Abstract
We experimentally and theoretically examined the conformation, pH and temperature dependence of the CH2 stretching frequencies of glycine (gly) in solution and in the crystalline state. To separate the effects of the amine and carboxyl groups on the CH2 stretching frequencies we examined the Raman spectra of 2,2,2-d3-ethylamine (CD3-CH2-NH2) and 3,3,3-d3-propionic acid (CD3-CH2-COOH) in D2O. The symmetric (νsCH2) and asymmetric (νasCH2) stretching frequencies show a significant dependence on gly conformation. We quantified the relation between the frequency splitting (Δ = νasCH2 − νsCH2) and the ξ angle which determines the gly conformational geometry. This relation allows us to determine the conformation of gly directly from the Raman spectral frequencies. We observe a large dependence of the νsCH2 and νasCH2 frequencies on the ionization state of the amine group, which we demonstrate theoretically results from a negative hyperconjugation between the nitrogen lone pair and the C-H anti-bonding orbitals. The magnitude of this effect is maximized for C-H bonds trans to the nitrogen lone pair. In contrast, a small dependence of the CH2 stretching frequencies on the carboxyl group ionization state arises from delocalization of electron density from carboxyl oxygen to C-H bonding orbitals. According to our experimental observations and theoretical calculations the temperature dependence of the νsCH2 and νasCH2 of gly is due to the change in the hydrogen bonding strength of the amine/carboxyl groups to water.
Introduction
Vibrational spectroscopy is a powerful technique to study the conformations of peptides and proteins. Vibrational spectroscopy provides a unique opportunities to study fast protein folding dynamics1–4 and unordered states of polypeptides chains.5 Vibrational spectra are highly informative on molecular structure due to the extreme sensitivity of certain vibrational bands or so-called “conformational markers”, to the small structural alterations such as bond lengths, dihedral angles, and hydrogen bonding patterns.
The most commonly used markers for the polypeptide backbone conformation analysis are the amide bands. The Amide I band (primarily C=O stretching of the peptide bond) is used for the IR spectroscopy secondary structure elucidation.1,6 The Amide II, Amide III and CαH bending vibrations, observed in Raman spectra, have been shown to be even more valuable for peptide secondary structure analysis.3,4,7,8 There should be other conformationally sensitive vibrations which can be used to expand the informational context of vibrational spectroscopy.
The CH (or deuterated CD) stretch is a potential candidate for use as a conformational marker to study the secondary structure of polypeptide chains. It has recently been shown theoretically9,10 that the CαH (CαD) bond stretching frequency depends on the ψ and φ Ramachandran angles.
CH stretching vibrations have previously been used to determine the structure of small organic molecules. Isolated C-D stretching frequencies have also been used in the conformational analysis of alkylamino chains,13 and monosaccharides.14 Good correlations have been experimentally found between isolated methyl CH stretching frequencies and HCH angles.11
The origin of the CH conformational sensitivity is not well understood. It is generally agreed that the CH stretching frequencies depend almost solely on the C-H bond lengths because these vibrations are essentially decoupled from other vibrations. Previous work has quantified the relationship between CH stretching frequencies and C-H bond lengths in various organic compounds12,15 This work includes McKean, Bellamy and others16,17 extensive IR spectroscopy studies in 60’s and 70’s which examined the factors influencing CH stretching frequencies
In the present study we focus our attention on gly, the smallest amino acid, which has a hydrogen atom instead of a side chain. There is no coupling or overlap of the gly CH2 stretches in proteins and peptides with the CH stretches of the adjacent amino acid residue side chains. In addition, the CH2 stretches of gly are unaffected by Fermi resonances because of the significant downshift of the CH2 scissoring vibration. The two CH stretches of the gly CH2 group couple with each other to form a high-frequency asymmetric and a low-frequency symmetric CH2 stretching components, which appear as a doublet in the Raman spectra. The magnitude of the frequency splitting of this doublet depends on the extent of vibrational coupling which in turn is determined by the C-H bond length difference.
The unique flexibility of gly makes it an essential structural element of many proteins, determining protein folding pathways, tertiary structure and biological function. Gly is frequently found in the turn and loop structures, which play an important role in polypeptide chain collapse during the early stages of folding. Gly accelerates loop formation compared to other amino acids.18 Gly rich flexible motifs are often impossible to characterize by x-ray crystallography and 2D NMR.19 Thus, there is a great need to develop new structural methods to determine the gly residue conformations.
In this paper we investigate the gly CH2 stretching frequency dependence on amino acid conformation and ionization state in order to develop methodologies for the conformational analysis of gly residues in polypeptides and proteins.
Experimental methods
Sample Preparation
For the pD measurements anhydrous gly (Sigma Chemicals) was dissolved in D2O (Cambride Isotope Laboratories Inc). Low and high pD samples were prepared by addition of DCl or NaOD solutions (Sigma Chemicals). 2,2,2-d3-ethylamine hydrochloride was obtained from Medical Isotopes Inc. and 3,3,3-d3-propionic acid from Cambride Isotope Laboratories Inc. Crystals of Gly•HCl, Gly•HNO3, 3Gly•H2SO4 (TGS) were obtained by slow evaporation of water solutions of stoichiometric mixtures of gly and the corresponding acid. All acids were purchased from J. T. Baker Inc. Crystal structures were determined by using x-ray crystallography.
Raman measurements
All Raman measurements were performed using 488 nm Ar-ion Laser (Coherent Inc.) excitation. Scattered light was collected using a back-scattering geometry, dispersed by a single monochromator and collected using a Princeton Instruments Spec-10:400B CCD camera (Roper Scientific). A 488 nm holographic notch filter (Kaiser Optical Systems Inc.) was used for Raleigh rejection. Typical accumulation times were ~ 2 min. A temperature controlled fused silica cell (20 mm path length, Starna Cell Inc.) was used for solutions. A custom made, rotating metal cell was used for solid powder samples to avoid light-induced degradation under continuous irradiation. The powder was pressed into a circular groove cut in the rotating metal cylinder.
Computational methods
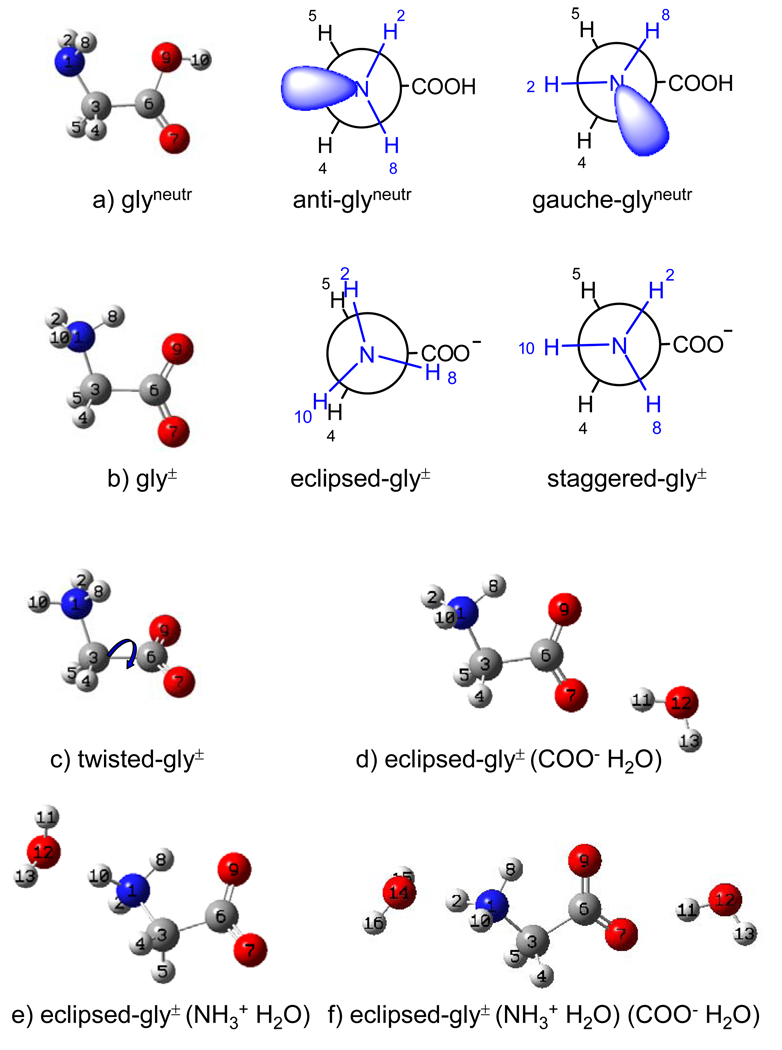
We optimized the geometries and calculated the vibrational frequencies, normal mode compositions, molecular orbital analysis, and charge distributions of a series of gly conformers in neutral and zwitterionic forms. We also performed the calculations for zwitterionic gly hydrogen bonded to one or two water molecules (Fig.1).
Figure 1.
Calculated conformers of gly: a) anti and gauche rotamers of neutral gly; b) eclipsed and staggered rotamers of zwitterionic gly; c) non-planar conformer (ξ = 60°) of zwitterionic gly; d) eclipsed zwitterionic gly hydrogen bonded to the acceptor water molecule, e) eclipsed zwitterionic gly hydrogen bonded to the donor water molecule, f) eclipsed zwitterionic gly hydrogen bonded simultaneously to the acceptor and donor water molecules. All conformers except c) are planar ξ = 0°.
