Abstract
A group of 204 adult patients was entered into a prospective, randomized trial comparing FK506/prednisone with FK506/azathioprine/prednisone after renal transplantation between August 1, 1991 and October 11, 1992. The purpose of the study was to see if the addition of azathioprine would reduce the incidence of rejection and improve graft survival. The recipient population was unselected, with 61 (30%) patients undergoing retransplantation, 37 (18%) having a panel-reactive antibody greater than 40%, and 33 (16%) over 60 years of age. The mean recipient age was 43.8 ± 13.7 years (range 17.6–78). The mean donor age was 34.0 ± 20.1 years (range 0.3–75); 13% of the cadaveric kidneys were from pediatric donors less than 3 years of age and were transplanted en bloc. The mean cold ischemia time was 31.4 ± 8.4 hr. Living donors were the source of 13% of the kidneys. The mean follow-up was 22 ± 4 months (range 12–29). Overall one-year actual patient survival was 94%. Overall one-year actual graft survival was 87%. Patients starting on double therapy had a one-year actual patient survival of 96% and a one-year actual graft survival of 92%. Patients starting on triple therapy had a one-year actual patient survival of 91% (P = ns compared with double therapy), and a one-year actual graft survival of 82% (P < 0.02, compared with double therapy). Overall results with first cadaver transplants included a one-year actual patient survival of 94% and one-year actual graft survival of 88%, with no differences between double and triple therapy. The overall incidence of rejection was 48%, with 54% in the double therapy group and 41% in the triple therapy group (P < .07). The incidence of steroid-resistant rejection requiring antilymphocyte therapy (OKT3 or ATGAM) was 13%, and was not different between the double and triple therapy groups. The mean serum creatinine was 1.8 ± 0.8 mg/dl. The mean BUN was 33 ± 21 mg/dl, with no significant difference between the therapy groups. The mean serum cholesterol was 192 ± 49 mg/dl. A total of 56% of the patients are off prednisone, and 35% of the patients are not taking any antihypertensive medications. Other complications included cytomegalovirus—14%; new-onset diabetes—16% (half of which was reversible); and posttransplant lymphoproliferative disorder—1%. There was a high incidence of crossover between the two groups, 27% of the patients in the double therapy group requiring the addition of azathioprine, and 45% of the patients in the triple therapy group requiring its discontinuation (usually temporary). These results show that FK506 is an excellent immunosuppressive agent after renal transplantation and that azathioprine is not routinely effective as a third agent. A high quality of life resulted from the ability to use no (56%) or low-dose maintenance steroids.
FK506 (Tacrolimus-Prograf) is a new immunosuppressive agent (1–3) that has been recently approved by the Food and Drug Administration for use after liver transplantation (4–7). Promising clinical experiences with this drug have also been described in heart (8), lung (9), intestine (10), and islet (11) transplant patients. In renal transplantation, the initial studies, while encouraging, seemed to suggest that FK506 resulted in equivalent patient and graft survival when compared with cyclosporine-based regimens (5, 12–14). The differences were seen in secondary issues, such as an increased freedom from chronic steroids, a somewhat lower need for antihypertensive medications, and significantly lower serum cholesterol levels (13, 14). On the basis of these findings, a prospective randomized trial was begun in August 1991, comparing two FK506-based regimens—with and without azathioprine. The purpose was to see if the addition of azathioprine would help to improve the primary outcomes and patient and graft survival, and decrease the incidence of rejection. Early reports of this trial suggested that overall graft survival under FK506 was improving with experience, but that the benefit of azathioprine was unclear (15,16). The data presented here reflect a minimum of one year of follow-up in the first 204 patients entered into this randomized trial, with actual survival calculations.
MATERIALS AND METHODS
Between August 1, 1991 and October 11, 1992, 204 patients were entered into a randomized trial comparing FK506/prednisone and FK506/azathioprine/prednisone. Inclusion and exclusion criteria, the details of randomization, and the Immunosuppressive protocol have been previously described (15, 16). The patient population was unselected and represented virtually all of the adults undergoing renal transplantation alone at the University of Pittsburgh Medical Center during this period. There were a high percentage of retransplantations (61 [30%]), sensitized recipients (PRA >40%—37 [18%]), and older patients (age >60–33 [16%]). There were 28 (14%) black, 4 (2%) Asian, and 2 (1%) hispanic recipients. The mean recipient age was 43.8 ± 13.7 years (range 17.6–78), and the mean donor age was 34.0 ± 20.1 years (range 0.3–75). A total of 178 (87%) transplantations were with cadaveric kidneys, and 26 (13%) were with living-donor kidneys. Of the cadaveric transplantations, 24 (13%) were with pediatric en bloc kidneys from donors 3 years of age or younger. The mean cold ischemia time for the cadaveric cases was 31.4 ± 8.4 hr. There were 7 (3%) 6-antigen-match and 13 (6%) 0-antigen-mismatch cases.
