Abstract
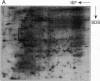
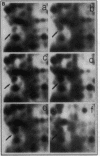
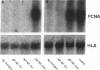
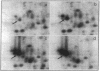
Mycobacteria have been implicated in the pathogenesis of autoimmunity. To determine the potential effect of mycobacterial antigens on peripheral blood mononuclear cells (PBMC), we analyzed PBMC incubated with the acetone-precipitable fraction of Mycobacterium tuberculosis (APMT) for changes in cellular protein expression. Two-dimensional gel analysis showed induction of a 36-kD polypeptide identified as proliferating cell nuclear antigen (PCNA), a known autoantigen, after incubation with AP-MT. PCNA plays a role in cell proliferation and is expressed as a late growth regulated factor. However, its synthesis in response to AP-MT was induced as an early event. The early induction of PCNA was regulated at a posttranscriptional level and was restricted to T cells. Treatment of PBMC with known T cell mitogens, namely PHA, anti-CD3 antibodies, and staphylococcal superantigens failed to induce an early PCNA increase. The distinct characteristics of the AP-MT effect on PCNA expression suggest a separate mechanism of induction in response to AP-MT, compared with the late increase observed in response to mitogens. The induction of PCNA in response to mycobacterial antigens may represent a pathogenically relevant mechanism in autoimmunity.
Full text
PDF


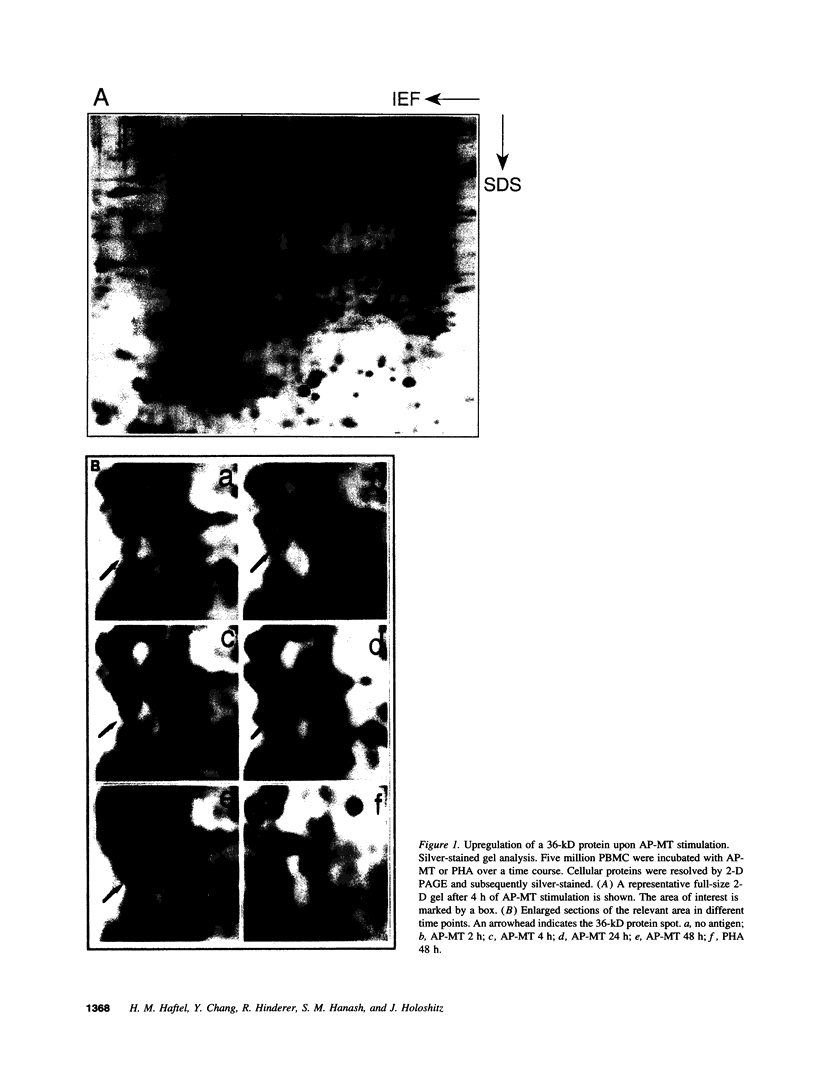
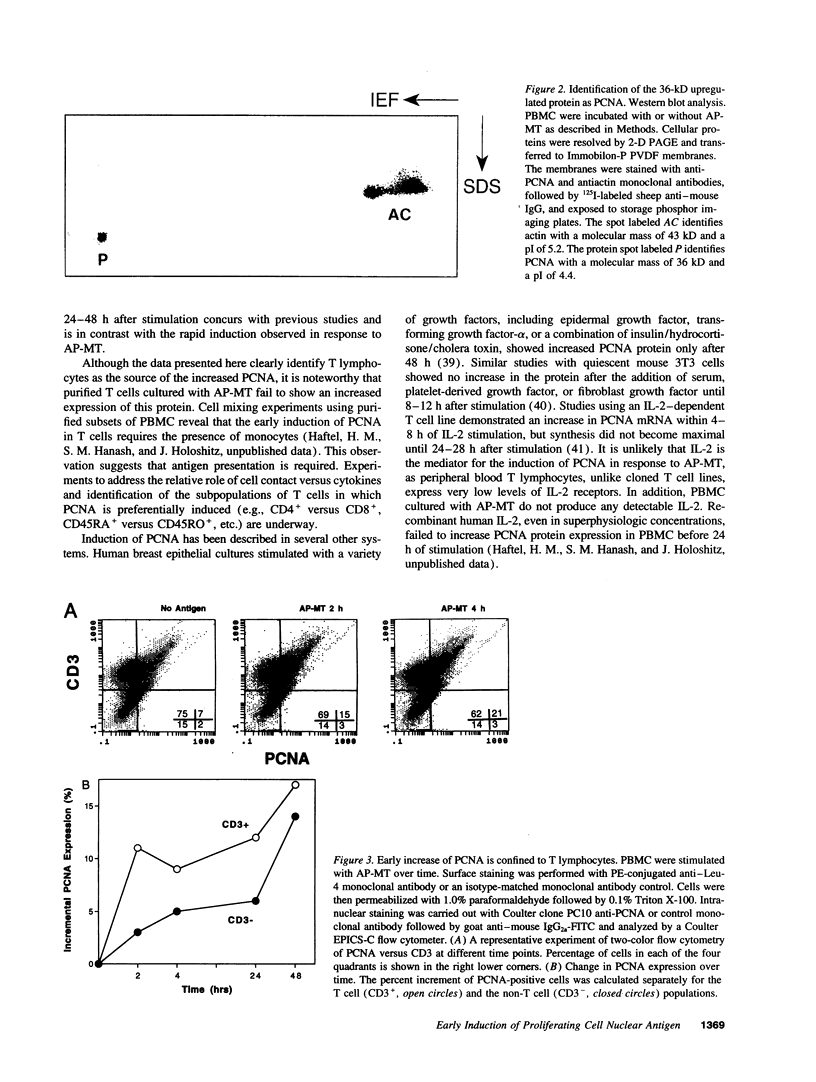
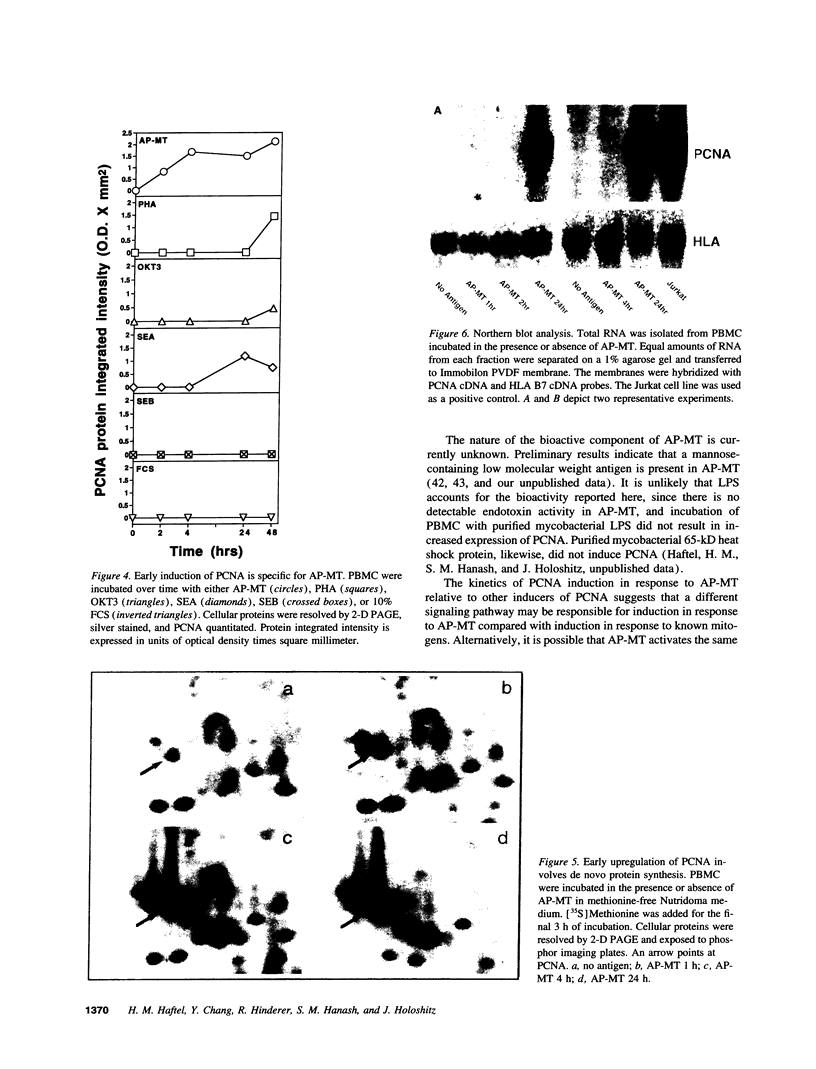
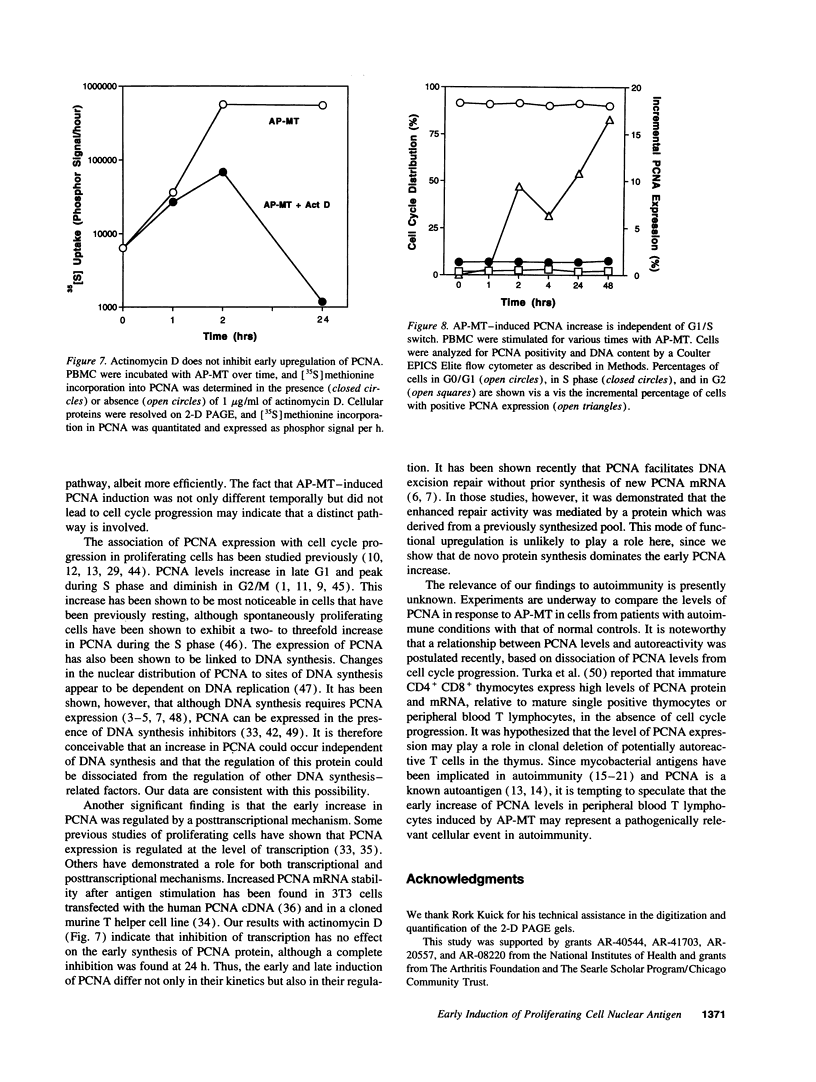

