Abstract
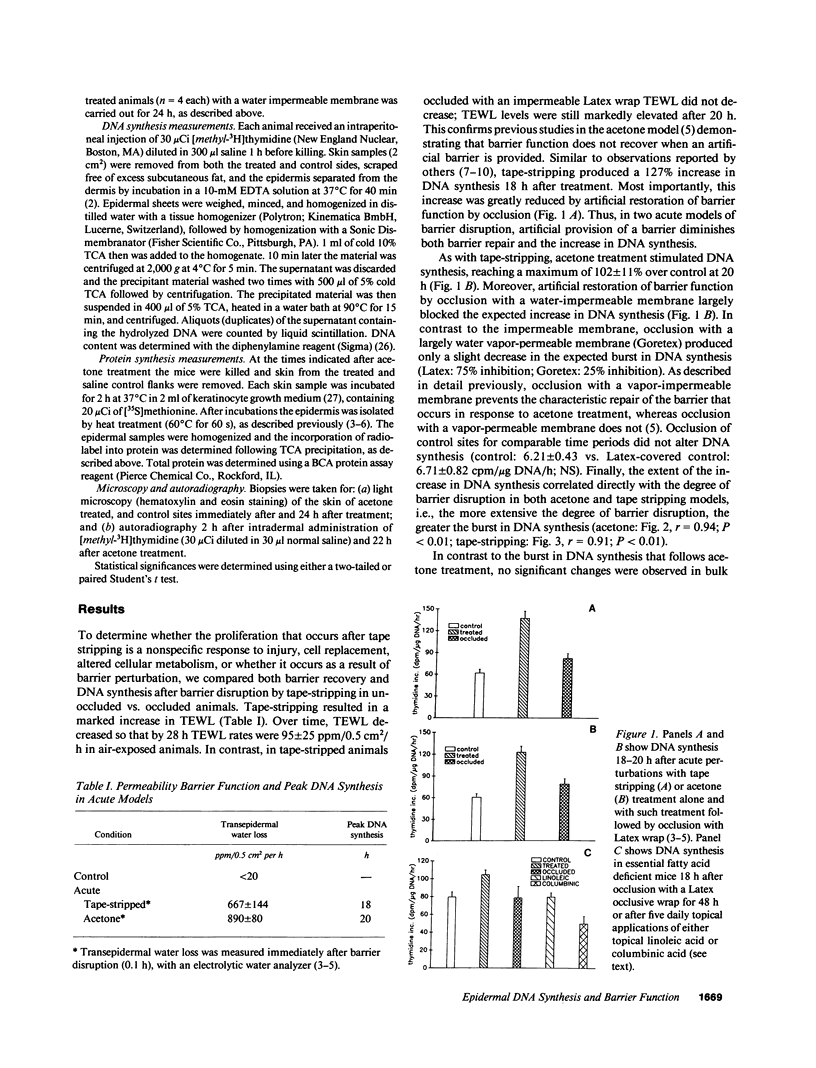
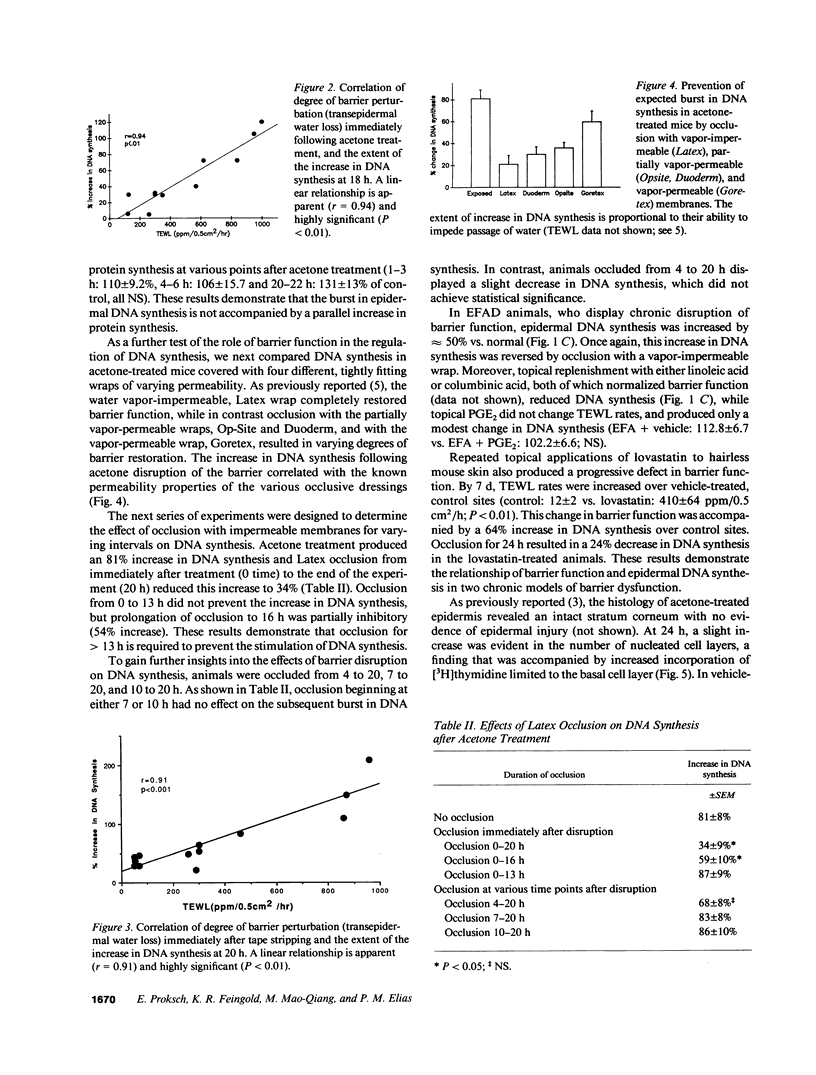
We examined the possibility that the cutaneous permeability barrier regulates epidermal DNA synthesis in two acute and two chronic models of barrier perturbation. In animals treated topically with acetone, DNA synthesis is increased 102%, in tape-stripped animals 127%, in essential fatty acid deficient animals 50%, and in animals chronically treated with topical lovastatin 64%. This linkage between disturbances in barrier function and increased DNA synthesis is further supported by specific and correlative observations: (a) in these disparate models, artificial replacement of the barrier with a water-impermeable membrane inhibits the expected increase in DNA synthesis; (b) the extent of the burst in DNA synthesis is proportional to the degree of barrier abrogation; (c) the inhibition of DNA synthesis by membranes is directly related to the degree of permeability of these occlusive membranes, i.e., the more impermeable the greater the degree of inhibition; (d) topical treatment with lipids that restore barrier function corrects the increase in DNA synthesis; and (e) barrier abrogation with acetone produces an increase in epidermal DNA synthesis without altering bulk protein synthetic rates in contrast to events known to follow injury or cell replacement. Autoradiographic studies show that the increase in DNA synthesis after acetone treatment is limited to the epidermal basal layer. This constellation of findings strongly suggests that cutaneous barrier function is one factor that regulates epidermal DNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Brown B. E. The mammalian cutaneous permeability barrier: defective barrier function is essential fatty acid deficiency correlates with abnormal intercellular lipid deposition. Lab Invest. 1978 Dec;39(6):574–583. [PubMed] [Google Scholar]
- Elliott W. J., Sprecher H., Needleman P. Physiologic effects of columbinic acid and its metabolites on rat skin. Biochim Biophys Acta. 1985 Jun 14;835(1):158–160. doi: 10.1016/0005-2760(85)90043-8. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Brown B. E., Lear S. R., Moser A. H., Elias P. M. Effect of essential fatty acid deficiency on cutaneous sterol synthesis. J Invest Dermatol. 1986 Nov;87(5):588–591. doi: 10.1111/1523-1747.ep12455835. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Man M. Q., Proksch E., Menon G. K., Brown B. E., Elias P. M. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991 Feb;96(2):201–209. doi: 10.1111/1523-1747.ep12461153. [DOI] [PubMed] [Google Scholar]