There were more older patients (>60 years) in the triple therapy group (22% vs. 11%, P < .04) and more living-donor cases in the double therapy group (18% vs. 8%, P < .04). The two groups were otherwise similar with regard to donor and recipient characteristics. The protocol was reviewed and approved by the Institutional Review Board of the University of Pittsburgh, and was renewed on a yearly basis.
Statistical Methods
The standard two-sample t test was used to test differences in means while differences m proportions were tested using Pearson’s chi-square test of association.
Patient survival was calculated from the date of kidney transplantation until death, and graft survival from the date of kidney transplantation until graft failure, retransplantation, or patient death. Survival curves were generated using the Kaplan-Meier (product-limit) method and were compared using the generalized Wilcoxon (Breslow) test. A multivariate Cox’s regression analysis was performed to adjust the relative risk of graft failure between the two groups based on age of recipient (over 60 years) and living-donor cases. A stepwise procedure was performed to identify high-risk patients for graft failure using all available information collected. A P value less than .05 was considered statistically significant. All analyses were performed according to intention-to-treat, unless otherwise stated.
RESULTS
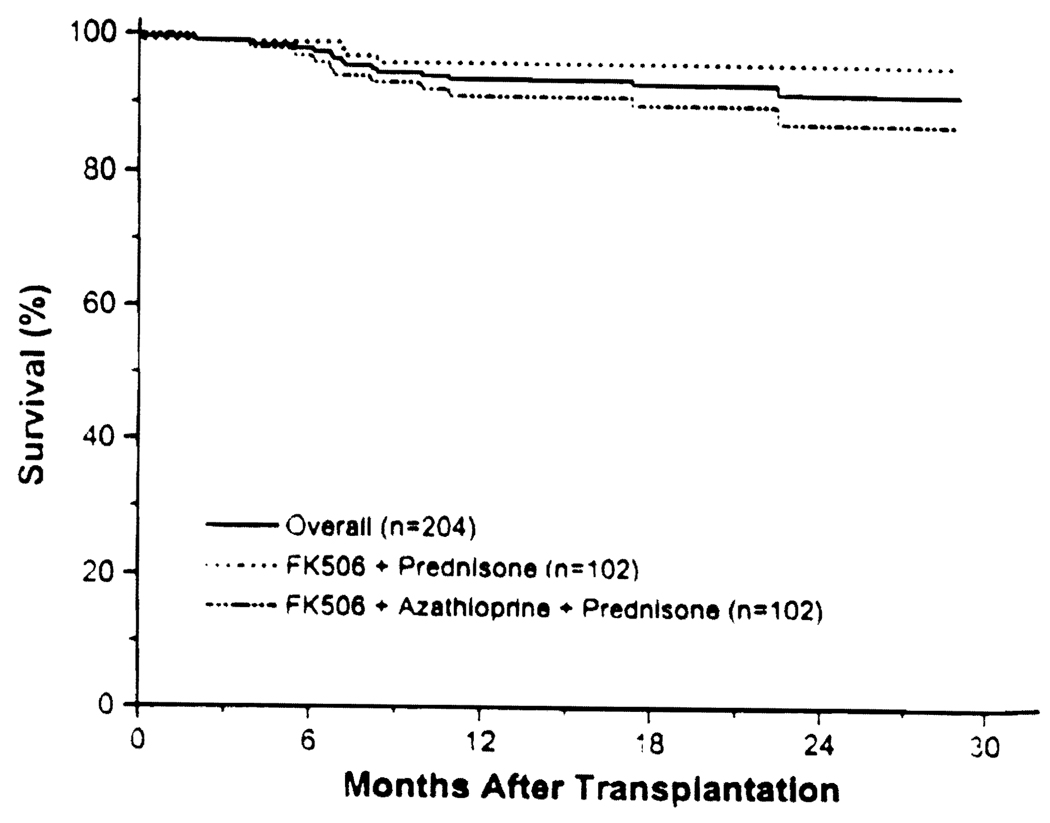
The mean follow-up was 22 ± 4 months (range 12–29). The overall actual one-year patient survival was 94%; in the double therapy group, it was 96%, and in the triple therapy group, it was 91% (Fig. 1; P = 0.10).