All calculations were carried out at the DFT level of theory20–22 employing the B3LYP exchange functional23–25 and 6-311+G(d,p) basis set. All frequencies were calculated at the harmonic approximation and scaled by 0.98.26,27 The presence of the solvent water was modeled using the Polarizable Continuum Model (PCM) initially devised by Tomasi and coworkers.28–30 The PCM calculations were performed using the integral equation formalism model30 (IEFPCM) and the Bondii’s atomic radii. Atomic charges were calculated using the Atoms in Molecule (AIM) algorithm31–33 as implemented in Gaussian’98. Orbital occupancies and hybridization analysis of Natural Local Molecular Orbitals were obtained through the Natural Bond Orbital (NBO) Analysis. All calculations except the AIM charge calculations were performed with the Gaussian’03 calculational package.34 Normal mode compositions for the calculated vibrational frequencies were obtained from the Gaussian output files employing the GAR2PED program.35
Experimental Results
The conformational dependence of the CH2 stretching frequencies on carboxyl group orientation of gly in the solid state
We investigated the structures of gly found in the Cambridge Structural Database (CSD):
Gly hydrochloride (Gly•HCl). In this crystal all heavy atoms lie almost in the same plane.36
Gly nitrate (Gly•HNO3). Here gly is bent.37
Triglycine sulphate (3Gly•H2SO4), TGS. In this crystal structure two gly molecules have geometries close to planar while one gly molecule is bent.38
In these structures the amine groups are protonated (-NH3+). Thus, rotation about the N-C bond should not significantly affect the CH2 stretching frequencies.
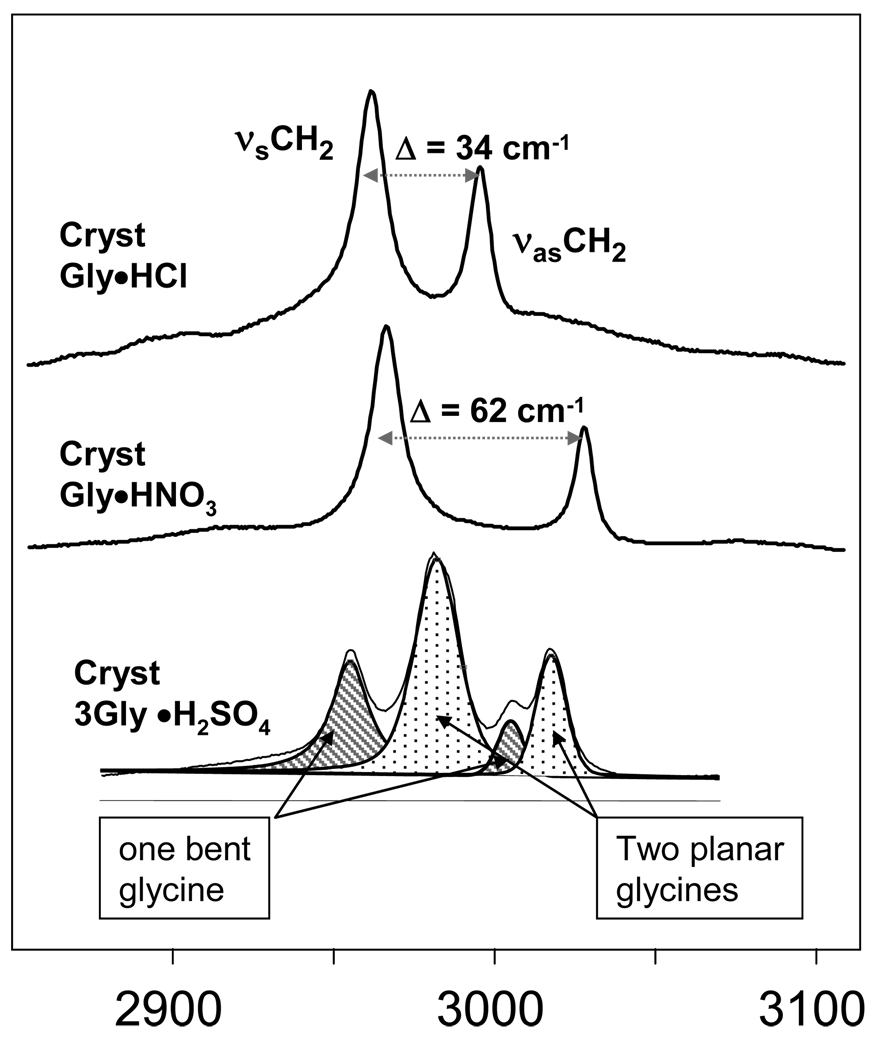
There is a remarkable difference in the frequencies and the frequency splitting between the CH2 symmetric (νsCH2) and asymmetric (νasCH2) stretching frequencies for these three samples. The Raman spectrum of crystalline Gly•HCl shows the symmetric and asymmetric stretching bands at νsCH2 = 2962 cm−1 and νasCH2 = 2996 cm−1, with a splitting Δ of ~ 34 cm−1. Crystalline Gly•HNO3 has a much larger splitting, Δ ~ 62 cm−1 between the νsCH2 = 2966 cm−1 and νasCH2 = 3028 cm−1 bands, mainly due to the upshift of the νasCH2. TGS has 3 nonequivalent gly in the crystal unit cell. In TGS we observe two different doublets of νsCH2 and νasCH2.
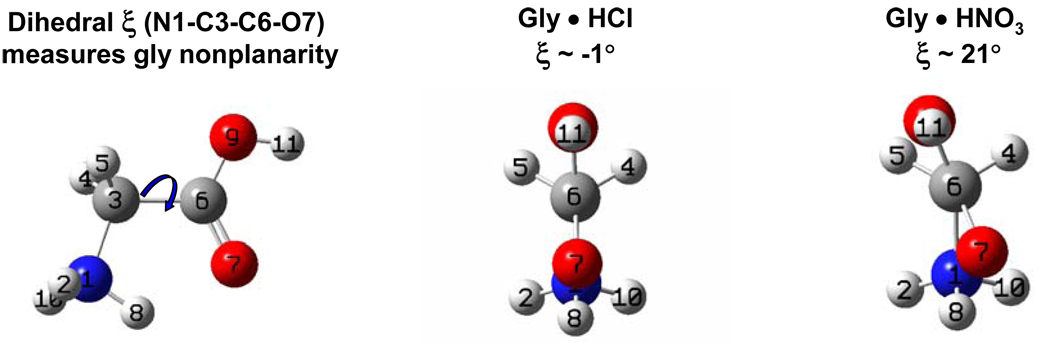
X-ray crystallographic data shows (Fig. 3) that in Gly•HCl crystals all heavy atoms (N-C-COO) lie almost in the same plane and the two C-H bonds are symmetrically disposed about this plane. The dihedral angel ξ, defined by atoms N-C-C-Ocis to N (since carboxyl group has two oxygens, ξ is defined by the one which is the closest (cis) to the nitrogen). The ξ angle indicates the deviation of gly from the planar conformation through rotation around the C-C bond. When ξ = 0° all heavy atoms in the gly molecule lie in the same plane (Figure 3). In contrast, the -COOH group of the Gly•HNO3 crystal is rotated such that ξ ~ 21°. In this case the C-H bonds are not symmetrically disposed on both sides of the COO plane, which results in greater splitting between the νsCH2 and νasCH2 frequencies.
Figure 3.
Dihedral angle ξ measures planarity of gly molecule. If ξ ~ 0°, the molecule is planar and the C-H bonds are symmetric with respect to the carboxyl group plane, as in case of Gly•HCl. In Gly•HNO3 the carboxyl is rotated ~ 21° with respect to the N1-C3 bond making the C-H bonds nonequivalent.
In TGS crystals, two of the gly molecules are almost planar (ξ = 4° and = 5°), while the third one is bent ξ ~ 21°. Thus, we assign the more intense, less split doublet (2982 cm−1 and 3017 cm−1) to the two planar gly molecules and the less intense more split doublet (2956 cm−1 and 3005 cm−1) to the third gly.
pD and temperature dependences of the CH2 stretching frequencies of gly in solution
We investigated the influence of the ionization state of the carboxyl and amine groups on the CH2 stretching vibrations. Deuteration of the amine in gly molecule significantly downshifts the N-D stretches which removes overlap or coupling between C-H and N-D stretches and simplifies the interpretation of the Raman spectra.
