Abstract
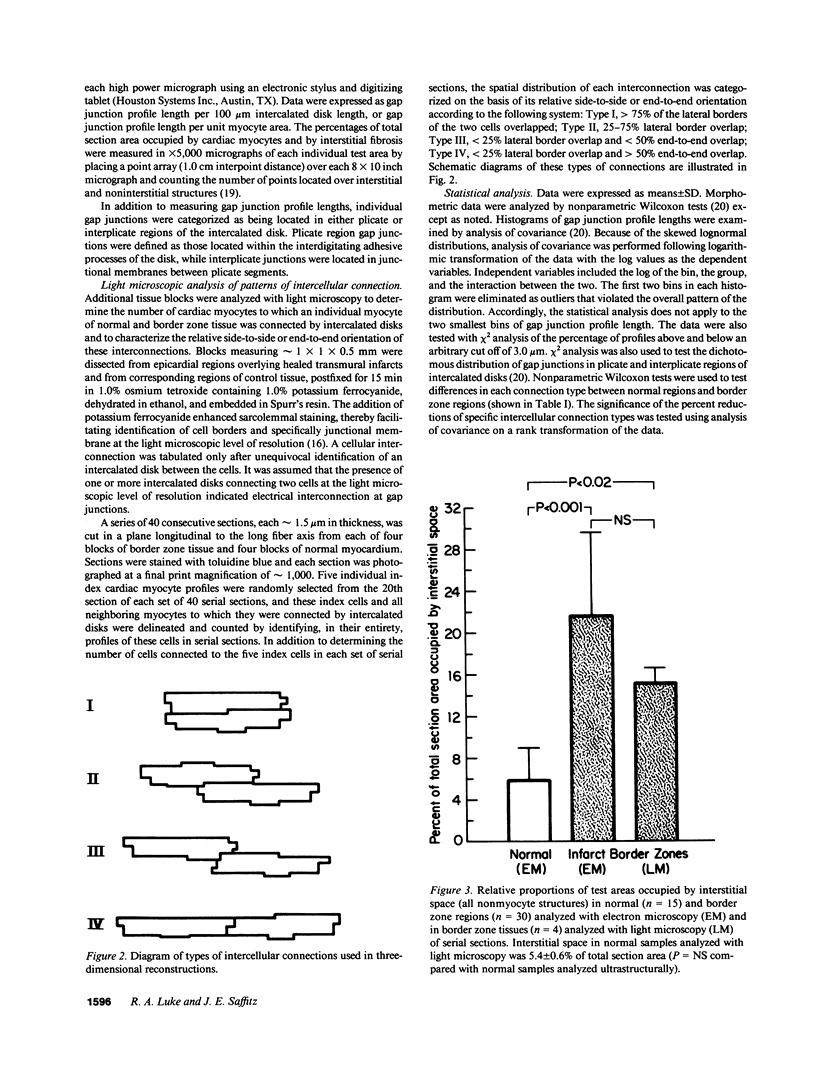
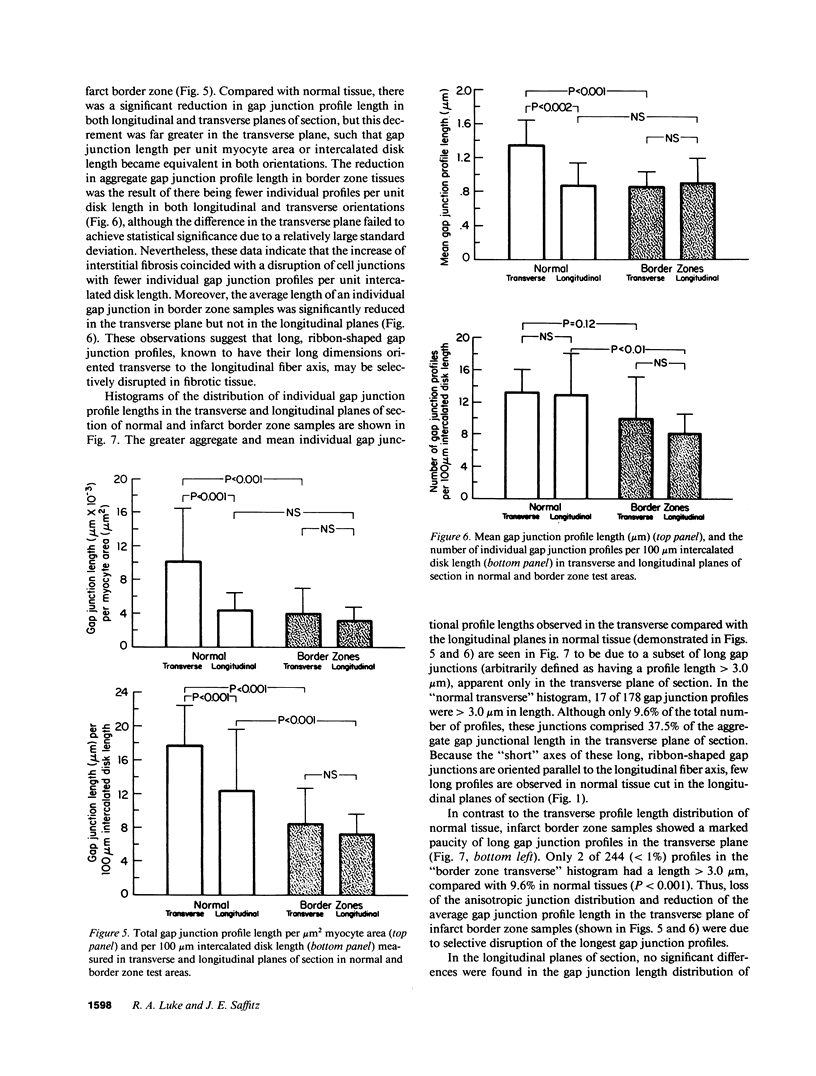
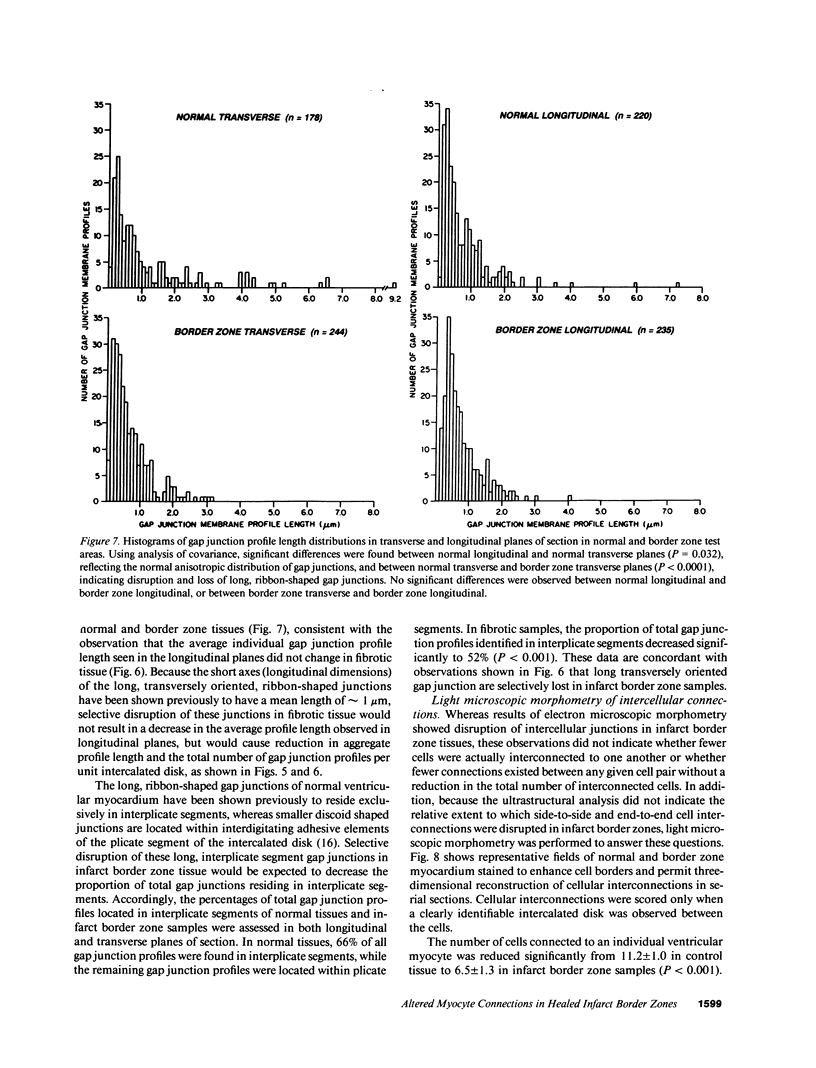
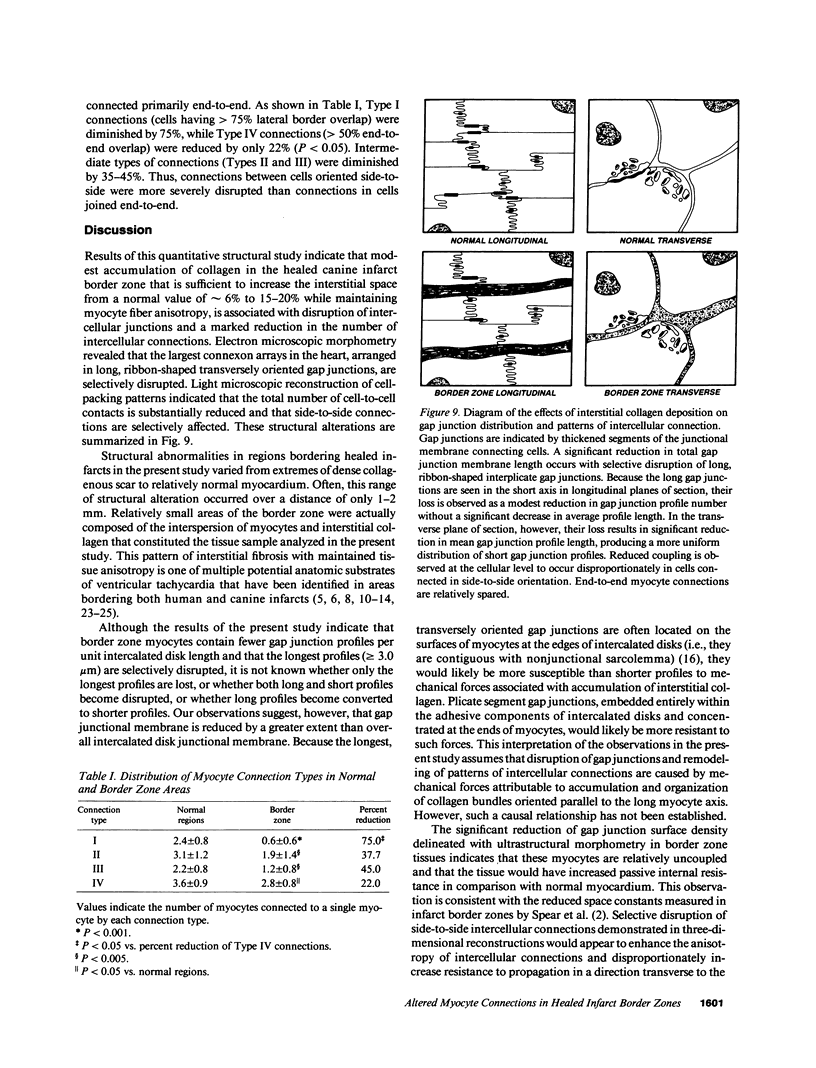
Remodeling of myocyte interconnections may be an important determinant of ventricular tachycardia in regions bordering healed infarcts. We used quantitative electron microscopy to characterize the distribution of gap junctions in 10 canine left ventricles 3-10 wk after coronary occlusion. In three normal canine left ventricles analyzed ultrastructurally, myocardial gap junctions were distributed anisotropically; gap junction profile length was significantly greater in the transverse than in longitudinal planes of section. In infarct border zone tissues, the normal anisotropic distribution was completely abolished and fewer gap junctions per unit intercalated disk length were observed. Analysis of individual gap junction profile length distributions revealed selective disruption of the largest gap junctions that collectively comprised only 9.6% of total junction profiles, but encompassed nearly 40% of aggregate gap junction length in the transverse plane of section. Three-dimensional reconstructions of myocyte interconnections by high resolution quantitative light microscopy of serial sections demonstrated a reduction in the number of cells connected by intercalated disks to a single myocyte from 11.2 +/- 1.0 in normal myocardium to 6.5 +/- 1.3 in border zone tissues (P less than 0.001). Connections of cells in primarily side-to-side apposition were reduced by 75%, whereas primarily end-to-end connections were reduced by only 22% (P less than 0.05). These alterations would disproportionately enhance axial resistivity in the transverse direction, potentially contributing to development of reentrant arrhythmias.