FIGURE 1.
Patient survival.
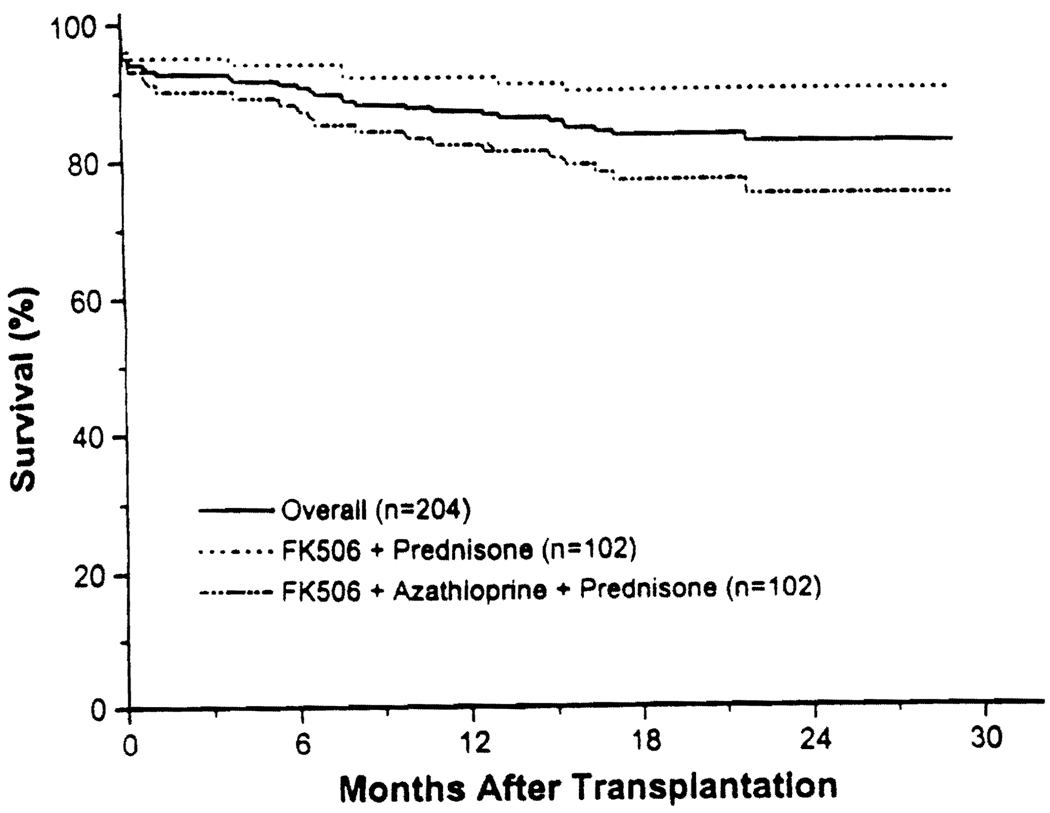
The overall one-year actual graft survival was 87%. In the double therapy group, it was 92%, and in the triple therapy group, it was 82% (Fig. 2; P < 0.02). For first cadaver transplants, the one-year actual graft survival was 88%; in the double therapy group, it was 90%, and in the triple therapy group, it was 87% (P = ns). Comparative one-year actual graft survivals in specific subgroups are shown in Table 1. Triple therapy was associated with poorer one-year graft survival in cadaveric cases, in patients undergoing retransplantation, in patients with PRAs >40%, in patients with immediate graft function, in patients who experienced rejection, in recipients who did not receive pediatric en bloc kidneys, in nonblack recipients, and in cases where the donor or the recipient was less than 60 years of age. First transplants, living-donor cases, patients with PRAs <40%, patients receiving pediatric en bloc kidneys, patients experiencing ATN, patients not experiencing rejection, black recipients, and donors or recipients over 60 years of age showed no difference between double and triple therapy. With regard to specific subgroups, the only significant variable was the presence of ATN, which was associated with significantly worse one-year graft survival. In all of the other subgroups, no difference was seen—i.e., outcome after retransplantation was similar to that seen with first transplants; patients with high PRAs did as well as with patients with low PRAs; blacks did as well as nonblacks: patients who had rejection were not significantly different from patients who did not have rejection; and so on (Table 1).