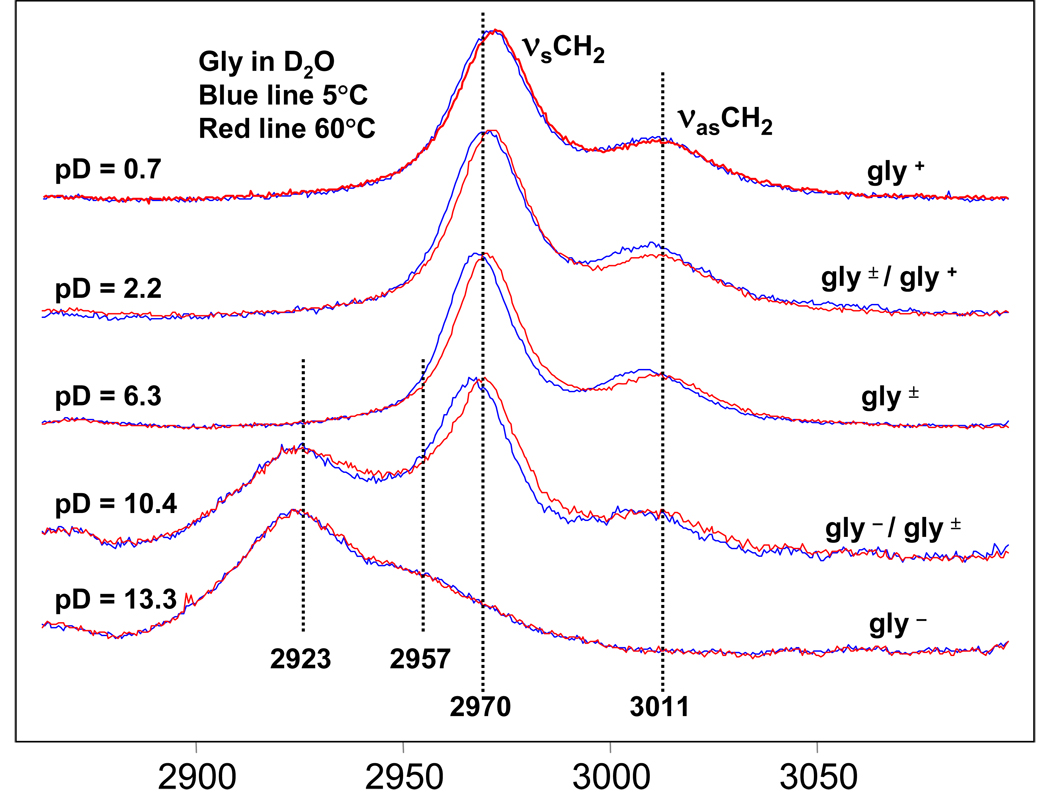
Figure 4 shows the 488 nm Raman spectra of gly in D2O at various pD values at 5°C and 60°C. At low pD values gly is cationic D3N+-CH2-COOD (gly+), while at pD values close to neutral gly is zwitterionic D3N+-CH2-COO− (gly±), and at high pD values gly is anionic D2N-CH2-COO− (gly−).
Figure 4.
488 nm excitation Raman spectra of gly solution in D2O at pD= 0.7, 2.2, 6.3, 10.4, 13.3. For each solution spectra were accumulated at two temperatures 5°C and 60°C.
pD Dependence
For the low pD (gly+) and neutral pD values (gly±) the CH2 stretching region Raman spectra are very similar, indicating that the ionization state of the carboxyl group has little effect on the CH2 group stretching frequencies. The CH2 symmetric stretch is at ~ 2970 cm−1 and is ~ 4 times more intense than the CH2 asymmetric stretch at ~ 3010 cm−1.
For pD values close to or above the pKa value of the gly amine group (pD = 9.8) a peak appears at lower frequency ~ 2920 cm−1, which must be due to C-H bond weakening due to interaction with the lone pair of the unprotonated amine group (-ND2), as discussed in detail below.
The frequency splitting between νsCH2 and νasCH2 is ~ 41 cm−1 for gly+ and gly±, while for gly− it is smaller (Δ ~ 34 cm−1).
Thus, the C-H bonds stretching frequencies depend only on the ionization state of the terminal amine with a little influence of the ionization state of the carboxyl.
Temperature dependence
Figure 4 and Table 1 indicate the temperature dependence of the CH2 stretching frequencies of gly in D2O.
Table 1.
Temperature dependence of the Raman CH2 stretching frequencies of gly in D2O
| Solution | T°C | νsCH2 / cm−1 | Δν/ΔT sym | νasCH2 / cm−1 | Δν/ΔT asym |
|---|---|---|---|---|---|
| D2O pD = 0.7 |
5 | 2971.2 | 0.016 | 3011.2 | 0.025 |
| 60 | 2972.1 | 3012.6 | |||
| D2O pD = 2.2 |
5 | 2969.9 | 0.027 | 3009.3 | 0.055 |
| 60 | 2971.4 | 3012.3 | |||
| D2O pD = 6.3 |
5 | 2968.1 | 0.038 | 3008.6 | 0.064 |
| 60 | 2970.2 | 3012.1 | |||
| D2O pD = 13.3 |
5 | 2923.1 | 0 | 2956.8 | 0 |
| 60 |
There is a significant temperature dependence of the symmetric and asymmetric CH2 stretching frequencies on pD. Temperature induced frequency shifts are larger at neutral pD than at high or low pD values. At pD = 13.3 the broad low frequency doublet is essentially temperature independent.
The stretching frequencies of gly methylene are affected by both the amine and carboxyl groups. In order to characterize the impact of the amine and carboxylic groups separately we investigated the CH2 stretching frequencies of 2,2,2-d3-ethylamine (CD3-CH2-NH2) and 3,3,3-d3-propionic acid (CD3-CH2-COOH) in D2O at different pD values and temperatures.
pD And temperature dependence of the CH2 stretching vibrations of CD3-CH2-COOD and CD3-CH2-ND2
pD Dependence
In CD3-CH2-ND2 and CD3-CH2-COOD, the CH2 is affected by changes in the ionization state of either the amine or carboxylic groups.
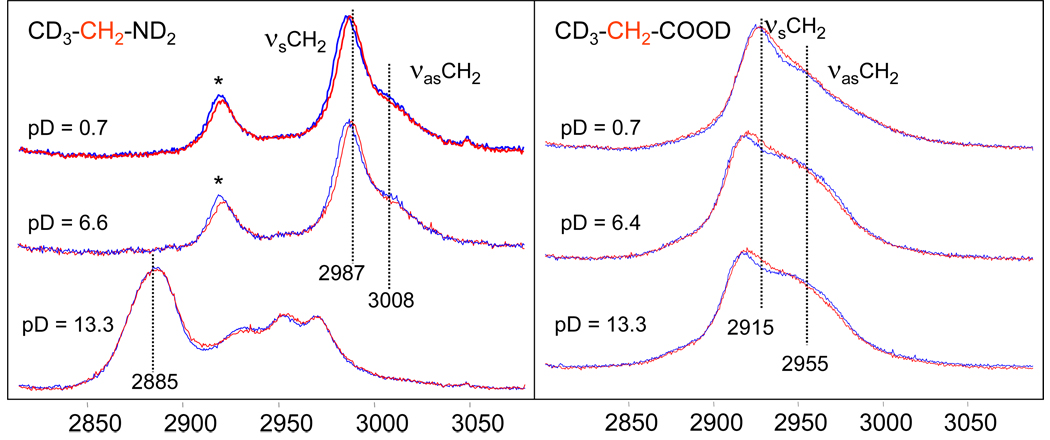
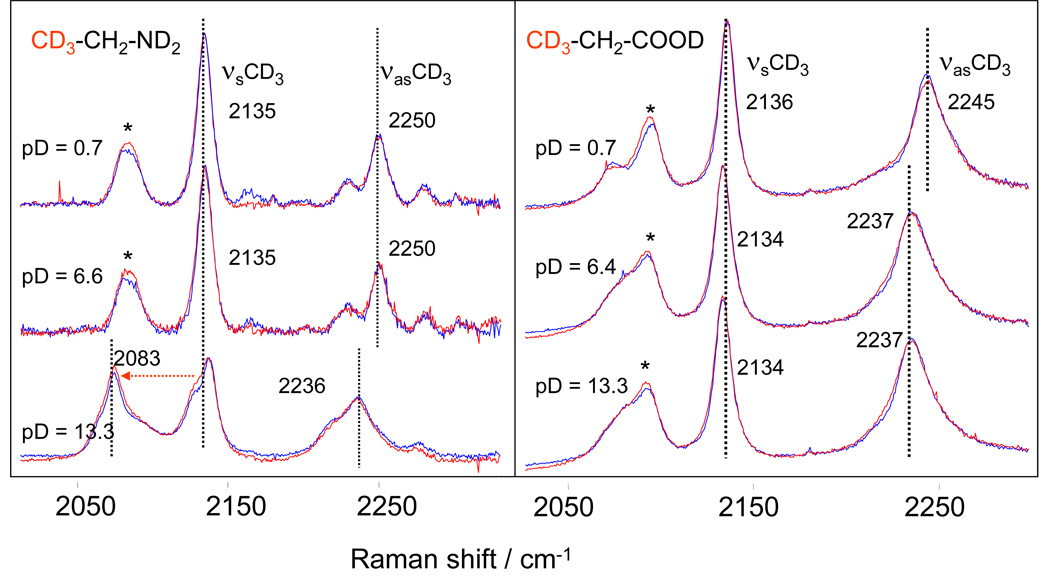
Figure 5 shows the CH2 stretching region of the Raman spectra of CD3-CH2-ND2 and CD3-CH2-COOD in D2O. At low and neutral pD values 2,2,2-d3-ethylamine is in its cationic form (CD3-CH2-ND3+). The νsCH2 and νasCH2 frequencies are 2987 cm−1 and 3008 cm−1, respectively. At high pD values 2,2,2-d3-ethylamine is in its neutral form (CD3-CH2-ND2) where the nitrogen possesses a lone pair of electrons. As in gly, the adjacent ND2 group significantly downshifts the CH2 stretching vibrations.
Figure 5.
CH2 stretching region of the Raman spectra of the 2,2,2-d3-ethyl amine and 3,3,3-d3-propionic acid in D2O at different pD values at 5°C (blue curve) and 60°C (red curve). Band marked with (*) at 2918 cm−1 in 2,2,2-d3-ethylamine is most likely due to Fermi resonance of the νsCH2 with the overtone of the CH2 scissoring. The splitting between symmetric and asymmetric CH2 stretches in CD3-CH2-ND3+ at pD = 0.7 and 6.6 is unusually small, ~ 19 cm−1 (an additional indication that νsCH2 is up-shifted due to Fermi resonance) In gly and propionic acid CH2 scissoring is at a significantly lower frequency than in ethylamine which removes the condition for Fermi resonance.
