–G747. doi: 10.1152/ajpgi.1989.257.5.G741. [DOI] [PubMed] [Google Scholar]
- Wheeler H. O., Mancusi-Ungaro P. L. Role of bile ducts during secretin choleresis in dogs. Am J Physiol. 1966 May;210(5):1153–1159. doi: 10.1152/ajplegacy.1966.210.5.1153. [DOI] [PubMed] [Google Scholar]
- Winterhager J. M., Stewart C. P., Heintze K., Petersen K. U. Electroneutral secretion of bicarbonate by guinea pig gallbladder epithelium. Am J Physiol. 1986 Apr;250(4 Pt 1):C617–C628. doi: 10.1152/ajpcell.1986.250.4.C617. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]



