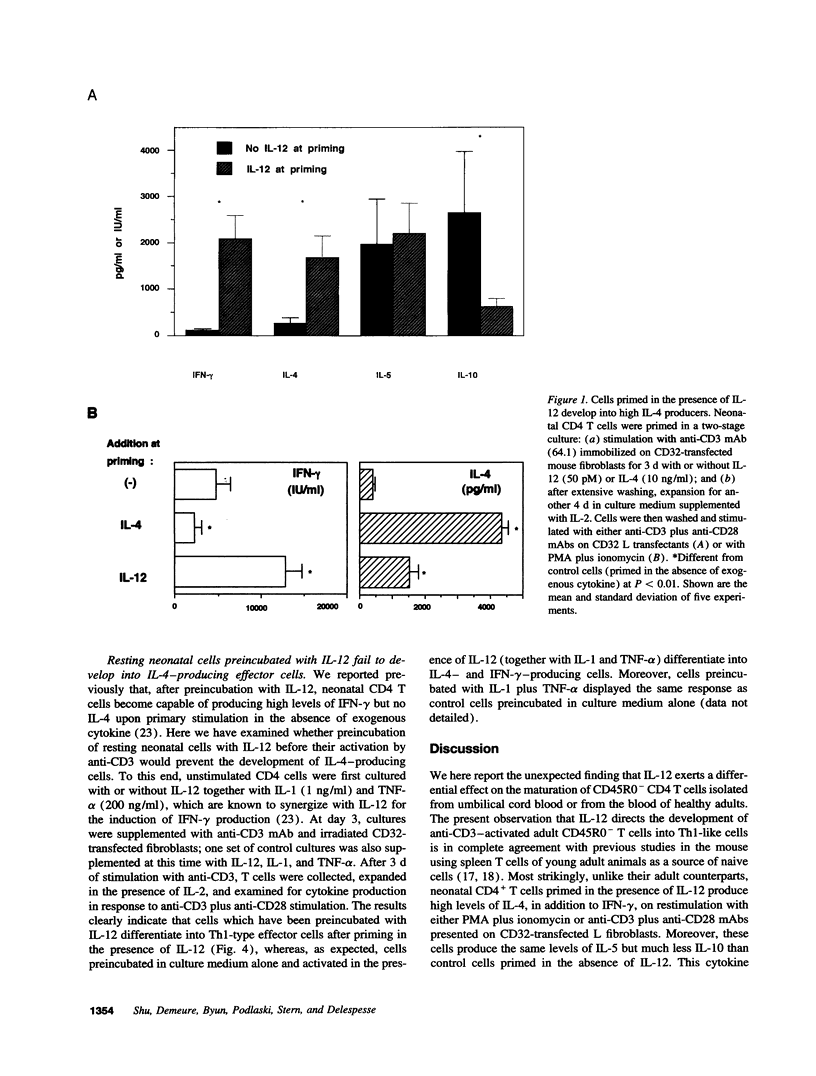
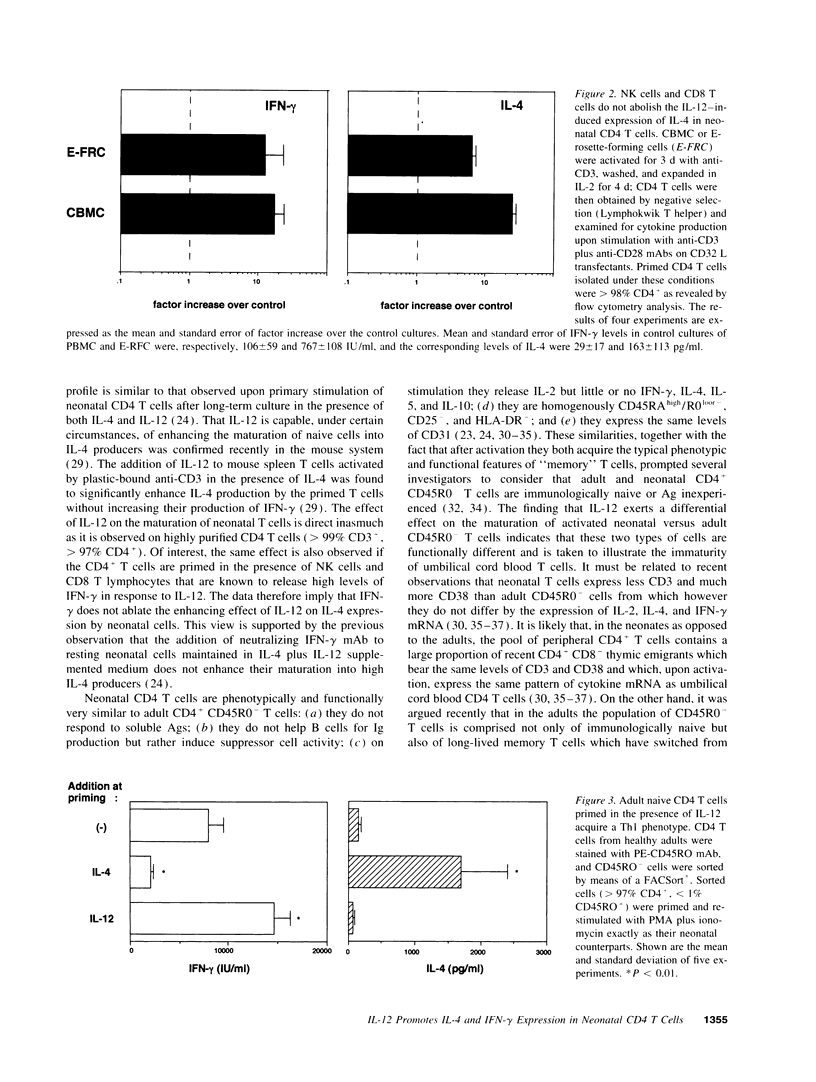
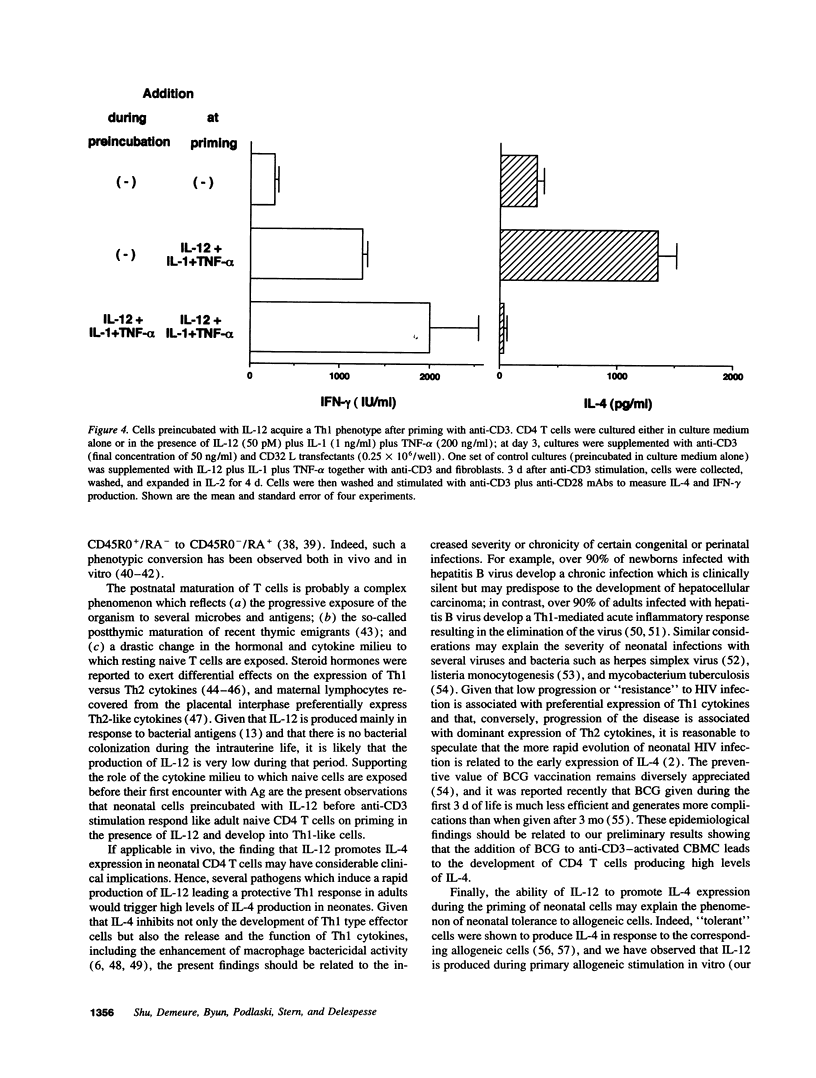
Abstract
It is now recognized that IL-12 plays a predominant role in protective immunity against intracellular pathogens by promoting the development of T helper type 1 (Th1) responses. We here report the unexpected observations that IL-12 exerts differential effects on the maturation of "native" human CD4 T cells isolated from umbilical cord blood or from the blood of healthy adults. After priming in the presence of IL-12, naive cells of adult donors, defined as CD45R0- CD4+ T cells, acquire a Th1 phenotype whereas neonatal cells develop into effector cells producing high levels of IL-4 in addition to IFN-gamma. This effect of IL-12 on neonatal T cells is direct inasmuch as it is observed on highly purified CD4 T cells, however, it is not inhibited by CD8 T cells and natural killer cells. Unstimulated neonatal T cells which have been preincubated with IL-12 before the priming behave like adult T cells and acquire a Th1 phenotype after stimulation in the presence of IL-12. Given that IL-4 is a potent antagonist of Th1 responses, the finding that IL-12 promotes the maturation of neonatal T cells into IL-4 producers may explain the increased susceptibility of neonates to intracellular pathogens and should be taken into account for the development of vaccines to be used in the perinatal period.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz D., Durez P., Gerard C., Donckier V., Amraoui Z., Velu T., Goldman M. Neonatal induction of transplantation tolerance in mice is associated with in vivo expression of IL-4 and -10 mRNAs. Transplant Proc. 1993 Feb;25(1 Pt 1):312–313. [PubMed] [Google Scholar]
- Bell E. B. Function of CD4 T cell subsets in vivo: expression of CD45R isoforms. Semin Immunol. 1992 Feb;4(1):43–50. [PubMed] [Google Scholar]
- Beverley P. C. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992 Feb;4(1):35–41. [PubMed] [Google Scholar]
- Clerici M., DePalma L., Roilides E., Baker R., Shearer G. M. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest. 1993 Jun;91(6):2829–2836. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Dudley D. J., Araneo B. A. Regulation of murine lymphokine production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur J Immunol. 1990 Apr;20(4):793–802. doi: 10.1002/eji.1830200413. [DOI] [PubMed] [Google Scholar]