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardinal R., Vermeulen M., Shenasa M., Roberge F., Page P., Hélie F., Savard P. Anisotropic conduction and functional dissociation of ischemic tissue during reentrant ventricular tachycardia in canine myocardial infarction. Circulation. 1988 May;77(5):1162–1176. doi: 10.1161/01.cir.77.5.1162. [DOI] [PubMed] [Google Scholar]
- Dillon S. M., Allessie M. A., Ursell P. C., Wit A. L. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988 Jul;63(1):182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- Friedman P. L., Fenoglio J. J., Wit A. L. Time course for reversal of electrophysiological and ultrastructural abnormalities in subendocardial Purkinje fibers surviving extensive myocardial infarction in dogs. Circ Res. 1975 Jan;36(1):127–144. doi: 10.1161/01.res.36.1.127. [DOI] [PubMed] [Google Scholar]
- Gardner P. I., Ursell P. C., Fenoglio J. J., Jr, Wit A. L. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985 Sep;72(3):596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- Gerdes A. M., Kasten F. H. Morphometric study of endomyocardium and epimyocardium of the left ventricle in adult dogs. Am J Anat. 1980 Dec;159(4):389–394. doi: 10.1002/aja.1001590405. [DOI] [PubMed] [Google Scholar]
- Hoyt R. H., Cohen M. L., Saffitz J. E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ Res. 1989 Mar;64(3):563–574. doi: 10.1161/01.res.64.3.563. [DOI] [PubMed] [Google Scholar]
- Kramer J. B., Saffitz J. E., Witkowski F. X., Corr P. B. Intramural reentry as a mechanism of ventricular tachycardia during evolving canine myocardial infarction. Circ Res. 1985 May;56(5):736–754. doi: 10.1161/01.res.56.5.736. [DOI] [PubMed] [Google Scholar]