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alder H., Yoshinouchi M., Prystowsky M. B., Appasamy P., Baserga R. A conserved region in intron 1 negatively regulates the expression of the PCNA gene. Nucleic Acids Res. 1992 Apr 11;20(7):1769–1775. doi: 10.1093/nar/20.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asero R., Origgi L., Crespi S., Bertetti E., D'Agostino P., Riboldi P. Autoantibody to proliferating cell nuclear antigen (PCNA) in SLE: a clinical and serological study. Clin Exp Rheumatol. 1987 Jul-Sep;5(3):241–246. [PubMed] [Google Scholar]
- Bravo R., Bellatin J., Celis J. E. [35S]-methionine labelled polypeptides from HELA cells. Coordinates and percentage of some major polypeptides. Cell Biol Int Rep. 1981 Jan;5(1):93–96. doi: 10.1016/0309-1651(81)90162-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Induction of the nuclear protein 'cyclin' in quiescent mouse 3T3 cells stimulated by serum and growth factors. Correlation with DNA synthesis. EMBO J. 1984 Dec 20;3(13):3177–3181. doi: 10.1002/j.1460-2075.1984.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. D., Ottavio L., Travali S., Lipson K. E., Baserga R. Transcriptional and posttranscriptional regulation of the proliferating cell nuclear antigen gene. Mol Cell Biol. 1990 Jul;10(7):3289–3296. doi: 10.1128/mcb.10.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Romzek N. C., Jia Y., Wagner L., Vila L. M., Chen S. J., Wilson J. M., Karp D. R. MHC-independent presentation of mycobacteria to human gamma delta T cells. Int Immunol. 1993 Nov;5(11):1437–1443. doi: 10.1093/intimm/5.11.1437. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Vila L. M., Keroack B. J., McKinley D. R., Bayne N. K. Dual antigenic recognition by cloned human gamma delta T cells. J Clin Invest. 1992 Jan;89(1):308–314. doi: 10.1172/JCI115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Keim D., Hailat N., Hodge D., Hanash S. M. Proliferating cell nuclear antigen expression in childhood acute leukemia. Blood. 1990 Sep 1;76(5):985–990. [PubMed] [Google Scholar]