- Demeure C. E., Wu C. Y., Shu U., Schneider P. V., Heusser C., Yssel H., Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J Immunol. 1994 May 15;152(10):4775–4782. [PubMed] [Google Scholar]
- Denich K., Börlin P., O'Hanley P. D., Howard M., Heath A. W. Expression of the murine interleukin-4 gene in an attenuated aroA strain of Salmonella typhimurium: persistence and immune response in BALB/c mice and susceptibility to macrophage killing. Infect Immun. 1993 Nov;61(11):4818–4827. doi: 10.1128/iai.61.11.4818-4827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. Production of inflammatory cytokines in the intestinal lamina propria. Immunol Res. 1991;10(3-4):239–246. doi: 10.1007/BF02919699. [DOI] [PubMed] [Google Scholar]
- Gately M. K. Interleukin-12: a recently discovered cytokine with potential for enhancing cell-mediated immune responses to tumors. Cancer Invest. 1993;11(4):500–506. doi: 10.3109/07357909309018881. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli R., Rambotti P., Cernetti C., Velardi A., Spinozzi F., Tabilio A., Martelli M. F., Grignani F., Davis S. A mature thymocyte-like phenotypic pattern on human cord circulating T-lymphoid cells. J Clin Immunol. 1984 Nov;4(6):461–468. doi: 10.1007/BF00916576. [DOI] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Schoenhaut D. S., Dwyer C. M., McComas W., Motyka R., Nabavi N., Wolitzky A. G., Quinn P. M., Familletti P. C. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Harris D. T., Schumacher M. J., Locascio J., Besencon F. J., Olson G. B., DeLuca D., Shenker L., Bard J., Boyse E. A. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993 May 1;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbert M. R., L'age-Stehr J., Mitchison N. A. Antigen presentation, loss of immunological memory and AIDS. Immunol Today. 1993 Jul;14(7):340–344. doi: 10.1016/0167-5699(93)90232-A. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Ildirim I., Sapan N., Cavuşoğlu B. Comparison of BCG vaccination at birth and at third month of life. Arch Dis Child. 1992 Jan;67(1):80–82. doi: 10.1136/adc.67.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991 Jan;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Mosmann T. R., Guilbert L., Tuntipopipat S., Wegmann T. G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993 Nov 1;151(9):4562–4573. [PubMed] [Google Scholar]