- Luke R. A., Beyer E. C., Hoyt R. H., Saffitz J. E. Quantitative analysis of intercellular connections by immunohistochemistry of the cardiac gap junction protein connexin43. Circ Res. 1989 Nov;65(5):1450–1457. doi: 10.1161/01.res.65.5.1450. [DOI] [PubMed] [Google Scholar]
- Richards D. A., Blake G. J., Spear J. F., Moore E. N. Electrophysiologic substrate for ventricular tachycardia: correlation of properties in vivo and in vitro. Circulation. 1984 Feb;69(2):369–381. doi: 10.1161/01.cir.69.2.369. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Barr R. C., Johnson E. A., Kootsey J. M. Cardiac extracellular potentials. Analysis of complex wave forms about the Purkinje networks in dogs. Circ Res. 1973 Oct;33(4):465–473. doi: 10.1161/01.res.33.4.465. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C., Heidlage J. F. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988 Apr;62(4):811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986 Mar;58(3):356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Dolber P. C., Kootsey J. M., Sommer J. R., Mosher C. E., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res. 1982 Feb;50(2):175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Geselowitz D. B., Barr R. C., Kootsey J. M., Johnson E. A. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981 Jan;48(1):39–54. doi: 10.1161/01.res.48.1.39. [DOI] [PubMed] [Google Scholar]
- Spear J. F., Michelson E. L., Moore E. N. Cellular electrophysiologic characteristics of chronically infarcted myocardium in dogs susceptible to sustained ventricular tachyarrhythmias. J Am Coll Cardiol. 1983 Apr;1(4):1099–1110. doi: 10.1016/s0735-1097(83)80112-0. [DOI] [PubMed] [Google Scholar]