FIGURE 2.
Graft survival.
TABLE 1.
One year actual graft survival
| 2-Drug | 3-Drug | Total | 2 vs. 3 | Subgroup | |
|---|---|---|---|---|---|
| Overall | 92% | 82% | 87% | P < .02 | |
| 1st Cadaveric | 90% | 87% | 88% | NS | |
| 1st Transplant | 90% | 87% | 89% | NS | NS |
| Retransplantation | 97% | 72% | 84% | P < .02 | |
| Cadaveric | 92% | 82% | 87% | P < .03 | NS |
| Living-donor | 94% | 88% | 92% | NS | |
| PRA <40% | 91% | 84% | 87% | NS | NS |
| PRA >40% | 100% | 77% | 86% | P < .04 | |
| Adult kidneys | 93% | 83% | 88% | P < .02 | NS |
| En bloc | 80% | 79% | 79% | NS | |
| No ATN | 100% | 88% | 93% | P < .001 | P < .0001 |
| ATN | 81% | 71% | 77% | NS | |
| Rejection | 91% | 76% | 85% | P < .03 | NS |
| No rejection | 94% | 87% | 90% | NS | |
| Donors >60 years | 77% | 80% | 78% | NS | NS |
| Donors <60 years | 94% | 83% | 88% | P < .02 | |
| Black | 92% | 87% | 89% | NS | NS |
| Nonblack | 92% | 82% | 87% | P < .02 | |
| Recipients <60 years | 93% | 83% | 88% | P < .02 | NS |
| Recipients >60 years | 82% | 82% | 82% | NS |
A multivariate analysis was performed, using Cox’s proportional hazards model. Two variables were associated with an increased likelihood of graft failure: the presence of ATN (relative risk 4.32 [95% confidence interval 2.10–8.88], P < .0001), and initial immunosuppression with triple therapy (relative risk 2.83 [95% confidence interval 1.35–5.93], P < .006).
The mean serum creatinine, calculated creatinine clearance, and BUN were 1.8 ± 0.8 mg/dl, 55 ± 25 ml/min, and 33 ± 21 mg/dl, and were not significantly different between double and triple therapy patients (Table 2).
TABLE 2.
Renal function
| 2-Drug | 3-Drug | Total | |
|---|---|---|---|
| Creatinine | 1.9 ± 0.8 | 1.8 ± 0.8 | 1.8 ± 0.8 mg/dl |
| Creatinine clearance | 55 ± 24 | 56 ± 26 | 55 ± 25 mg/dl |
| BUN | 33 ± 22 | 33 ± 21 | 33 ± 21 mg/dl |
The incidence of acute rejection was 48%; in the double therapy group it was 54%, and in the triple therapy group it was 41%. This difference did not quite reach statistical significance (P < .07). The incidence of rejection in specific subgroups is shown in Table 3. In cadaveric cases, there was less rejection with triple therapy than with double therapy (43% vs. 61%, P < .02). In specific subgroups, retransplant patients had more rejection than recipients of first transplants; cadaveric cases had more rejection than living-donor cases; high-PRA patients had more rejection than low-PRA patients; black patients had more rejection than nonblacks; patients with ATN had more rejection than patients without ATN. Over 70% of the rejections were responsive to steroids and adjustment in the FK506 dosage. Antilymphocyte therapy was needed for steroid-resistant rejection in 13% of patients; there was no difference between double and triple therapy.
TABLE 3.
