Abstract
The collagen-tailed form of acetylcholinesterase (ColQ-AChE) is the major if not unique form of the enzyme associated with the neuromuscular junction (NMJ). This enzyme form consists of catalytic and non-catalytic subunits encoded by separate genes, assembled as three enzymatic tetramers attached to the three-stranded collagen-like tail (ColQ). This synaptic form of the enzyme is tightly attached to the basal lamina associated with the glycosaminoglycan perlecan. Fasciculin-2 is a snake toxin that binds tightly to AChE. Localization of junctional AChE on frozen sections of muscle with fluorescent Fasciculin-2 shows that the labeled toxin dissociates with a half-life of about 36 h. The fluorescent toxin can subsequently be taken up by the muscle fibers by endocytosis giving the appearance of enzyme recycling. Newly synthesized AChE molecules undergo a lengthy series of processing events before final transport to the cell surface and association with the synaptic basal lamina. Following co-translational glycosylation the catalytic subunit polypeptide chain interacts with several molecular chaperones, glycosidases and glycosyltransferases to produce a catalytically active enzyme that can subsequently bind to one of two non-catalytic subunits. These molecular chaperones can be rate limiting steps in the assembly process. Treatment of muscle cells with a synthetic peptide containing the PRAD attachment sequence and a KDEL retention signal results in a large increase in assembled and exportable AChE, providing an additional level of post-translational control. Finally, we have found that Pumilio2, a member of the PUF family of RNA-binding proteins, is highly concentrated at the vertebrate neuromuscular junction where it plays an important role in regulating AChE translation through binding to a highly conserved NANOS response element in the 3′-UTR. Together, these studies define several new levels of AChE regulation in electrically excitable cells.
Keywords: Fasciculin-2, AChE turnover, Synapse, Molecular chaperones, Protein folding, AChE assembly, RNA-binding protein, Translational regulation
1. Introduction
The complex mechanisms underlying the regulation of acetylcholinesterase (AChE) expression at sites of nerve–muscle contact are still being elucidated. The synaptic form of the enzyme, consisting of three catalytic tetramers associated with the collagen-like tail (ColQ), is highly concentrated at the neuromuscular junction (NMJ), both intracellularly and on the cell surface associated with the synaptic basal lamina at regions of nerve–muscle contact [1–3]. While much research has focused on the transcriptional regulation of this important enzyme, much less is know about the post-transcriptional events that lead to its expression and localization at synapses. This paper will focus on recent studies from our laboratory on the early events of AChE biogenesis and the several levels of translational and post-translational controls that affect the expression of active enzyme at the neuromuscular junction.
2. Early events in the assembly of AChE
We have previously shown that the enzyme is synthesized on the rough endoplasmic reticulum where it is rapidly assembled into dimers and tetramers, and only later assembled into collagen-tailed molecules [4]. Most of the newly synthesized enzyme, however, is catalytically inactive and rapidly degraded by the ERAD pathway [5,6]. These molecules are sensitive to the endoglycosidase Endo-H, indicating residence in the endoplasmic reticulum and/or the early Golgi apparatus [6]. The subset of AChE molecules that subsequently mature to catalytically active enzyme become resistant to Endo-H, indicating transport to and transit through the Golgi apparatus. In contrast to the exported enzyme that reaches the cell surface, we now show that the newly synthesized catalytically active molecules are very unstable and are rapidly inactivated by high temperatures, proteases and reducing agents suggesting that they transit an intermediate stage where the molecules are incompletely folded. When cells expressing AChE are treated with DTT, and the enzyme allowed to refold, only those molecules originally destined for activation regain catalytic activity, suggesting a rate limiting step in the folding process. One possible candidate for this rate limiting step is the non-catalytic subunit itself, either ColQ or the transmembrane anchor PRiMA. Co-expression of the catalytic and non-catalytic ColQ subunits in primary cells or transfected cell lines shows that the non-catalytic subunits rescue AChE from ERAD degradation in addition to promoting assembly. In fact, treatment of the cells with peptides containing both the PRAD sequence [7,8] and the ER retention signal sequence KDEL alone are capable of rescuing AChE from degradation. These results have led to the development of specific peptides designed to rescue AChE following synthesis as a possible therapy for exposure the nerve agents and organophosphate type pesticides.