- Fisher L. B., Maibach H. I. Physical occlusion controlling epidermal mitosis. J Invest Dermatol. 1972 Jul;59(1):106–108. doi: 10.1111/1523-1747.ep12625873. [DOI] [PubMed] [Google Scholar]
- Fisher L. B., Maibach H. I. The effect of occlusive and semipermeable dressings on the mitotic activity of normal and wounded human epidermis. Br J Dermatol. 1972 Jun;86(6):593–600. doi: 10.1111/j.1365-2133.1972.tb05074.x. [DOI] [PubMed] [Google Scholar]
- Fritsch P. O., Pohlin G., Längle U., Elias P. M. Response of epidermal cell proliferation to orally administered aromatic retinoid. J Invest Dermatol. 1981 Sep;77(3):287–291. doi: 10.1111/1523-1747.ep12482459. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Grice K. A., Bettley F. R. Skin water loss and accidental hypothermia in psoriasis, ichthyosis, and erythroderma. Br Med J. 1967 Oct 28;4(5573):195–198. doi: 10.1136/bmj.4.5573.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubauer G., Elias P. M., Feingold K. R. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989 Mar;30(3):323–333. [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Elias P. M. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res. 1987 Jun;28(6):746–752. [PubMed] [Google Scholar]
- Hartop P. J., Prottey C. Changes in transepidermal water loss and the composition of epidermal lecithin after applications of pure fatty acid triglycerides to skin of essential fatty acid-deficient rats. Br J Dermatol. 1976 Sep;95(3):255–264. doi: 10.1111/j.1365-2133.1976.tb07012.x. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., van der Beek A. Effects of topical application of fatty acids. Prog Lipid Res. 1981;20:219–224. doi: 10.1016/0163-7827(81)90041-2. [DOI] [PubMed] [Google Scholar]
- Lowe N. J. Essential fatty acid deficient hairless mouse: the effects of topical agents on the epidermis. Br J Dermatol. 1977 Jul;97(1):39–47. doi: 10.1111/j.1365-2133.1977.tb15425.x. [DOI] [PubMed] [Google Scholar]
- Lowe N. J., Stoughton R. B. Essential fatty acid deficient hairless mouse: a model of chronic epidermal hyperproliferation. Br J Dermatol. 1977 Feb;96(2):155–162. doi: 10.1111/j.1365-2133.1977.tb12537.x. [DOI] [PubMed] [Google Scholar]
- Marks F., Bertsch S., Fürstenberger G. Ornithine decarboxylase activity, cell proliferation, and tumor promotion in mouse epidermis in vivo. Cancer Res. 1979 Oct;39(10):4183–4188. [PubMed] [Google Scholar]