Rejection
| 2-Drug | 3-Drug | Total | 2 vs. 3 | Subgroup | |
|---|---|---|---|---|---|
| Overall | 54% | 41% | 48% | NS | |
| 1st Transplant | 49% | 36% | 43% | NS | P < .04 |
| Retransplantation | 66% | 53% | 59% | NS | |
| Cadaveric | 61% | 43% | 51% | P < .02 | P < .007 |
| Living-donor | 22% | 25% | 23% | NS | |
| PRA <40% | 49% | 35% | 43% | NS | P < .002 |
| PRA >40% | 80% | 64% | 70% | NS | |
| No ATN | 43% | 34% | 39% | NS | P < .001 |
| ATN | 69% | 54% | 62% | NS | |
| Black | 85% | 67% | 75% | NS | P < .002 |
| Nonblack | 49% | 37% | 43% | NS | |
| Donors >60 years | 62% | 50% | 57% | NS | NS |
| Donors < 60 years | 53% | 40% | 46% | NS | |
| Recipients <60 years | 55% | 45% | 50% | NS | NS |
| Recipients >60 years | 46% | 27% | 33% | NS |
The incidence of initial nonfunction, defined as a lack of allograft urine output or a need for dialysis within the first week after transplantation, was 38%; in the double therapy group it was 41%, and in the triple therapy group it was 34% (P = ns). The incidence of initial nonfunction in specific subgroups is shown in Table 4. Not surprisingly, cadaveric recipients had more ATN than living-donor recipients, and increasing cold ischemia time was associated with an increasing incidence of ATN. Blacks also had more ATN than nonblacks.
TABLE 4.
ATN
| 2-Drug | 3-Drug | Total | 2 vs. 3 | Subgroup | |
|---|---|---|---|---|---|
| Overall | 41% | 34% | 38% | NS | |
| 1st Transplant | 37% | 31% | 34% | NS | NS |
| Retransplantation | 52% | 41% | 46% | NS | |
| Cadaveric | 48% | 37% | 42% | NS | P < .001 |
| Living-donor | 11% | 0 | 8% | NS | |
| PRA <40% | 40% | 29% | 35% | NS | NS |
| PRA >40% | 47% | 55% | 51% | NS | |
| Black | 54% | 60% | 57% | NS | P < .03 |
| Nonblack | 39% | 30% | 35% | NS | |
| Donors >60 years | 54% | 50% | 52% | NS | NS |
| Donors <60 years | 39% | 33% | 36% | NS | |
| Recipients <60 years | 42% | 31% | 37% | NS | NS |
| Recipients >60 years | 36% | 46% | 42% | NS | |
| CIT | |||||
| <12 hr | 11% | 0 | 8% | NS | P < .001 |
| >24 hr | 0 | 29% | 16% | NS | |
| >24 hr | 46% | 30% | 39% | NS | |
| >36 hr | 79% | 53% | 63% | NS |
The incidence of cytomegalovirus disease or infection was 14%; all were treated with gancyclovir. The CMV incidence for the 4 different donor/recipient serologic combinations is shown in Table 5. The highest incidence, 38%, was in the seropositive donor/seronegative recipient group (P < .00001). All patients received high-dose acylovir prophylaxis; CMV hyperimmune globulin was also given to patients in the seropositive donor/seronegative recipient group.
TABLE 5.
CMV
| 2-Drug | 3-Drug | Total | 2 vs. 3 | Subgroup | |
|---|---|---|---|---|---|
| Overall | 12% | 15% | 14% | NS | |
| + → − | 30% | 47% | 38% | NS | |
| + → + | 3% | 8% | 6% | NS | P < .00001 |
| − → − | 11% | 15% | 13% | NS | |
| − → + | 10% | 7% | 8% | NS |
The incidence of posttransplant lymphoproliferative disorder (PTLD) was 1% (1 patient in each immunosuppressive group). In both cases, the PTLD disappeared with reduction of immunosuppression and initiation of gancyclovir therapy, and renal function was maintained. In addition, there was one case of Kaposi’s sarcoma in a patient on triple therapy who was lost to follow-up after returning home outside the United States. It resolved after discontinuation of immunosuppression, but the patient eventually lost her allograft.
The incidence of new onset diabetes was 16%, 22% in the double therapy group, and 10% in the triple therapy group (P < .04). Half these patients were able to be weaned off insulin once the FK506 and steroid dosages were reduced-thus, the incidence of chronic new-onset insulin dependence was 8%; 13% in the double therapy group and 4% in the triple therapy group (P < .05).
Crossover was seen frequently. In the double therapy group, 27% of patients received azathioprine at one time or another, and virtually all of these patients were permanently switched to triple therapy. In the triple therapy group, 45% of patients were taken off azathioprine at one time or another, and 11% remain off azathioprine permanently. The main reason for conversion from double to triple therapy was rejection, and the main reason for conversion from triple to double therapy was neutropenia or liver dysfunction. The one-year actual patient and graft survivals in patients currently on double therapy were 94% and 86%; in patients currently on triple therapy, they were 93% and 88% (P = ns).