The Raman spectrum of the 2,2,2-d3-ethylamine at pD = 13.3 consists of four bands in the CH2 stretching region which are due to the free rotation about the C-N bond and presence of both gauche- and ant-i rotamers. The gauche form is slightly more stable (~ 0.3 kcal/mol) and gauche-anti rotational barrier is about 2 kcal/mol for the vapor phase.39 The assignment of the high pD spectrum will be given late in the discussion after getting additional information from theoretical calculations.
The 3,3,3-d3-propionic acid CH2 stretching vibrations (νsCH2 ~ 2915 cm−1 and νasCH2 ~ 2955 cm−1) show a weak frequency dependence on solution pD. At neutral and high pD, the anionic CD3-CH2-COO− CH2 stretching bands are somewhat broader than are those of the protonated species at low pD.
Temperature dependence
High pD neutral 2,2,2-d3-ethylamine (CD3-CH2-ND2) shows no CH2 stretching temperature dependence (figure 5). In the cationic form (at low and neutral pD values) the CH2 symmetric stretches upshift with temperature similar to that of the CH2 symmetric stretch of gly±, while the temperature shift of the CH2 asymmetric stretch is 2-fold smaller. Table 2 shows the CH2 stretching frequencies and their temperature dependence.
Table 2.
Temperature dependence of the CH2 νs and νas of 2,2,2-d3-ethylamine in D2O
| pD | T°C | νsCH2 / cm−1 | Δν/ΔT sym | νasCH2 / cm−1 | dν/dT asym |
|---|---|---|---|---|---|
| 0.7 | 5 | 2985.7 | 0.033 | 3006.3 | 0.033 |
| 60 | 2987.5 | 3008.3 | |||
| 6.6 | 5 | 2986.1 | 0.033 | 3007.5 | 0.022 |
| 60 | 2987.9 | 3008.7 |
The temperature induced CH2 stretching frequency shifts in 2,2,2-d3-ethylamine are similar to those of zwitterionic gly (compare Table 1 and Table 2). 3,3,3-d3-propionic acid, in contrast, shows little temperature dependence of the CH2 stretching frequencies for any solution pD values. Thus, we can conclude that the amine group dominates the temperature dependence of the CH2 stretching frequencies in gly.
pD and temperature dependence of the CD3 stretching frequency in CD3-CH2-COOD and CD3-CH2-ND2
Deuteration of the methyl group in CD3-CH2-ND2 and CD3-CH2-COOD separates the originally overlapping methylene and methyl stretching vibrations, allowing us to unambiguously observe these vibrations in different solution conditions.
Figure 6 shows the CD3 stretching region for 2,2,2-d3-ethylamine and 3,3,3-d3-propionic acid at different pD values and temperatures. For both compounds, the CD3 stretching frequencies depend on the carboxyl or amine ionization states.
Figure 6.
CD3 stretching region of the 2,2,2-d3-ethyl amine and 3,3,3-d3-propionic acid in D2O at different pD values at 5°C (blue curve) and 60°C (red curve). CD3 stretches show a clear dependence on the ionization state of the amine and carboxylic group even though the CD3 group is not directly linked to the carboxyl or amine groups. Frequencies of the νsCD3 and νasCD3 for both 2,2,2-d3-ethylamine and 3,3,3-d3-propionic acid do not show any temperature dependence at any pD values. Band marked by (*) is an overtone of CD3 asymmetric bending (2δasym CD3) enhanced due to Fermi resonance with intense CD3 symmetric stretch.40
At pD = 0.7 and pD = 6.6 the 2,2,2-d3-ethylamine cation (CD3-CH2-NH3+) shows νsCD3 frequencies of 2135 cm−1 and νasCD3 frequencies of 2250 cm−1 (Fig. 6). In contrast, at pD = 13.3 the νsCD3 vibration of neutral CD3-CH2-NH2 splits in two bands. One remains near ~ 2135 cm−1 while the other downshifts 52 cm−1 to 2083 cm−1. The νasCD3 of the neutral form downshifts ~ 14 cm−1 to 2236 cm−1 and broadens. For 3,3,3-d3-propionic acid the νasCD3 downshifts ~ 8 cm−1 (from 2245 cm−1 to 2237 cm−1) as the carboxylic acid (pD = 0.7) becomes a carboxylate anion (pD = 6.4 and 13.3).
From these data we conclude that the CD3 stretches in CD3-CH2-NH2 and CD3-CH2-COOD show the pD-induced frequency shifts of about the same magnitude as do the CH2 stretches, despite the fact that the CD3 group is not directly attached to the NH2 or COOH groups. It should be noted, however, that although the CD3 group is not directly bound to the amine or carboxyl group, it is very close to them; in their equilibrium conformations. The CD3 deuterium atoms are closer to the carbonyl oxygen or nitrogen lone pairs than are the CH2 group hydrogens.
In contrast to CH2 stretching vibrations the CD3 stretches show no temperature dependence for any pD value for both 2,2,2-d3-ethylamine and 3,3,3-d3-propionic acid. In both these compounds the CH2 group is directly linked to amine or carboxyl groups by a σ-bond while the CD3 group is only spatially close. This indicates that in order to show a CH2 stretching frequency temperature dependence the CH2 group must be directly linked to the amine or carboxyl group.
Theoretical calculations
The effect of carboxyl group orientation on the CH2 stretching frequencies
The experimental results indicate a dependence of the CH2 stretching frequencies on carboxyl group orientations. Although in real crystals the range of available conformations is limited because gly prefer to be in planar conformations where ξ is small,41 theoretical calculations allow us to explore a much broader range of conformations. Thus, we theoretically modeled the conformational dependence of CH2 vibrations frequencies upon the ξ angle for neutral gly. We fixed the orientation of the nitrogen lone pair with respect to the CH2 group to rule out any possible impact of the nitrogen lone pair orientation.
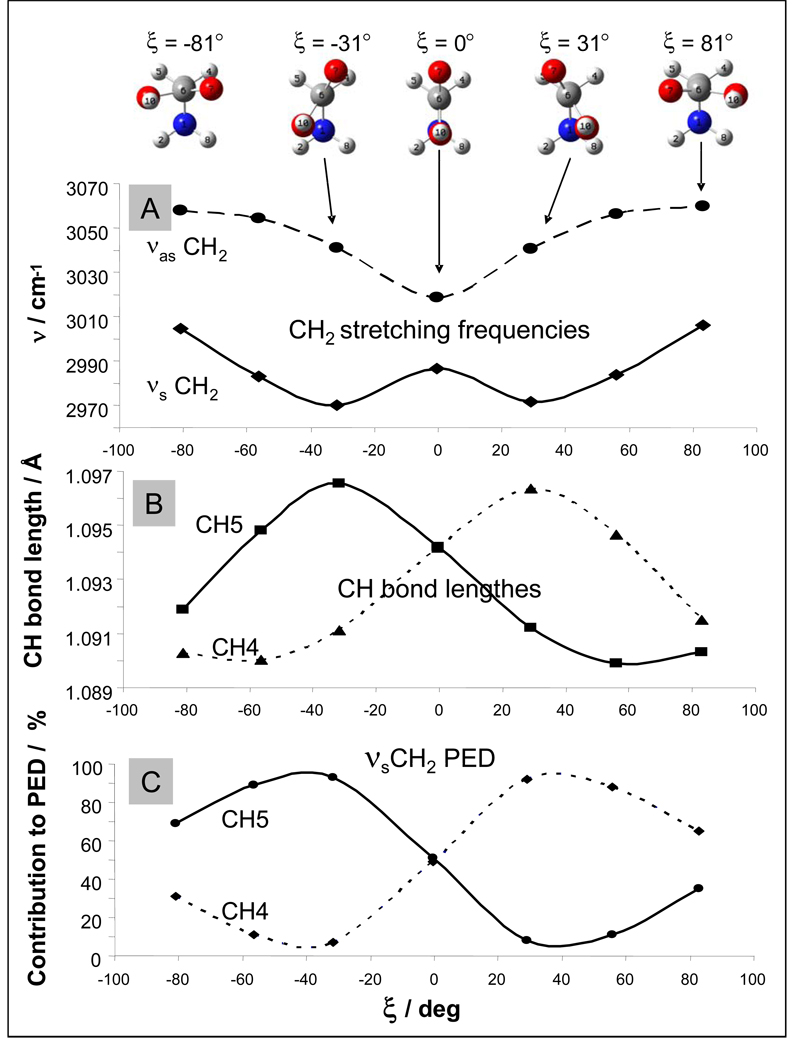
As expected from experiments, our theoretical results show that the νasCH2 and νsCH2 frequencies significantly depend on ξ (Fig. 7A), and these frequencies correlate with the C-H bond lengths (Fig 7B).
Figure 7.
Calculated ξ angular dependence of (A) gly CH2 stretching frequencies, (B) C-H bond lengths, and (C) normal mode composition (%) of CH2 symmetric stretch. Frequencies are scaled to 0.98.
For ξ = 0°the C-H bonds symmetrically arrange with respect to the carboxyl group plane and have identical bond lengths (Fig. 7B). This results in the minimum calculated frequency splitting between the νasCH2 and νsCH2 vibrations (Δ0°, calc = 33 cm−1). At ξ = ± 31° one of the C-H bonds is almost perpendicular to the carboxyl plane, while the other lies within the COO plane. These conformations have the maximal frequency splitting between the νs and νas CH2 stretching frequencies and the largest differences in C-H bond lengths. At ξ = ± 81° the νsCH2 and νasCH2 frequencies are at their maximum values, both C3-H4 and C3-H5 bonds have their shortest bond lengths and both lie almost within the COO plane (Fig. 7, top).