- McCullough J. L., Schreiber S. H., Ziboh V. A. Cell proliferation kinetics of epidermis in the essential fatty acid deficient rat. J Invest Dermatol. 1978 Jun;70(6):318–320. doi: 10.1111/1523-1747.ep12543484. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Moser A. H., Brown B. E., Elias P. M. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985 Apr;26(4):418–427. [PubMed] [Google Scholar]
- Menton D. N. The effects of essential fatty acid deficiency on the skin of the mouse. Am J Anat. 1968 Mar;122(2):337–355. doi: 10.1002/aja.1001220211. [DOI] [PubMed] [Google Scholar]
- PINKUS H. Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping. J Invest Dermatol. 1951 Jun;16(6):383–386. doi: 10.1038/jid.1951.45. [DOI] [PubMed] [Google Scholar]
- Petzoldt D. G., Braun-Falco O., Wenig K. H. Effects of plastic-foil-occlusion on psoriatic lesions. Arch Klin Exp Dermatol. 1970;238(2):160–168. doi: 10.1007/BF00519901. [DOI] [PubMed] [Google Scholar]
- Schwarz T., Luger T. A. Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B. 1989 Oct;4(1):1–13. doi: 10.1016/1011-1344(89)80097-1. [DOI] [PubMed] [Google Scholar]
- Siegenthaler U., Laine A., Polak L. Studies on contact sensitivity to chromium in the guinea pig. The role of valence in the formation of the antigenic determinant. J Invest Dermatol. 1983 Jan;80(1):44–47. doi: 10.1111/1523-1747.ep12531034. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D. Role of cholesterogenesis and isoprenoid synthesis in DNA replication and cell growth. J Lipid Res. 1984 Dec 15;25(13):1462–1468. [PubMed] [Google Scholar]
- VANSCOTT E. J., EKEL T. M. KINETICS OF HYPERPLASIA IN PSORIASIS. Arch Dermatol. 1963 Oct;88:373–381. [PubMed] [Google Scholar]
- WILLIAMS M. G., HUNTER R. Studies on epidermal regeneration by means of the strip method. J Invest Dermatol. 1957 Dec;29(6):407–413. doi: 10.1038/jid.1957.116. [DOI] [PubMed] [Google Scholar]
- Weinstein G. D., Van Scott E. J. Autoradiographic analysis of turnover times of normal and psoriatic epidermis. J Invest Dermatol. 1965 Oct;45(4):257–262. doi: 10.1038/jid.1965.126. [DOI] [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. The extracellular matrix of stratum corneum: role of lipids in normal and pathological function. Crit Rev Ther Drug Carrier Syst. 1987;3(2):95–122. [PubMed] [Google Scholar]
- Ziboh V. A., Hsia S. L. Effects of prostaglandin E 2 on rat skin: inhibition of sterol ester biosynthesis and clearing of scaly lesions in essential fatty acid deficiency. J Lipid Res. 1972 Jul;13(4):458–467. [PubMed] [Google Scholar]