The mean FK506 dosage was 10.3 ± 5.8 mg/day (0.15 ± 0.10 mg/kg/day) and was not different between the 2 treatment groups. The mean FK506 level was 0.88 ± 0.72 ng/ml and was also not different between the 2 groups.
A total of 56% of the patients have been weaned off steroids, 57% in the double therapy group and 56% in the triple therapy group; 5% had steroids withdrawn and then restarted because of rejection—none of these patients lost their allograft. The mean prednisone dose was 3.7 ± 5.6 mg/day; in patients still on steroids, it was 7.8 ± 5.7 mg/day.
A total of 35% of the patients were off antihypertensive medications—30% in the double therapy group and 40% in the triple therapy group. The mean number of antihypertensive medications required was 1.0 ± 1.0, 1.1 ± 1.0 in the double therapy group and 0.9 ± 1.0 in the triple therapy group.
The mean serum cholesterol was 192 ± 49 mg/dl, and was not different between the two groups.
DISCUSSION
Current expectations in renal transplantation are high: there is a presumption that no more than 5–10% of patients will die within the first year after transplantation, and that no more than 15–25% of patients will lose their allograft within the first year (17). While these results are not perfect, they are considerably better than they were 15 years ago (18), and represent maturation of a field that barely existed 35 years ago. However, current outcomes offer little reason for complacency, and active investigation of new immunosuppressive agents is proceeding around the world. FK506, the farthest along of these agents, has already been demonstrated to be a superior drug for liver transplantation (4 –6, 7). Experience with kidney transplantation, including the data reported here, has suggested improving outcomes with FK506, in unselected patients, that equal or surpass the best results obtainable with conventional therapy (15, 16). Of perhaps greater significance is the ability to withdraw steroids in more than half the patients. Other trials, from Japan and the United States, have demonstrated excellent outcomes (19–21). If comparable results are seen with the ongoing American and European multicenter trials, this will confirm the utility of FK506 as a formidable addition to the immunosuppressive armamentarium in renal transplantation.
The goal of the current randomized trial was to assess the ability of preemptive azathioprine to reduce the incidence of rejection and safely improve graft survival beyond that achievable with FK506 and prednisone alone. While the addition of azathioprine was associated with less rejection, particularly in cadaveric recipients, the reduction was not significant—and in fact, overall graft survival was worse in patients starting on triple therapy. There was a high incidence of crossover in both treatment limbs, but nearly twice as many from triple to double as from double to triple therapy. Thus the routine administration of azathioprine as a third agent was not advantageous. Nevertheless, about one quarter of the patients who self-selected to delayed azathioprine were thought to have derived benefit from it.
The apparent superiority of FK506 and prednisone alone and the outstanding results in patients with a higher-than-average risk profile raises questions about the wisdom of polypharmaceutical immunosuppression as complex as in the ongoing American multicenter randomized trial comparing cyclosporine and FK-506 for renal transplantation. In these trials, a sequential four-drug regimen is being used, beginning with induction antilymphocyte therapy and azathioprine. If azathioprine is confirmed to be without value in these trials, it may be that one of the new agents on the horizon such as mycophenolate mofetil (RS-61443) (22), brequinar (23), rapamycin (24), leflunomide (25), or deoxyspergualin (26) will be an effective third agent. It is noteworthy that even with cyclosporine convincing controlled studies showing the value of triple or quadruple therapy versus cyclosporine—prednisone are not available (27–29).
The side effects of FK506 are similar to those seen with cyclosporine, the principal ones being nephrotoxicity (30–34), neurotoxicity (35), and diabetogenicity (36). These are all dose-related and largely reversible with dose reduction. The infectious profile is also similar to that observed in past experience (14), although in liver recipients, the mortality from infectious complications has been significantly less (7). Hirsutism and gingival hyperplasia do not occur with FK506 (12–14). The long-term liability of hypercholesterolemia and refractory arterial hypertension have been reduced in recipients of various organs—a particular advantage for pediatric renal (37, 38) and heart recipients (39). One-third of the adults in the present series require no antihypertensive medications.
Our global assessment is that FK506 is a highly effective agent for renal transplant patients, once its nuances have been mastered. The addition of azathioprine to the combination of FK506 and prednisone was not uniformly advantageous, although there are some patients who may have benefited from the secondary use of azathioprine for specific indications. Further improvements in the short-term—and particularly the long-term—outlook after renal transplantation may depend more on biologic immune modulation, as with the adjuvant administration of donor bone marrow that has been reported elsewhere (40).