- Spear J. F., Michelson E. L., Moore E. N. Reduced space constant in slowly conducting regions of chronically infarcted canine myocardium. Circ Res. 1983 Aug;53(2):176–185. doi: 10.1161/01.res.53.2.176. [DOI] [PubMed] [Google Scholar]
- Surawicz B. Ventricular arrhythmias: why is it so difficult to find a pharmacologic cure? J Am Coll Cardiol. 1989 Nov 15;14(6):1401–1416. doi: 10.1016/0735-1097(89)90374-4. [DOI] [PubMed] [Google Scholar]
- Ursell P. C., Gardner P. I., Albala A., Fenoglio J. J., Jr, Wit A. L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- Waxman H. L., Sung R. J. Significance of fragmented ventricular electrograms observed using intracardiac recording techniques in man. Circulation. 1980 Dec;62(6):1349–1356. doi: 10.1161/01.cir.62.6.1349. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Allessie M. A., Bonke F. I., Lammers W., Smeets J., Fenoglio J. J., Jr Electrophysiologic mapping to determine the mechanism of experimental ventricular tachycardia initiated by premature impulses. Experimental approach and initial results demonstrating reentrant excitation. Am J Cardiol. 1982 Jan;49(1):166–185. doi: 10.1016/0002-9149(82)90292-2. [DOI] [PubMed] [Google Scholar]
- Zuanetti G., Hoyt R. H., Corr P. B. Beta-adrenergic-mediated influences on microscopic conduction in epicardial regions overlying infarcted myocardium. Circ Res. 1990 Aug;67(2):284–302. doi: 10.1161/01.res.67.2.284. [DOI] [PubMed] [Google Scholar]
- de Bakker J. M., van Capelle F. J., Janse M. J., Wilde A. A., Coronel R., Becker A. E., Dingemans K. P., van Hemel N. M., Hauer R. N. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988 Mar;77(3):589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]