The normal mode composition indicates that the extent of coupling between the two C-H stretching vibrations depends on the difference in C-H bond lengths. For planar gly (ξ = 0°) with equivalent C-H bonds, both C-H stretching vibrations contribute equally to symmetrical and asymmetrical components of CH2 stretching (Fig. 7C). At ξ = ± 31°, where the C-H bond length difference is the largest, the coupling is smallest, and each calculated C-H stretching mode is an almost local vibration. The longer the C-H bond length, the more it contributes to the low-frequency symmetric vibration and vice versa.
Our experimental data and our theoretical modeling show that the proximity of the C-H bond to the carboxyl group oxygen results in significant upshift of the corresponding CH stretching frequencies due to C-H bond shortening.
As shown above, the carboxyl group orientation affects the C-H bond length. To study the carboxyl group orientation effect on the gly electronic structure, we calculated the AIM charge distributions for the ξ = 0°, ξ = 31°, ξ = 81° gly conformers. The calculated charge distributions indicate that the C-H bond length decrease results from transfer of electronic density from the carboxyl group oxygen to the nearest methylene hydrogen which decreases the oxygen negative charge and decreases the hydrogen positive charge. The magnitude of this effect depends on the distance between the C=O and C-H bonds. At ξ = 0°, where both hydrogens are equidistant from the COOH plane, the two methylene hydrogens have equal charge (Table 3). At ξ = 81° both hydrogens are closer to the COOH plane than in the ξ = 0° conformer.
Table 3.
AIM charge distributions for selected ξ conformations
| ξ = 0° | ξ = 81° | ξ = 31° | |
|---|---|---|---|
| N1 | −1.014 | −1.001 | −1.012 |
| H2 | 0.366 | 0.368 | 0.369 |
| C3 | 0.365 | 0.367 | 0.366 |
| H4 | 0.052 | 0.044 | 0.047 |
| H5 | 0.052 | 0.041 | 0.051 |
| C6 | 1.503 | 1.493 | 1.501 |
| O7 | −1.208 | −1.199 | −1.203 |
| H8 | 0.366 | 0.370 | 0.365 |
| O9 | −1.122 | −1.121 | −1.124 |
| H10 | 0.641 | 0.064 | 0.641 |
Consequently, the hydrogens will have similar, less positive charges than for the ξ = 0° conformer. In addition, the negative charges on both oxygens are decreased compared to the ξ = 0° conformer. In the ξ = 31° conformer, the hydrogen atom of the C-H bond closest to the COOH plane has less positive charge than does the other, while the negative charge on the oxygen decreases.
Theoretical modeling of the pD dependence of CH2 stretching frequency. Effect of the amine and carboxyl ionization states and orientations
We calculated the geometry, electronic structure properties, vibrational frequencies and normal mode compositions for a series of gly conformers in neutral and zwitterionic forms in solution. Both neutral and zwitterionic forms were calculated in an implicit solvent with the dielectric constant of water modeled by PCM. PCM was employed to both stabilize the zwitterion which is unstable in gas phase calculations, and to account for the macroscopic effects of water. We calculated neutral gly.
Amine group effect
The nitrogen lone pair impact on the C-H stretching frequencies of organic compounds has been referred to as either the "trans effect of lone pair", “negative hyperconjugation” or “the Bohlmann effect”.16,42 C-H bonds within the same CH3 or CH2 linked to an atom carrying a lone pair of electrons often have different lengths.43 Some C-H bonds are substantially longer which results in large frequency downshifts (up to 150 cm−1). This phenomenon is especially prominent in cases with nitrogen or oxygen lone pair electrons. The specific influence of the lone pair is confirmed by the disappearance of C-H bond lengthening when the lone pair was removed.
It is generally agreed that the trans-C-H bond weakening occurs because of partial transfer of the lone pair electrons to the vacant σ* orbital of the C-H bond.44 The stretching frequency of the C-H bond trans to the lone pair is significantly decreased compared to that of the gauche C-H bond.45,46 It should be noted that “lone pair trans effect” is quite general, also occurring for OH, NH, etc.46
Transfer of electronic density to a σ* orbital is expected to be particularly favored when the acceptor orbital presents a smooth, nodeless character in the region of the donor orbital as do σ*C-H orbitals. The best donor orbital for such interaction would be a diffuse lone pair nonbonding orbital, such as the nitrogen lone pair. From the shapes of these orbitals, simple consideration suggests that the n-σ* interaction is optimized in a linear “end-on” arrangement. Thus, the strongest n-σ* interactions would occur for the C-H bond trans to the lone pair, since this particular orientation gives a linear arrangement of the nitrogen lone pair relative to the C-H antibonding orbital (Fig. 1).
We examined the effect of the nitrogen lone pair on two conformations of neutral gly with different orientations of the CH2 group relative to the NH2 group (Fig. 1a). In anti-glyneut the nitrogen lone pair is gauche to both C-H bonds, where both C-H bonds are approximately equidistant from the lone pair. In contrast, in gauche-glyneut one of C-H bond (C3-H4) is gauche to the nitrogen lone pair, while the other (C3-H5) is trans. In zwitterionic gly such orientations are not differentiated because of the lack of the nitrogen lone pair.
For neutral gly our calculations show that anti-glyneut has equivalent C-H bonds, while in gauche-glyneut the C3-H5 bond trans to the nitrogen sp3 lone pair orbital is significantly elongated, while the other bond is slightly contracted compared to anti-glyneut (Table 4). In zwitterionic gly both C-H bonds have equal length. The zwitterionic C-H bonds shorten because of the proximity of the positively charged NH3+ group.
Table 4.
Calculated C-H bond lengths, C-H stretching frequencies†, normal mode compositions, and AIM charge distributions for neutral, zwitterionic gly conformer, and the hydrogen bonded zwitterionic gly-water conformers
| glyneut | gly± | gly±-Water Complexes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| anti-glyneut | gauche-glyneut | eclipsed-gly± | staggered-gly± | twisted-gly± | eclipsed-gly± | eclipsed-gly± | eclipsed-gly± | ||
| l(C-H), Å | C-H4 | 1.096 | 1.095 | 1.090 | 1.091 | 1.089 | 1.091 | 1.090 | 1.090 |
| C-H5 | 1.096 | 1.101 | 1.090 | 1.091 | 1.091 | 1.090 | 1.090 | 1.091 | |
| Freq, cm−1* | νsCH2 | 2962 | 2902 | 3015 | 3007 | 3008 | 3011 | 3017 | 3012 |
| νasCH2 | 2995 | 2983 | 3069 | 3063 | 3074 | 3063 | 3073 | 3065 | |
| PED, % | νsCH2 | C-H5 s (51) C-H4 s (48) |
C-H5 s (97) C-H4 s (3) |
C-H4 s (50) C-H5 s (48) |
C-H5 s (50) C-H4 s (49) |
C-H5 s (67) C-H4 s (33) |
C-H4 s (55) C-H5 s (44) |
C-H4 s (52) C-H5 s (47) |
C-H4 s (56) C-H5 s (44) |
| νasCH2 | C-H4 s (51) C-H5 s (48) |
C-H4 s (97) C-H5 s (3) |
C-H5 s (51) C-H4 s (49) |
C-H4 s (50) C-H5 s (49) |
C-H4 s (66) C-H5 s (33) |
C-H5 s(55) C-H4 s (44) |
C-H5 s(52) C-H4 s (48) |
C-H5 s(56) C-H4 s (44) |
|
| AIM Charges | N1 | −1.013 | −1.003 | −0.980 | −0.962 | −0.959 | −1.010 | −0.979 | −1.010 |
| H2 | 0.364 | 0.364 | 0.468 | 0.473 | 0.473 | 0.455 | 0.469 | 0.504 | |
| C3 | 0.359 | 0.348 | 0.244 | 0.249 | 0.261 | 0.250 | 0.247 | 0.253 | |
| H4 | 0.052 | 0.055 | 0.083 | 0.080 | 0.070 | 0.073 | 0.086 | 0.079 | |
| H5 | 0.052 | 0.034 | 0.083 | 0.080 | 0.076 | 0.076 | 0.086 | 0.076 | |
| C6 | 1.508 | 1.523 | 1.654 | 1.660 | 1.647 | 1.648 | 1.655 | 1.649 | |
| O7 | −1.204 | −1.204 | −1.257 | −1.263 | −1.254 | −1.261 | −1.235 | −1.239 | |
| H8 | 0.364 | 0.361 | 0.482 | 0.472 | 0.473 | 0.469 | 0.481 | 0.468 | |
| O9 | −1.134 | −1.128 | −1.241 | −1.256 | −1.257 | −1.246 | −1.231 | −1.236 | |
| H10 | 0.642 | 0.641 | 0.468 | 0.469 | 0.473 | 0.502 | 0.470 | 0.456 | |
Frequencies are scaled by 0.98
The C-H stretching frequencies differ between anti-glyneut and gauche-glyneut. In anti-glyneut the νsCH2 is calculated at 2962 cm−1, while in gauche-glyneut this vibration downshifts by 60 cm−1 compared to that in anti-glyneut. The frequency splitting between the symmetrical and asymmetrical components of CH2 also differs in these structures. In anti-glyneut, where both C-H bonds are equal, the split is 33 cm−1, while in gauche-glyneut the split is a much larger 81 cm−1 (Table 4). Our Raman spectra of gly at pD=13.3 shows a 34 cm−1 spliting between the two CH2 stretching peaks which agrees well with the frequencies calculated for anti-glyneut. This suggests that at high pH gly exists mainly in the anti-glyneut conformation. However, the breadth of both CH2 stretching bands indicates the existence of additional conformations in solution.