Acknowledgments
We would like to thank Regina Fenton, RN, BSN, CCTC, Loraine Kaminski, RN, Deborah Good, RN, BSN, CCTC, Holly Woods, RN, CCTC, Jareen Flohr, RN, BSN, Sue Bauder, RN, Janice Zagari, RN, BSN, Jennifer Ovesney, RN, BSN, and Sharon Orlofske, RN, for their help with patient care; Janet Schmelzer for her help with data entry and organization; David Krakosky for his help with graph and slide preparation; Kate Carr for her help with slide preparation; and Karen Toler for her help with typing the manuscript and table and slide preparation.
Footnotes
Presented at the 13th Annual Meeting of the American Society of Transplant Physicians. May 16–18, 1994. Chicago, IL.
REFERENCES
- 1.First International Workshop on FK506: A Potential Breakthrough in Immunosuppression, October, 1987, Gothenburg, Sweden. Transplant Proc. 1987;19 suppl 6:3. [PMC free article] [PubMed] [Google Scholar]
- 2.Second International Workshop on FK506: A Potential Breakthrough in Immunosuppression—Clinical Implications, February 1990, Barcelona, Spain. Transplant Proc. 1990;22 suppl 1:5. [PubMed] [Google Scholar]
- 3.Proceedings of the First International Congress on FK506, December, Pittsburgh. Transplant Proc. 1991;23:2709. [PubMed] [Google Scholar]
- 4.Fung JJ, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK506 vs. cyclosporine. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Fung JJ, Starzl TE. Liver, kidney, and thoracic organ transplantation under FK506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klintmalm G The U.S. Multi-center FK506 Liver Study Group. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after liver transplantation. N Engl J Med. 1994;331:1110. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 7.Todo S, Fung JJ, Starzl TE, et al. Single-center experience with primary orthotopic liver transplantation under FK506 immunosuppression. Ann Surg. doi: 10.1097/00000658-199409000-00006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armitage JM, Kornos RL, Fung JJ, Starzl TE. The clinical trial of FK506 as a primary and rescue immunosuppression in adult cadaveric transplantation. Transplant Proc. 1991;23:3054. [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith BP, Bando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57:848. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without the colon. Transplantation. 1994;57:840. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricordi C, Tzakis A, Carroll P, et al. Human islet allotransplantation under FK506. Transplant Proc. 1991;23:3207. [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Fung JJ, Jordan M, et al. Kidney transplantation under FK506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro R, Todo S, Starzl TE. Kidney transplantation under FK506 immunosuppression. Transplant Proc. 1991;23:920. [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro R, Jordan ML, Scantlebury V, et al. FK506 in clinical kidney transplantation. Transplant Proc. 1991;23:3065. [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro R, Jordan M, Scantlebury V, et al. Randomized trial of FK506/prednisone vs. FK506/azathioprine/prednisone after renal transplantation: preliminary report. Transplantation Proc. 1993;25:669. [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro R, Jordan M, Scantlebury V, et al. A prospective, randomized trial of FK506 in renal transplantation—a comparison between double and triple drug therapy. Clin Transplant. 1994;8:508. [PMC free article] [PubMed] [Google Scholar]
- 17.Cecka JM, Terasski PI. In: Clinical transplants 1992. Terasaki PI, Cecks JM, editors. Los Angeles: UCLA Tissue Typing Laboratory; 1993. p. 1. [Google Scholar]
- 18.Teresaki PI, Toyotome A, Mickey MR, et al. In: Clinical kidney transplant 1985. Terasaki PI, editor. Los Angeles: UCLA Tissue Typing Laboratory; 1985. p. 1. [Google Scholar]
- 19.Japanese FK506 Study Group. Japanese study of FK506 on kidney transplantation: the benefit of monitoring the whole blood FK506 concentration. Transplant Proc. 1991;23:3085. [PubMed] [Google Scholar]
- 20.Japanese FK506 Study Group. Japanese study of FK506 on kidney transplantation: results of late phase II study. Transplant Proc. 1993;25:649. [PubMed] [Google Scholar]