- Kurki P., Lotz M., Ogata K., Tan E. M. Proliferating cell nuclear antigen (PCNA)/cyclin in activated human T lymphocytes. J Immunol. 1987 Jun 15;138(12):4114–4120. [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Macdonald-Bravo H., Bravo R. Induction of the nuclear protein cyclin in serum-stimulated quiescent 3T3 cells is independent of DNA synthesis. Exp Cell Res. 1985 Feb;156(2):455–461. doi: 10.1016/0014-4827(85)90552-x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Moriuchi T., Koji T., Nakane P. K. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 1987 Mar;6(3):637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Monaghan P., Perusinghe N. P., Nicholson R. I., McClelland R. A., O'Hare M. J., Lane D. P., Jayatilake H., Gusterson B. A. Growth factor stimulation of proliferating cell nuclear antigen (PCNA) in human breast epithelium in organ culture. Cell Biol Int Rep. 1991 Jul;15(7):561–570. doi: 10.1016/0309-1651(91)90003-2. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Gulle H., Kaufmann S. H., Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990 May;20(5):1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Plesnila N., Lipford G. B., Kromer S., Deusch K., Wagner H. A lectin-binding, protease-resistant mycobacterial ligand specifically activates V gamma 9+ human gamma delta T cells. J Immunol. 1992 Jan 15;148(2):575–583. [PubMed] [Google Scholar]
- Pope R. M., Wallis R. S., Sailer D., Buchanan T. M., Pahlavani M. A. T cell activation by mycobacterial antigens in inflammatory synovitis. Cell Immunol. 1991 Mar;133(1):95–108. doi: 10.1016/0008-8749(91)90182-b. [DOI] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Sabath D. E., Monos D. S., Lee S. C., Deutsch C., Prystowsky M. B. Cloned T-cell proliferation and synthesis of specific proteins are inhibited by quinine. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4739–4743. doi: 10.1073/pnas.83.13.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman-Appasamy P., Cohen K. S., Prystowsky M. B. Interleukin 2-induced expression of proliferating cell nuclear antigen is regulated by transcriptional and post-transcriptional mechanisms. J Biol Chem. 1990 Nov 5;265(31):19180–19184. [PubMed] [Google Scholar]
- Shipman P. M., Sabath D. E., Fischer A. H., Comber P. G., Sullivan K., Tan E. M., Prystowsky M. B. Cyclin mRNA and protein expression in recombinant interleukin 2-stimulated cloned murine T lymphocytes. J Cell Biochem. 1988 Nov;38(3):189–198. doi: 10.1002/jcb.240380306. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Takasaki Y., Deng J. S., Tan E. M. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981 Dec 1;154(6):1899–1909. doi: 10.1084/jem.154.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi L., Bravo R. Changes in cyclin/proliferating cell nuclear antigen distribution during DNA repair synthesis. J Cell Biol. 1988 Nov;107(5):1623–1628. doi: 10.1083/jcb.107.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L. A., Gratiot-Deans J., Keim D., Bandukwala R., Green J., Strahler J., Hanash S. M. Elevated proliferating cell nuclear antigen levels in immature thymocytes. Dissociation from cell cycle progression. J Immunol. 1993 Apr 1;150(7):2746–2752. [PubMed] [Google Scholar]
- Weinberg D. H., Kelly T. J. Requirement for two DNA polymerases in the replication of simian virus 40 DNA in vitro. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9742–9746. doi: 10.1073/pnas.86.24.9742. [DOI] [PMC free article] [PubMed] [Google Scholar]