3. Localizing AChE to the neuromuscular junction
The synaptic form of acetylcholinesterase is tightly associated with the synaptic basal lamina, however the molecular mechanism(s) underlying its attachment at sites of nerve–muscle contact are still poorly understood. COLQ-AChE is assembled intracellularly and transported to the cell surface where it transiently associates with the extracellular matrix [9]. At this early stage the enzyme can readily be detached with high salt solutions or heparin. However, during the subsequent 2–3 h, the attachment becomes stronger and the enzyme can no longer be removed even with ionic detergents or 8 M urea suggesting that the enzyme becomes covalently attached [10]. Most of the enzyme in vivo is also strongly associated with the extracellular matrix. AChE appears to be localized at the NMJ through its interactions with perlecan that in turn is attached to dystroglycan [11–13]. Several studies have shown direct binding of AChE to perlecan, both in vitro and in vivo, and mice lacking perlecan are also null for the synaptic AChE. Perlecan is a multifunctional heparin sulfate proteoglycan that is also concentrated on the synaptic basal lamina, and the carboxyl terminal domain of ColQ is necessary and sufficient for its attachment. Several mutations in the C-terminal domain of COLQ indicate that this region of the collagenic tail is essential for AChE localization at the synapse [14–16]. To determine whether the entire collagenic tail was necessary for attachment, or only the C-terminal domain, we generated several fusion proteins consisting of GFP fused to varying lengths of COLQ. The proteins were then expressed in HEK293, COS7 or primary avian skeletal muscle cells and tested for their ability to bind to the cell surface. The results indicate that a single C-terminal domain alone with the collagenic domain is sufficient to target COLQ-AChE to the cell surface (Lewis Kimbell, unpublished observations).
Another possible ColQ-protein interaction has been described by Cartaud and colleagues [17] who suggested that the putative agrin receptor MuSK is also a receptor for ColQ-AChE and was required for AChE binding to the neuromuscular junction. Using a ColQ-GFP fusion protein expression to emulate expression of ColQ-AChE, and co-transfection with MuSK, the authors demonstrate that the surface clusters of ColQ-GFP co-distribute with endogenous perlecan and exogenous MuSK in COS-7 cells. In contrast, COS-7 cells transfected with ColQ-GFP alone did not exhibit cell surface clusters. These interesting studies remain to be repeated and a direct interaction between MuSK and ColQ-AChE remains to be demonstrated.
4. Association and dissociation of Fasciculin-2 with junctional AChE
To further study the association of AChE with the extracellular matrix we began studies by labeling the enzyme with fluorescent Fasciculin-2 (Alexa 488-Fas2), however the fluorescent probe dissociates from the enzyme in solution and can be taken up by the cells via endocytosis. Direct labeling of AChE on frozen tissue sections, followed by incubation in serum-containing medium, showed that the Alexa 488-Fas2 dissociated from the enzyme with a half-life of about 36 h measured by recording the loss of fluorescence at the NMJ (Figs. 1 and 2). Most of the fluorescence intensity could be restored by relabeling the NMJ, indicating that the enzyme remained attached during this time course. Free Alexa 488-Fas2, at concentrations used to label the NMJs, is rapidly taken up by skeletal muscle cells where it co-localizes with rhodamine dextran, a marker of endocytic vesicles. It thus appears that Alexa 488-Fas2 dissociates more rapidly from AChE than AChE does from the NMJ. The observation that much of the enzyme can be relabeled after the fluorescent Fas2 comes off indicates that the enzyme is strongly attached to the synapse, consistent with previously published studies from several laboratories.
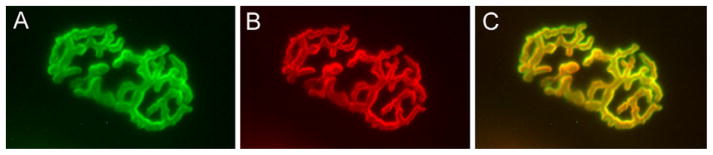
Fig. 1.