- 21.Neylan J, Whelchel J, Laskow D, et al. Adverse events in the comparative dose finding trial of FK506 in primary renal transplantation. Presented at the 13th Annual Meeting of the American Society of Transplant Physicians; May 16–18, 1993; Chicago, IL. [Google Scholar]
- 22.Sollinger HW, Deierhoi MH, Belzer FO, Diethelm AG, Kauffman RS. RS-61443—A phase I clinical trial and pilot rescue study. Transplantation. 1992;53:428. doi: 10.1097/00007890-199202010-00031. [DOI] [PubMed] [Google Scholar]
- 23.Makowka L, Chapman F, Cramer DV. Historical development of brequinar sodium as a new immunosuppressive drug for transplantation. Transplant Proc. 1993;25:2. [PubMed] [Google Scholar]
- 24.Morris RE. Rapamycin: FK506’s fraternal twin or distant cousin? Immunol Today. 1991;12:137. doi: 10.1016/S0167-5699(05)80040-4. [DOI] [PubMed] [Google Scholar]
- 25.Chong AS-F, Gebel H, Finnegan A, et al. Leflunomide, a novel immunomodulatory agent: in vitro analyses of the mechanism of immunosuppression. Transplant Proc. 1993;25(1):747. [PubMed] [Google Scholar]
- 26.Morris RE. ± 15-deoxyspergualin: A mystery wrapped within an enigma. Clin Transplant. 1991;5:530. [Google Scholar]
- 27.Brinker KR, Dickerman RM, Gonwa TA, et al. A randomized trial comparing double-drug and triple-drug therapy in primary cadaveric renal transplants. Transplantation. 1990;50:43. doi: 10.1097/00007890-199007000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Lindholm A, Albrechtsen D, Tufveson G, Karlberg I, Persson NH, Groth CG. A randomized trial of cyclosporine and prednisone versus cyclosporine, azathioprine, and prednisolone in primary cadaveric renal transplantation. Transplantation. 1992;54:624. doi: 10.1097/00007890-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Cecka JM, Young WC, Teresaki PI. Analyses of the UNOS scientific renal transplant registry at three years—early events affecting transplant success. Transplantation. 1992;53(1):59. doi: 10.1097/00007890-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 30.McCauley J, Takaya S, Fung J, Tzakis A, Abu-Elmagd K, Jain A, Todo S, Starzl TE. The question of FK506 nephrotoxicity after liver transplantation. Transplant Proc. 1991;23:1444. [PMC free article] [PubMed] [Google Scholar]
- 31.Starzl TE, Abu-Elmagd K, Tzakis A, Fung JJ, Porter KA, Todo S. Selected topics on FK506, with special references to rescue of extrahepatic whole organ grafts, transplantation of “forbidden organs,” side effects, mechanisms, and practical pharmacokinetics. Transplant Proc. 1991;23:914. [PubMed] [Google Scholar]
- 32.Starzl TE. FK506 versus cyclosporine. Transplant Proc. 1993;25:511. [PMC free article] [PubMed] [Google Scholar]
- 33.Demetris AJ, Banner B, Fung JJ, Shapiro R, Jordan M, Starzl M. Histopathology of human renal allograft function under FK506: a comparison with cyclosporine. Transplant Proc. 1991;23:944. [PMC free article] [PubMed] [Google Scholar]
- 34.Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506: clinical significance and comparison with cyclosporine. Am J Surg Pathol. 1993;17:60. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro R, Fung JJ, Jain AB, Parks P, Todo S, Starzl TE. The side effects of FK506 in humans. Transplantation Proc. 1990;22 suppl 1:35. [PMC free article] [PubMed] [Google Scholar]
- 36.Scantlebury V, Shapiro R, Fung JJ, et al. New onset of diabetes in FK506 vs. cyclosporine-treated kidney transplant recipients. Transplant Proc. 1991;23:3169. [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis D, Shapiro R, Jordan ML, et al. Comparison of FK506 and cyclosporine regimens in pediatric renal transplantation. Pediatr Nephrol. 1994;8:193. doi: 10.1007/BF00865477. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro R, Scantlebury V, Jordan ML, et al. FK506 in pediatric kidney transplantation—primary and rescue experience. Kidney Int. doi: 10.1007/BF00867683. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armitage JM, Fricker FJ, Del Nido P, Cipriani L, Starzl TE. The clinical trial of FK506 as primary and rescue immunosuppression in pediatric cardiac transplantation. Transplantation Proc. 1991;23:3058. [PMC free article] [PubMed] [Google Scholar]
- 40.Fontes P, Rao AS, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]