C-H bond length changes impact the coupling between the CH2 stretching vibrations (Table 4). In anti-glyneut both C-H bonds contribute equally to the asymmetric and symmetric components. In contrast, in gauche-glyneut the two C-H vibrations are uncoupled. The high frequency vibration is almost a pure stretch of the shorter C-H bond, while the low-frequency band is almost a pure stretch of the longer C-H bond.
In zwitterion gly the C-H bonds have the same length, but are shorter than in anti-glyneut. Consequently, both symmetric and asymmetric stretches of CH2 occur at higher frequencies (Table 4) The zwitterion calculated CH2 54 cm−1 frequency splitting is larger than for the anti-glyneut conformer. We also observe a larger frequency splitting of the CH2 stretching vibrations at lower pD than at higher pD.
The charge distribution on the CH2 group (Table 4) indicates that in the zwitterion each C-H bond is more polar than in the neutral form. This increased bond polarity results from the nitrogen negative inductive effect, which shortens the zwitterionic C-H bonds. In anti-glyneut, the C-H bond lengths and charges on both methylene hydrogens are equal. In gauche-glyneut, different bond lengths and charge distributions occur. The longest C-H bond hydrogen atom is less positive than the other, whereas the positive charge is almost equal to that in anti-glyneut. In gauche-glyneut the decreased hydrogen positive charge indicates an increased electronic density transferred from the nitrogen lone pair, since the nitrogen negative charge is decreased compared to that in anti-glyneut. The correlation of an increased electronic density with a bond length elongation indicates that the electronic density was accepted by an orbital with non- or anti-bonding character; the NBO analysis shows an increased occupancy of the C-H σ* MO (Table 5). This interaction elongates the corresponding C-H bond resulting in a downshift of the C-H stretching frequency in the gly anion compared to the zwitterion or cation, where the lone pair is removed by protonation. This explains the strong dependence of C-H stretching frequencies on the ionization state of the gly amino group.
Table 5.
NBO calculated occupancies of selected molecular orbitals of methylene, amino, and carboxyl groups in neutral and zwitterionic gly conformers
| glyneut | gly± | gly±-Water Complexes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| anti-glyneut |
gauche- glyneut |
eclipsed-gly± |
staggered- gly± |
twisted-gly± ξ=60° |
eclipsed-gly± NH3 H2O |
eclipsed-gly± COO− H2O |
eclipsed gly± NH3 H2O, COO− H2O |
|||
| MO Occupancy | σ | C-H4 | 1.964 | 1.963 | 1.978 | 1.976 | 1.985 | 1.978 | 1.978 | 1.978 |
| C-H5 | 1.964 | 1.969 | 1.978 | 1.976 | 1.979 | 1.978 | 1.978 | 1.978 | ||
| σ* | C-H4 | 0.0157 | 0.0139 | 0.0083 | 0.0096 | 0.0107 | 0.0094 | 0.0083 | 0.0084 | |
| C-H5 | 0.0157 | 0.0287 | 0.0083 | 0.0096 | 0.0096 | 0.0085 | 0.0083 | 0.0094 | ||
| LP N | 1.950 | 1.958 | N/A | |||||||
| COO− | LP2 O7 | 1.863 | 1.864 | 1.884 | 1.885 | 1.897 | 1.886 | 1.874 | 1.875 | |
| LP3 O9 | N/A | 1.631 | 1.616 | 1.613 | 1.629 | 1.825 | 1.612 | |||
| LP2 O9 | 1.799 | 1.797 | 1.872 | 1.892 | 1.893 | 1.876 | 1.869 | 1.873 | ||
| BD CO7 | 1.996 | 1.996 | 1.996 | 1.996 | 1.988 | 1.996 | 1.832 | 1.993 | ||
| NH3+ | BD N-H2 | N/A | 1.994 | 1.993 | 1.993 | 1.994 | 1.994 | 1.993 | ||
| BD N-H8 | N/A | 1.993 | 1.994 | 1.994 | 1.993 | 1.993 | 1.993 | |||
| BD N-H10 | N/A | 1.994 | 1.994 | 1.993 | 1.993 | 1.994 | 1.994 | |||
The effect of carboxyl group in zwitterionic gly
Comparison of the solution gly Raman spectra measured at pD = 0.7 (cationic form) and pD = 6.3 (zwitterionic form) show that the carboxyl group ionization does not significantly affect the CH stretching vibrations. The frequency shift of both C-H stretching vibrations does not exceed 3 cm−1 (see Table 1). The charge distribution calculated for the ξ = 0° (staggered) and ξ = − 60° (twisted) conformations of gly zwitterion (Table 4) indicates that the C-H bond length decrease is caused by redistribution of electronic density from the C=O bond lying in the same plane with the C-H bond. Analysis of the molecular orbital occupancy (Table 5) shows a significantly increased occupancy of the bonding orbital of the short C3-H4 bond and a decreased O7 lone pair molecular orbital occupancy. The 0.002 Ǻ bond length difference between the C-H bonds of twisted-gly± prevents coupling between the two CH stretches. PED indicates that two CH stretching frequencies calculated for twisted-gly± result from almost pure vibrations of the individual C-H bonds, while in staggered-gly± the CH stretching motions are coupled into symmetric and asymmetric vibrations (Table 4). In twisted-gly± the C-H bond contraction results in an 11 cm−1 upshift of the high-frequency CH stretch, while the low-frequency CH stretch is equal to that in staggered-gly±.
Temperature dependence of the CH2 and CD3 stretches
As shown above the electronic configuration of the nitrogen atom and spatial orientation of the carboxyl group have a large impact on the neighboring CH2 bond lengths and stretching frequencies. Hydrogen bonding to -NH3+/-NH2 and -COOH/-COO− of gly affects the electronic configurations of these groups which, in turn, change the CH2 bond strengths, which shift the CH2 stretching frequencies.
To examine the effect of water hydrogen bonding on the C-H frequencies, we calculated the geometries and electronic properties of gly zwitterion (ξ = 0°), the zwitterion with water attached to the NH3+ group and the zwitterion with water attached to the COO− site. We also examined gly zwitterion with waters attached to both NH3+ and COO−.
Water hydrogen bonding to the gly -COO− terminus does not change the C-H bond lengths significantly. However, it does result in a slight frequency increase of both the symmetric (2 cm−1) and asymmetric (4 cm−1) C-H stretching vibrations (Table 4). Hydrogen bonding to the NH3+ site elongates both C-H bonds more significantly. As a result, the frequencies of the symmetric and asymmetric C-H stretching vibrations downshift by 4 and 6 cm−1 respectively. Two waters added to COO− and NH3+ groups results in an increase in C-H bond lengths and frequency downshifts of the symmetric (4 cm−1) and asymmetric (6 cm−1) stretches.
The MO occupancy listed in Table 5 shows that the water hydrogen bonded to -NH3+ decreases the electronic density of the N-H bonding orbitals and increases the electronic density of the C-H σ* orbitals. In contrast, water hydrogen bonding to COO− did not significantly change the C-H bond electronic density (Table 4).
An additional important insight into the origin of the CH2 stretching temperature induced frequency shifts is evident from the CD3 stretching frequencies of CD3-CH2-ND2 and CD3-CH2-COOD. As shown above, the CD3 group, which is not directly connected to the amine or carboxyl, does not show any temperature induced frequency shifts but does show a significant dependence on the amine and carboxyl group ionization state. Thus, we conclude that the temperature dependence results from some inductive interaction through σ-bonds.
Our calculations show that water hydrogen bonding to the donor (-NH3+) group downshifts CH2 stretching frequencies while water hydrogen bonding to the acceptor group (-COO−) upshifts CH2 stretches. The deprotonated amine (-NH2) and the protonated carboxyl (-COOH) can serve as both hydrogen bond donors and acceptors. Thus, the temperature induced frequency shift of the CH2 stretches of gly in solution depends on interplay of different hydrogen bonding patterns. However, our experimental and theoretical results show that at the pH values close to neutral, hydrogen bonding to the gly -NH3+ group has dominant effect on the CH2 stretching frequencies.
CH2 stretching frequency splitting monitors gly non-planarity
The splitting between νsCH2 and νasCH2 stretching vibrational frequencies should depend less on environment than the frequencies themselves because the splitting depends mostly on C-H bond nonequivalence which depends upon the gly conformation. The actual doublet frequency can depend on the dielectric constant of the medium, hydrogen bonding to the amine/carboxyl groups etc. Such environmental factors will likely shift the frequencies of the symmetric and asymmetric stretches in the same direction without significantly changing the splitting between them. This makes the splitting a more reliable criterion for gly conformational analysis.
For the planar Gly•HCl molecules the calculated and observed splittings between the νasCH2 and νsCH2 vibrations are essentially identical at Δ ~ 33 cm−1. For gly nitrate, Gly•HNO3, the ξ ~ 21° deviation from planarity results in ΔGly•HNO3 cryst = 62 cm−1 (Fig. 3), while the calculated ξ = 21° splitting is Δ21°, calc ~ 56 cm−1. For TGS, the splitting between νasCH2 and νsCH2 of the more intense doublet (2982 cm−1 and 3017 cm−1) corresponding to almost planar gly pair is ΔTGS, planar ~ 35 cm−1, which again is very close to the calculated value. In contrast for bent gly the observed value is ΔTGS, bent ~ 45 cm−1 compared to a calculated Δξ = 19°, calculated = 53 cm−1. Thus, the splitting between the νasCH2 and νsCH2 stretching frequencies definitely depends upon the relative orientations of the CH2 and carboxylic groups. The small deviations between calculated and measured CH2 frequency splitting values in crystals may result from crystal packing forces which distort the gly molecules.