Labeling of neuromuscular junctions on frozen sections of skeletal muscle. Mouse gastronemius muscles were dissected and frozen in isopentane cooled with liquid nitrogen, then stored at −20°C. The muscles were sectioned using a cryostat at 20 μm and mounted on glass slides. The sections were stained with Alexa 555-conjugated α-bungarotoxin to label the acetylcholine receptors and Alexa 488-Fasciculin-2 to label AChE. The sections were then viewed using a Leica IM-6000 fluorescence microscope and sequential images captured using a Hamamatsu CCD camera and analyzed with Slidebook 4.0 image analysis software. (A) Neuromuscular junction stained with Alexa 488 Fas2; (B) NMJ stained with Alexa 555 to label nAChR; (C) Overlay of the two images showing congruence.
Fig. 2.
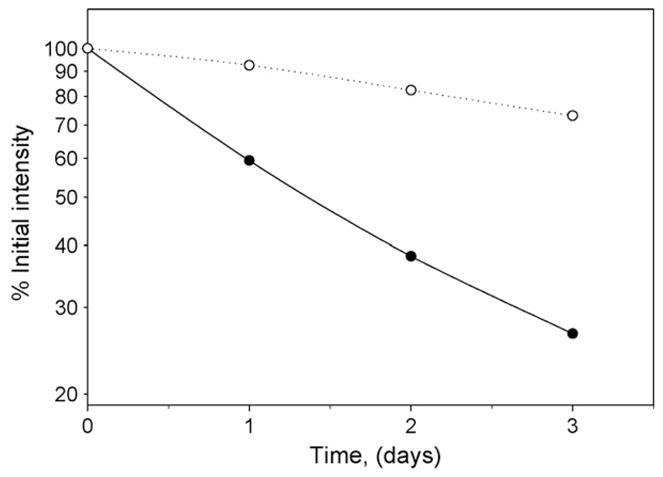
Half-life of dissociation of Fasciculin-2 from the NMJ sections. Images of neuromuscular junctions identical to those described in Fig. 1 were captured at 24 h intervals and stored. At the end of the experiment the fluorescence intensity of the NMJs was determined using the Slidebook 4.0 software. This is a representative graph showing the rate of dissociation of Fas2 and α-Btx from a single NMJ. The half-life of Fas2 dissociation is approximately 36 h whereas the dissociation half-life of α-Btx is closer to 8–10 days, as previously reported. Thus the rate of Fas2 dissociation from previously frozen skeletal muscle sections is essentially the same as reported for the recycling if AChE at the living NMJ of adult mice [22,23].
5. Role of molecular chaperones in the regulation of AChE assembly
Acetylcholinesterase in skeletal muscle is regulated in part by muscle activity. Synaptic AChE is assembled from two separate gene products encoding the catalytic subunit and the non-catalytic collagenic tail, ColQ, and therefore is predicted to require the assistance of several molecular chaperones to aid in correct folding and assembly. Using quail muscle cultures (QMC) we showed that ColQ-AChE expression is regulated by muscle activity at the post-translational level. In a search for post-transcriptional mechanisms that regulate AChE assembly and expression, we studied the role of molecular chaperones. Calnexin over-expression has previously been shown to enhance the expression of the nicotinic acetylcholine receptor [18]. To test this possibility for AChE we cloned and over-expressed the chicken endoplasmic reticulum chaperones Erp72 and protein disulfide isomerase (PDI), as well as tested canine calnexin effects in QMCs. Over-expression of each chaperone enhanced expression of junctional AChE, cell surface AChE activity and the number of cell surface AChE clusters on transfected cells. Over-expression of PDI had the most dramatic effect on ColQ-AChE expression with more than a 100% increase in the intracellular AChE pool and cell surface enzyme activity. In contrast, expression of all intracellular AChE forms in 5-day old QMCs was decreased after PDI inhibition with 1 mM bacitracin in culture. Moreover, primary QMCs transfected with PDI-shRNAs showed a significant decrease of ColQ-AChE together with a decrease in cell surface activity and number of surface AChE clusters. In addition, we found that the levels of PDI in QMCs are regulated by muscle activity and correlate directly with the levels of synaptic AChE expression. Thus ColQ-AChE expression can be regulated post-translationally by molecular chaperones at the level of individual subunit folding and/or at the level of assembly of catalytic and non-catalytic subunits. These results also suggest that newly synthesized proteins compete for chaperone assistance during the folding process.