- Locksley R. M. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis H. S., Alter M. J., Hadler S. C. Hepatitis B: evolving epidemiology and implications for control. Semin Liver Dis. 1991 May;11(2):84–92. doi: 10.1055/s-2008-1040427. [DOI] [PubMed] [Google Scholar]
- Michie C. A., McLean A., Alcock C., Beverley P. C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992 Nov 19;360(6401):264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Nutman T. B. Type 2 cytokines and negative immune regulation in human infections. Curr Opin Immunol. 1993 Aug;5(4):511–517. doi: 10.1016/0952-7915(93)90031-m. [DOI] [PubMed] [Google Scholar]
- Morris S. C., Madden K. B., Adamovicz J. J., Gause W. C., Hubbard B. R., Gately M. K., Finkelman F. D. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994 Feb 1;152(3):1047–1056. [PubMed] [Google Scholar]
- Peleman R., Wu J., Fargeas C., Delespesse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J Exp Med. 1989 Nov 1;170(5):1751–1756. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz G. A., Trounstine M. L., Moore K. W. Cloned and expressed human Fc receptor for IgG mediates anti-CD3-dependent lymphoproliferation. J Immunol. 1988 Sep 15;141(6):1891–1896. [PubMed] [Google Scholar]
- Peltz G. A role for CD4+ T-cell subsets producing a selective pattern of lymphokines in the pathogenesis of human chronic inflammatory and allergic diseases. Immunol Rev. 1991 Oct;123:23–35. doi: 10.1111/j.1600-065x.1991.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Powell T. J., Jr, Streilein J. W. Neonatal tolerance induction by class II alloantigens activates IL-4-secreting, tolerogen-responsive T cells. J Immunol. 1990 Feb 1;144(3):854–859. [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Rothstein D. M., Yamada A., Schlossman S. F., Morimoto C. Cyclic regulation of CD45 isoform expression in a long term human CD4+CD45RA+ T cell line. J Immunol. 1991 Feb 15;146(4):1175–1183. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Schmitt E., Hoehn P., Germann T., Rüde E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994 Feb;24(2):343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Dvorak A. M., Sharkis S. J., Kagey-Sobotka A., Niv Y., Finkelman F. D., Barbieri S. A., Galli S. J., Plaut M. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro C. N. Epidemiology of hepatitis B. Pediatr Infect Dis J. 1993 May;12(5):433–437. doi: 10.1097/00006454-199305000-00036. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Podlaski F. J., Hulmes J. D., Pan Y. C., Quinn P. M., Wolitzky A. G., Familletti P. C., Stremlo D. L., Truitt T., Chizzonite R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O., Ishizaka S. T. Ontogeny of T-cell function: alloreactivity appears earlier than reactivity against hapten-modified self and interleukin-2 production. Clin Immunol Immunopathol. 1982 May;23(2):202–214. doi: 10.1016/0090-1229(82)90108-8. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torimoto Y., Rothstein D. M., Dang N. H., Schlossman S. F., Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol. 1992 Jan 15;148(2):388–396. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993 Jul;14(7):335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- Whitley R. J. Neonatal herpes simplex virus infections: pathogenesis and therapy. Pathol Biol (Paris) 1992 Sep;40(7):729–734. [PubMed] [Google Scholar]
- Wilson M., Rosen F. S., Schlossman S. F., Reinherz E. L. Ontogeny of human T and B lymphocytes during stressed and normal gestation: phenotypic analysis of umbilical cord lymphocytes from term and preterm infants. Clin Immunol Immunopathol. 1985 Oct;37(1):1–12. doi: 10.1016/0090-1229(85)90129-1. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Demeure C. E., Gately M., Podlaski F., Yssel H., Kiniwa M., Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. I. Induction of IL-4-producing cells after long-term culture in the presence of IL-4 plus either IL-2 or IL-12. J Immunol. 1994 Feb 1;152(3):1141–1153. [PubMed] [Google Scholar]
- Wu C. Y., Demeure C., Kiniwa M., Gately M., Delespesse G. IL-12 induces the production of IFN-gamma by neonatal human CD4 T cells. J Immunol. 1993 Aug 15;151(4):1938–1949. [PubMed] [Google Scholar]