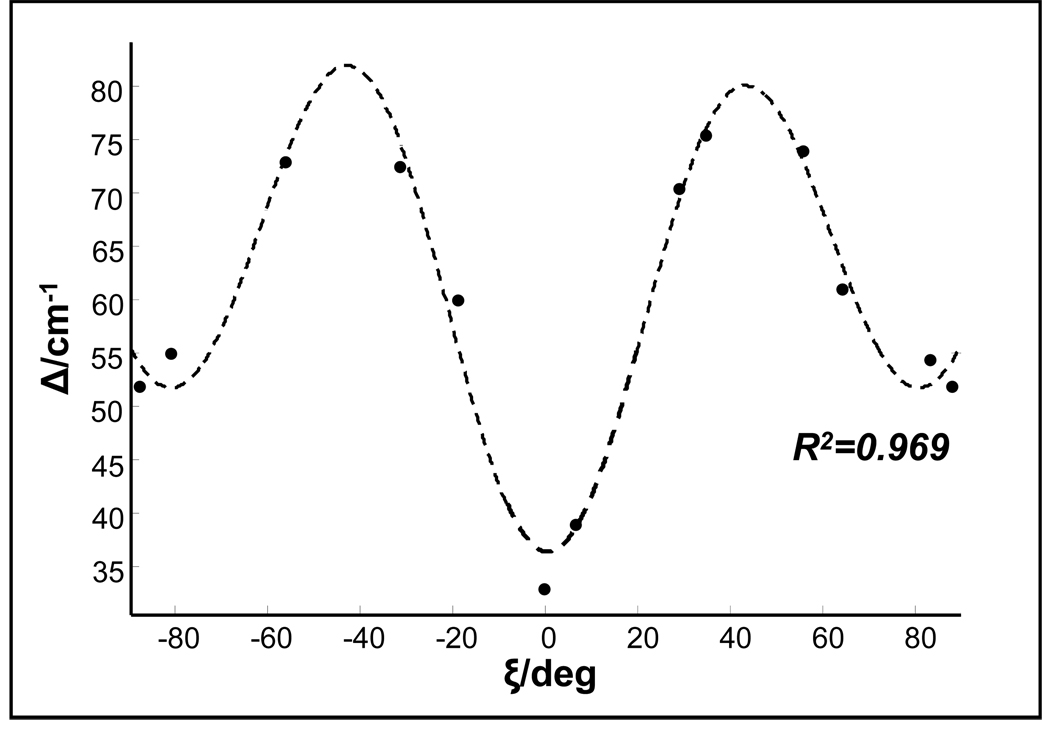
As we have shown both experimentally (Fig. 2) and theoretically (Fig. 7A), the frequency splitting between symmetrical and asymmetrical components of CH2 stretching depends on the value of ξ angle. Therefore, the frequency splitting (Δ) measured experimentally can be used to determine the ξ angle defined by the gly conformation. Figure 8 shows the frequency splitting calculated for gly conformers versus the ξ angle.
Figure 2.
Raman spectra of the CH stretching region of the crystalline gly hydrochloride (Gly•HCl), crystalline gly nitrate (Gly•HNO3), crystalline trigly sulphate or TGS (3Gly•H2SO4).
Figure 8.
Calculated ξ angular dependence of frequency splitting of the gly CH2 stretching. The dots represent calculated data points, the dashed line is the Fourier fit to the calculated results. Δ(ξ) = 60 − 10.6cos(0.04ξ) − 4.3sin(0.04ξ) − 21.3cos(0.08ξ) − 3.6sin(0.08ξ)
This correlation describes the frequency splitting change which results from the non-planarity of gly molecule, when the amide group is protonated and the nitrogen lone pair does not affect the CH2 stretching frequencies.
Conformational preferences of gly in solution
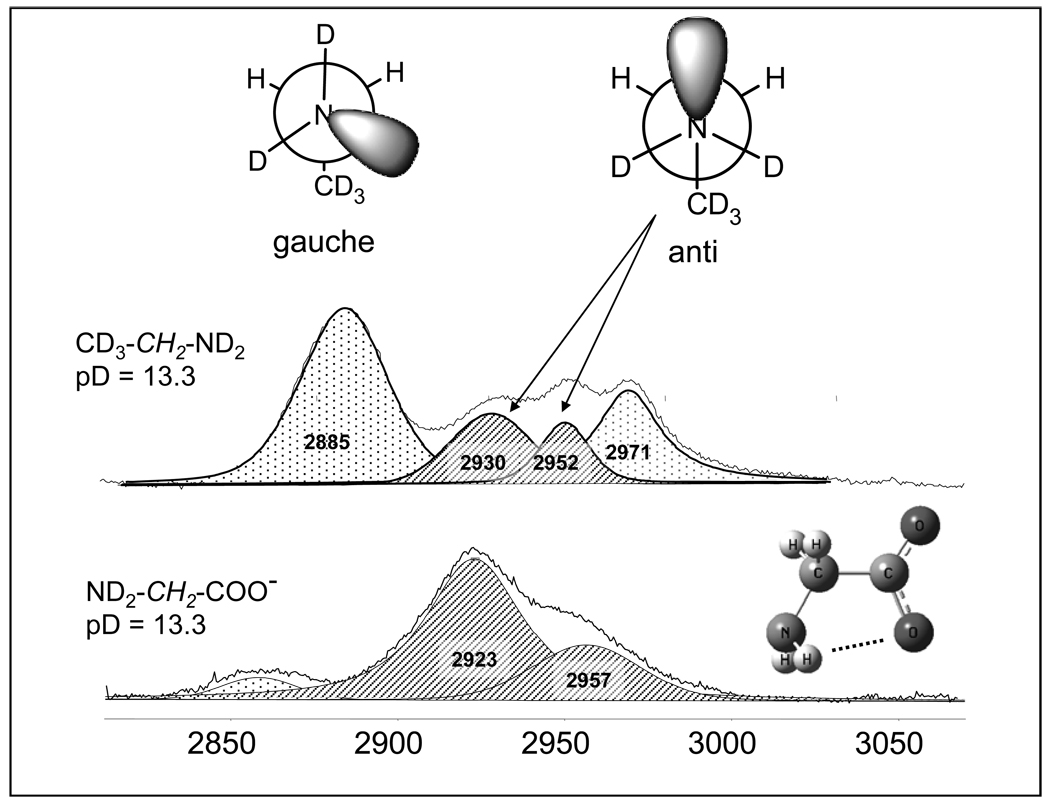
Figure 9 shows Raman spectra of 2,2,2-d3-ethylamine and gly in solution at pD = 13.3. Two staggered conformations – gauche and anti are populated in solution at room temperature which differ by a rotation about the C-N bond in ethylamine. In the gauche conformation the nitrogen lone pair is trans to a C-H bond which results in elongation of the trans C-H bond due to electron delocalization from the nitrogen lone pair to the C-H anti-bonding σ* orbital which significantly downshifts the C-H stretching frequency by ~ 100 cm−1 to ~2885 cm−1. The other C-H bond is slightly upshifted. Thus, the two most intense, most separated bands at ~2885 cm−1 and ~2971 cm−1 correspond to the uncoupled CH stretches of the gauche conformation of ethylamine. Interaction of the C-H bonds with the lone pair in the anti conformer modestly downshifts both CH stretching vibrations without increasing the frequency splitting between the symmetric and asymmetric components at ~ 2930 cm−1 and 2952 cm−1 are νsCH2 and νasCH2 of anti-ethylamine. Assuming that the areas of the bands are proportional to the population of the ethylamine rotamers in D2O allows us to calculate that the population of gauche conformers is ~ 71 % and the anti conformer is ~ 29 %. This distribution agrees well with that for n-propylamine in the gas phase as determined from the NH2 wagging and torsion bands in the IR spectra.47
Figure 9.
Fitted Raman spectrum (CH2 stretching region) of the CD3-CH2-ND2 and ND2-CH2-COO− solutions in D2O at pD = 13.3. Also shown are the two conformations of the ethylamine and for gly− in solution.
In contrast to ethylamine, gly− shows only two bands at ~ 2923 cm−1 (νsCH2) and 2957 cm−1 (νasCH2), with frequencies similar to those for the anti conformation of 2,2,2-d3-ethylamine. This indicates that the anti-gly− conformer dominates high pD solutions. This anti-conformational preference of gly− in solution may result from bifurcated intramolecular hydrogen bonding between the amine group hydrogens and the carboxylic group oxygen (Fig. 9) Such a hydrogen bond was shown to stabilize gas phase gly conformations48,49 and gly molecules in inert gas matrices at low temperatures.50 Hyperconjugation could also contribute to the increased stability of the gly anti- conformation.51
Conclusions
We examined the dependence of the CH2 stretching frequencies of glycine, 2,2,2-d3-ethylamine and 3,3,3-d3-propionic acid on conformation, pD and temperature by means of Raman spectroscopy (488 nm excitation) and DFT calculations.
Experimental data show a large dependence of the CH2 stretching frequencies on the ionization state of the amine group. We theoretically demonstrate that the high sensitivity of the νsCH2 and νasCH2 frequencies on the orientation and ionization state of the amine group results from a negative hyperconjugation between the nitrogen lone pair and anti-bonding C-H orbitals. This effect is maximal when a C-H bond is trans to the lone pair.