6. Translational control of AChE by the mRNA-binding protein Pumilio2
We recently found that the RNA-binding protein Pumilio2 is specifically localized at the neuromuscular synapse, whereas Pumilio1 has a more widespread distribution throughout the muscle fibers and is absent at the NMJ. PUM2 belongs to a highly conserved family of RNA-binding proteins known as the PUF family that are present from yeast to human and are characterized by a highly conserved C-terminal RNA-binding domain, composed of eight tandem repeats. This domain binds to a specific sequence motif at the 3′ untranslated region (3′-UTR) of certain mRNAs, known as NANOS response elements (NREs). Analysis of the AChE transcripts shows that all higher vertebrate species have the canonical Pumilio recognition sequence, the NANOS response element and a very highly conserved motif, located approximately 50 nucleotides downstream of the AChE stop codon. Alignment of the 3′-UTR sequences of human, mouse and rat AChE mRNA identified the presence of the canonic octamer NRE sequence. Co-immunoprecipitation studies indicate that PUM2 binds directly to the AChE mRNA isolated from tissue cultured muscle while PUM1 does not. Over-expression of PUM2 in tissue cultured muscle represses AChE translation, whereas knockdown of the PUM2 transcript with specific siRNAs increases AChE expression. Together, these studies suggest an important role for PUM2 in the translational regulation of AChE at the vertebrate neuromuscular synapse. In this context we envision two roles for the binding of PUM2 to the AChE transcript. First, PUM2 would be part of the molecular mechanism for physically localizing the AChE mRNA at or near the sites of translation. Second, PUM2 would be part of the actual translational control mechanism through activity-dependent de-repression, i.e., the release of translational inhibition, following the appropriate trans-synaptic signals.
Acetylcholinesterase transcripts are also more highly expressed at sites of nerve–muscle contact [19,20] where they are presumably regulated as well. The mechanisms underlying their localization and translational regulation are not known but presumably involve RNA-binding proteins such as PUM2 and the formation of ribonucleoprotein complexes. PUM2 binds directly to NREs within the 3′-UTR of target mRNA and recruits other co-factors like NANOS through its HD domain to form a translation repression complex capable of blocking the expression of that gene. We can thus conclude that PUM2 is binding to AChE mRNA forming a translational complex that can regulate AChE expression post-translationally at the neuromuscular synapse.
7. Summary
The results from several areas of research in our laboratory were presented. First, studies on the mechanism of ColQ-AChE attachment at the NMJ showed that the C-terminal cysteine-rich domain alone was sufficient to localize the enzyme on the cell surface. As part of our studies to determine the mechanism of attachment, we labeled the NMJs on frozen sections of skeletal muscle only to find the fluorescent toxin dissociating with a half-life of about 36 h. Thus this approach cannot be used for long term labeling studies of AChE, nor to study its turnover. Studies on the biogenesis and regulation of AChE showed that there is a strong post-transcriptional regulatory components including regulation at the level of protein folding and assembly as well as translation initiation. Finally, the mechanism underlying AChE mRNA localization at the NMJ was studied together with the role of PUM2, an RNA-binding protein that can repress AChE translation by binding to the NANOS response element located in the 3′-UTR of the AChE transcript. Ongoing studies in the laboratory are focused on determining the mechanism of translational activation of AChE by muscle activity.
Acknowledgments
This research was supported by grants from the National Institutes of Health and the Army Research Office to RLR. LMK was the recipient of an American Heart Association Florida and Puerto Rico Chapter Graduate Student Fellowship.