The C-H stretching frequency dependence on the carboxyl group ionization state is small, however carboxyl group orientation affects the CH2 symmetric and asymmetric stretching frequencies as well as their frequency splitting. The magnitude of frequency splitting between the νsCH2 and νasCH2 depends on the relative orientation of the CH2 and COOH/COO− groups. The calculated conformational dependence of the CH2 stretches frequency splitting (νasCH2 − νsCH2) agrees well with the experimental data obtained from gly crystals.
According to our experimental observations and theoretical calculations, the temperature dependence of the νsCH2 and νasCH2 of gly in solution is due to the change in hydrogen bonding strength of the amine and carboxyl groups to water. This effect has an inductive mechanism and occurs only for the CH2 directly connected to amine/carboxyl groups by a σ-bond. The magnitude of the frequency shifts varies with the ionization states of amine/carboxyl groups. At pD values close to neutral, hydrogen bonding to the protonated amine group (-NH3+) is likely to dominate temperature dependence of the CH2 stretches of gly.
CD3 stretching of the deuterated methyl of 2,2,2-d3-ethylamine and 3,3,3-d3-propionic acid show a significant dependence on the ionization state of the amine and carboxylic groups even though the CD3 group is separated by a CH2 group. It appears that the delocalization of the electron density from the amine and carboxyl groups to the CH/CD bonds occurs “through space” and requires interacting groups to be spatially close, but not necessarily linked by a covalent bond. Our results indicate that in D2O at high pD gly is predominantly in an anti conformation in contrast to 2,2,2-d3-ethylamine which exists in both anti and gauche conformers.
Acknowledgment
This work was supported by NIH Grant GM8RO1EB002053.
References
- 1.Huang C-Y, Getahun Z, Wang T, DeGrado WF, Gai F. Journal of the American Chemical Society. 2001;123:12111–12112. doi: 10.1021/ja016631q. [DOI] [PubMed] [Google Scholar]
- 2.Hamm P, Hochstrasser RM. Practical Spectroscopy. 2001;26:273–347. [Google Scholar]
- 3.Lednev IK, Karnoup AS, Sparrow MC, Asher SA. Journal of the American Chemical Society. 2001;123:2388–2392. doi: 10.1021/ja003381p. [DOI] [PubMed] [Google Scholar]
- 4.Mikhonin AV, Asher SA, Bykov SV, Murza A. Journal of Physical Chemistry B. 2007;111:3280–3292. doi: 10.1021/jp0654009. [DOI] [PubMed] [Google Scholar]
- 5.Shi Z, Chen K, Liu Z, Kallenbach NR. Chemical Reviews (Washington, DC, United States) 2006;106:1877–1897. doi: 10.1021/cr040433a. [DOI] [PubMed] [Google Scholar]
- 6.Brewer SH, Song B, Raleigh DP, Dyer RB. Biochemistry. 2007;46:3279–3285. doi: 10.1021/bi602372y. [DOI] [PubMed] [Google Scholar]
- 7.Chi Z, Chen XG, Holtz JSW, Asher SA. Biochemistry. 1998;37:2854–2864. doi: 10.1021/bi971160z. [DOI] [PubMed] [Google Scholar]
- 8.Mikhonin AV, Bykov SV, Myshakina NS, Asher SA. Journal of Physical Chemistry B. 2006;110:1928–1943. doi: 10.1021/jp054593h. [DOI] [PubMed] [Google Scholar]
- 9.Mirkin NG, Krimm S. Journal of Physical Chemistry A. 2004;108:10923–10924. [Google Scholar]
- 10.Mirkin NG, Krimm S. Journal of Physical Chemistry A. 2007;111:5300–5303. doi: 10.1021/jp070852m. [DOI] [PubMed] [Google Scholar]
- 11.McKean DC. Journal of Molecular Structure. 1976;34:181–185. [Google Scholar]
- 12.McKean DC, Duncan JL, Batt L. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 1973;29:1037–1049. [Google Scholar]
- 13.Ohno K, Nomura S-I, Yoshida H, Matsuura H. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 1999;55A:2231–2246. [Google Scholar]
- 14.Longhi G, Zerbi G, Paterlini G, Ricard L, Abbate S. Carbohydrate Research. 1987;161:1–22. [Google Scholar]
- 15.Thomas HD, Chen K, Allinger NL. Journal of the American Chemical Society. 1994;116:5887–5897. [Google Scholar]
- 16.McKean DC. Chemical Society Reviews. 1978;7:399–422. [Google Scholar]
- 17.Bellamy LJ. Advances in Infrared Group Frequencies, Vol. 2: The Infrared Spectra of Complex Molecules. (2nd Ed) 1980 [Google Scholar]
- 18.Krieger F, Moeglich A, Kiefhaber T. Journal of the American Chemical Society. 2005;127:3346–3352. doi: 10.1021/ja042798i. [DOI] [PubMed] [Google Scholar]
- 19.Vertessy BG. Proteins: Structure, Function, and Genetics. 1997;28:568–579. [PubMed] [Google Scholar]
- 20.Kohn W, Sham LJ. Physical Review. 1965;137:1697–1705. [Google Scholar]
- 21.Parr RG, Yang W. Density-functional theory of atoms and molecules. Oxford: Oxford Univ. Press; 1989. [Google Scholar]
- 22.Hohenberg P, Kohn W. Phys. Rev. 1964;136:B864. [Google Scholar]
- 23.Becke AD. Journal of Chemical Physics. 1993;98:5648–5652. [Google Scholar]
- 24.Lee C, Yang W, Parr RG. Physical Review B: Condensed Matter and Materials Physics. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 25.Miehlich B, Savin A, Stoll H, Preuss H. Chemical Physics Letters. 1989;157:200–206. [Google Scholar]
- 26.Irikura KK, Johnson RD, III, Kacker RN. Journal of Physical Chemistry A. 2005;109:8430–8437. doi: 10.1021/jp052793n. [DOI] [PubMed] [Google Scholar]
- 27.Halls MD, Velkovski J, Schlegel HB. Theoretical Chemistry Accounts. 2001;105:413–421. [Google Scholar]
- 28.Miertus S, Scrocco E, Tomasi J. Chemical Physics. 1981;55:117–129. [Google Scholar]
- 29.Cossi M, Barone V, Mennucci B, Tomasi J. Chemical Physics Letters. 1998;286:253–260. [Google Scholar]
- 30.Mennucci B, Tomasi J. Journal of Chemical Physics. 1997;106:5151–5158. [Google Scholar]
- 31.Bader RFW. Atoms in Molecules: A Quantum Theory. Oxford: Oxford Univ. Press; 1990. [Google Scholar]
- 32.Cioslowski J, Mixon ST. Journal of the American Chemical Society. 1991;113:4142–4145. [Google Scholar]
- 33.Cioslowski J. Chemical Physics Letters. 1992;194:73–78. [Google Scholar]
- 34.Frisch MJT, G W, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Revision C.01 ed. Wallingford CT: Gaussian, Inc.; 2004. [Google Scholar]
- 35.Martin JML, Alsenoy CV. Antwerpen: University of Antwerpen; 1995. [Google Scholar]
- 36.Al-Karaghouli AR, Cole FE, Lehmann MS, Miskell CF, Verbist JJ, Koetzle TF. Journal of Chemical Physics. 1975;63:1360–1366. [Google Scholar]
- 37.Narayanan P, Venkataraman S. Journal of Crystal and Molecular Structure. 1975;5:15–26. [Google Scholar]
- 38.Itoh K, Mitsui T. Ferroelectrics. 1973;5:235–251. [Google Scholar]
- 39.Manocha AS, Tuazon EC, Fateley WG. Journal of Physical Chemistry. 1974;78:803–807. [Google Scholar]
- 40.Robertson AHJ, McQuillan GP, McKean DC. Journal of the Chemical Society, Dalton Transactions: Inorganic Chemistry. 1995:3941–3954. [Google Scholar]
- 41.Ho BK, Brasseur R. BMC Structural Biology. 2005;5 doi: 10.1186/1472-6807-5-14. No pp. given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lii J-H, Chen K-H, Allinger NL. Journal of Physical Chemistry A. 2004;108:3006–3015. [Google Scholar]
- 43.Henbest HB, Meakins GD, Nicholls B, Wagland AA. J. Chem. Soc. 1957:1462–1464. [Google Scholar]
- 44.Hamlow HP, Okuda S, Nakagawa N. Tetrahedron Letters. 1964:2553–2559. [Google Scholar]
- 45.McKean DC, Ellis IA. Journal of Molecular Structure. 1975;29:81–96. [Google Scholar]
- 46.Bellamy LJ, Mayo DW. Journal of Physical Chemistry. 1976;80:1217–1220. [Google Scholar]
- 47.Sato N, Hamada Y, Tsuboi M. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 1987;43A:943–954. [Google Scholar]
- 48.Hu CH, Shen M, Schaefer HF., III Journal of the American Chemical Society. 1993;115:2923–2929. [Google Scholar]
- 49.Jensen JH, Gordon MS. Journal of the American Chemical Society. 1991;113:7917–7924. [Google Scholar]
- 50.Ivanov AY, Sheina G, Blagoi YP. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 1999;55A:219–228. doi: 10.1016/s1386-1425(02)00416-x. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Pu X, Zheng W, Wong N-B, Tian A. Chemical Physics Letters. 2003;370:147–153. [Google Scholar]