References
- 1.Legay C. Why so many forms of acetylcholinesterase? Microsc Res Tech. 2000;49:56–72. doi: 10.1002/(SICI)1097-0029(20000401)49:1<56::AID-JEMT7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993;41:31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- 3.Rotundo RL. Expression and localization of acetylcholinesterase at the neuromuscular junction. J Neurocytol. 2003;32:743–766. doi: 10.1023/B:NEUR.0000020621.58197.d4. [DOI] [PubMed] [Google Scholar]
- 4.Rotundo RL. Asymmetric acetylcholinesterase is assembled in the Golgi apparatus. Proc Natl Acad Sci USA. 1984;81:479–483. doi: 10.1073/pnas.81.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotundo RL. Biogenesis of acetylcholinesterase molecular forms in muscle: evidence for rapidly turning over catalytically inactive precursor pool. J Biol Chem. 1988;263(36):19398–19406. [PubMed] [Google Scholar]
- 6.Rotundo RL, Thomas K, Porter-Jordan K, Benson RJJ, Fernandez-Valle C, Fine RE. Intracellular transport, sorting, and turnover of acetylcholinesterase: evidence for endoglycosidase h-sensitive form in Golgi apparatus, sarcoplasmic reticulum, and clathrin-coated vesicles and its rapid degradation by a non-lysosomal mechanism. J Biol Chem. 1989;264(6):3146–3152. [PubMed] [Google Scholar]
- 7.Bon S, Coussen F, Massoulie J. Quaternary associations of acetyl-cholinesterase. II. The polyproline attachment domain of the collagen-tail. J Biol Chem. 1997;272(5):3016–3021. doi: 10.1074/jbc.272.5.3016. [DOI] [PubMed] [Google Scholar]
- 8.Bon S, Massoulie J. Quaternary associations of acetylcholinesterase. I. Oligomeric associations of T subunits with without the amino-terminal domain of the collagen-tail. J Biol Chem. 1997;272(5):3007–3015. doi: 10.1074/jbc.272.5.3007. [DOI] [PubMed] [Google Scholar]
- 9.Rossi SG, Rotundo RL. Transient interactions between collagen-tailed acetylcholinesterase and sulfated proteoglycans prior to immobilization on the extracellular matrix. J Biol Chem. 1996;271:1979–1987. doi: 10.1074/jbc.271.4.1979. [DOI] [PubMed] [Google Scholar]
- 10.Rossi SG, Rotundo RL. Localization of “non-extractable” acetyl-cholinesterase to the vertebrate neuromuscular junction. J Biol Chem. 1993 [PubMed] [Google Scholar]
- 11.Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci. 2002;5:119–123. doi: 10.1038/nn801. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J Cell Biol. 2001;152:435–450. doi: 10.1083/jcb.152.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng HB, Xie H, Rossi SG, Rotundo RL. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol. 1999;145:911–921. doi: 10.1083/jcb.145.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimbell LM, Ohno K, Engel AG, Rotundo RL. C-terminal and heparin-binding domains of collagenic tail subunit are both essential for anchoring acetylcholinesterase at the synapse. J Biol Chem. 2004;279:10997–11005. doi: 10.1074/jbc.M305462200. [DOI] [PubMed] [Google Scholar]
- 15.Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA. 1998;95:9654–9659. doi: 10.1073/pnas.95.16.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno K, Engel AG, Brengman J, Shen X, Heidenreich F, Vincent A, Milone M, Tan E, Demirci M, Walsh P, Nakano S, Akiguchi I. The spectrum of mutations causing end-plate acetylcholinesterase deficiency. Ann Neurol. 2000;47:162–170. [PubMed] [Google Scholar]
- 17.Cartaud A, Strochlic L, Guerra M, Blanchard B, Lambergeon M, Krejci E, Cartaud J, Legay C. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J Cell Biol. 2004;165:505–515. doi: 10.1083/jcb.200307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang W, Gelman MS, Prives JM. Calnexin-dependent enhancement of nicotinic acetylcholine receptor assembly and surface expression. J Biol Chem. 1997;272:28925–28932. doi: 10.1074/jbc.272.46.28925. [DOI] [PubMed] [Google Scholar]
- 19.Jasmin BJ, Lee RK, Rotundo RL. Compartmentalization of acetylcholinesterase mRNA and enzyme at the vertebrate neuromuscular junction. Neuron. 1993;11:467–477. doi: 10.1016/0896-6273(93)90151-g. [DOI] [PubMed] [Google Scholar]
- 20.Michel RN, Vu CQ, Tetzlaff W, Jasmin BJ. Neural regulation of acetylcholinesterase mRNAs at mammalian neuromuscular synapses. J Cell Biol. 1994;127:1061–1069. doi: 10.1083/jcb.127.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krejci E, Martinez P, Ameziane R, Akaaboune M. Acetylcholinesterase dynamics at the neuromuscular junction of live animals. J Biol Chem. 2006;281:10347–10354. doi: 10.1074/jbc.M507502200. [DOI] [PubMed] [Google Scholar]
- 23.Martinez P, Hume RI, Krejci E, Akaaboune M. In vivo regulation of acetylcholinesterase insertion at the neuromuscular junction. J Biol Chem. 2005;280:31801–31808. doi: 10.1074/jbc.M502874200. [DOI] [PubMed] [Google Scholar]